The Association between Apolipoprotein A-II and Metabolic Syndrome in Korean Adults: A Comparison Study of Apolipoprotein A-I and Apolipoprotein B
Article information
Abstract
Background
Apolipoprotein A-II (apoA-II) is the second-most abundant apolipoprotein in human high-density lipoprotein and its role in cardio metabolic risk is not entirely clear. It has been suggested to have poor anti-atherogenic or even pro-atherogenic properties, but there are few studies on the possible role of apoA-II in Asian populations. The aim of this study is to evaluate the role of apoA-II in metabolic syndrome (MetS) compared with apolipoprotein A-I (apoA-I) and apolipoprotein B (apoB) in Korean adults.
Methods
We analyzed data from 244 adults who visited the Center for Health Promotion in Pusan National University Yangsan Hospital for routine health examinations.
Results
The mean apoB level was significantly higher, and the mean apoA-I level was significantly lower, in MetS; however, there was no significant difference in apoA-II levels (30.5±4.6 mg/dL vs. 31.2±4.6 mg/dL, P=0.261). ApoA-II levels were more positively correlated with apoA-I levels than apoB levels. ApoA-II levels were less negatively correlated with homocysteine and high sensitivity C-reactive protein levels than apoA-I levels. The differences in MetS prevalence from the lowest to highest quartile of apoA-II were not significant (9.0%, 5.7%, 4.9%, and 6.6%, P=0.279). The relative risk of the highest quartile of apoA-II compared with the lowest quartile also was not significantly different (odds ratio, 0.96; 95% confidence interval, 0.95 to 1.04; P=0.956).
Conclusion
Compared with apoA-I (negative association with MetS) and apoB (positive association with MetS) levels, apoA-II levels did not show any association with MetS in this study involving Korean adults. However, apoA-II may have both anti-atherogenic and pro-atherogenic properties.
INTRODUCTION
Plasma high density lipoprotein (HDL) is very heterogeneous, but apolipoprotein A-I (apoA-I) and apolipoprotein A-II (apoA-II) are two main sub-populations of HDL [1]. The antiatherogenic properties of apoA-I, the major structural apolipoprotein of HDL, are widely known [2-5], but in the case of apoA-II, the second most abundant structural apolipoprotein associated with HDL [1], the role on these properties is controversial. There have been several studies, showing an inverse relationship between cardiovascular disease, including type 2 diabetes mellitus, and plasma apoA-II levels [6-9]; however, other studies have suggested that elevated apoA-II levels may be pro-atherogenic [10], or have shown no relationship between cardiovascular disease and apoA-II levels [11,12].
Recently, Onat et al. [13] showed that low serum apoA-II concentrations confer risk of metabolic syndrome (MetS) and type 2 diabetes mellitus among Turkish adults at 4 years follow-up. There are few studies about the association between apoA-II and MetS, a constellation of atherosclerotic cardiovascular disease risk factors [14], and the possible properties of apoA-II in cardiovascular risk are even less clear, especially in Asian populations. Therefore, we tried to investigate the relationship of apoA-II with MetS and other inflammatory markers in Korean adults and the possible roles of apoA-II in comparison with other well-known lipid markers, such as apoA-I (anti-atherogenic) and apoB (pro-atherogenic).
METHODS
Subjects and measurements
Our study subjects included 244 adults (20 to 70 years of age; 159 men and 85 women) who visited the Center for Health Promotion at Pusan National University Yangsan Hospital for routine health examinations between December 2009 and April 2010. All subjects had no history of coronary heart disease (CHD) or cerebro-vascular disease. Subjects who were being treated with lipid-modifying agents or on estrogen replacement therapy, and those with hyperthyroidism, hypothyroidism, liver disease (serum levels of aspartate aminotransferase [AST] or alanine aminotransferase [ALT] were greater than three times the upper limit of the reference range), serum triglyceride levels ≥ 400 mg/dL, or abnormal serum creatinine levels (male, ≥1.5 mg/dL; female ≥1.3 mg/dL) were excluded. Height and weight were measured while subjects wore light clothing without shoes. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared and was used as an estimate of overall adiposity. Waist circumference, an estimate of central obesity, was measured with a soft tape on standing subjects midway between the lowest rib and the iliac crest. Blood pressure was measured on the right arm with subjects in a sitting position after a 5-minute rest. Venous blood was sampled after an overnight fast. Fasting glucose was measured by the glucose oxidase method (Synchron LX-20; Beckman Coulter Inc., Fullerton, CA, USA). Standard liver enzyme, total cholesterol, HDL cholesterol (HDL-C), and serum triglyceride (TG) concentrations were measured with an autoanalyzer using an enzymatic colorimetric method (Hitachi 7600; Hitachi Ltd., Tokyo, Japan). Serum levels of apoA-I, apoA-II, and apoB were measured by turbidimetric immuno assay using the automatic immunoassay analyzer Roche E-170 (Roche, Basel, Switzerland). The metabolic syndrome criteria used were those of the modified, revised NCEP-ATP III (using the WHO Western Pacific Region obesity criteria and defining glucose levels as ≥100 mg/dL [5.24 mmol/L]) [15,16], which defines metabolic syndrome as the presence of at least three of the following five traits: 1) Abdominal obesity: waist circumference ≥90 cm in men, ≥80 cm in women, 2) Hypertriglyceridemia (HyperTG): ≥150 mg/dL, (1.70 mmol/L), 3) Low HDL-C: <40 mg/dL (1.03 mmol/L) in men and <50 mg/dL (1.29 mmol/L) in women, 4) Hypertension: systolic blood pressure ≥130 mm Hg or diastolic pressure ≥85 mm Hg or on anti-hypertensive medication, 5) High fasting glucose: ≥100 mg/dL (5.54 mmol/L) or under treatment for diabetes. This study was approved by the Ethical Committee of Pusan National University Yangsan Hospital in Yangsan, Korea.
Statistical analysis
All data are given as mean±standard deviation for continuous variables. Median values are also indicated in the cases of AST, ALT, TG, and high sensitivity C-reactive protein (hs-CRP), which have skewed distributions. Components of MetS and other clinical characteristics were compared between subjects with and without MetS using an independent-sample Student's t-test and Mann-Whitney U test in variables with a skewed distribution. To evaluate the association between apoA-II and other variables, including apoA-I and apoB, we performed Pearson's partial correlation coefficients analyses, adjusting for age and sex. Because triglycerides and hs-CRP levels had skewed distributions, values were log-transformed before statistical analysis. The total subjects were divided into quartiles based on the distribution of apoA-I, apoA-II, and apoB. The prevalence of MetS in each quartile was indicated as a percentage and compared using the chi-square test. Finally, the odd ratios and corresponding 95% confidence intervals for the highest quartile of apoA-I, apoA-II, and apoB were calculated as an estimate of the relative risk of MetS by logistic regression analyses, with the lowest quartile used as the reference category. Statistical analyses were performed with an SPSS statistical package (SPSS Inc., Chicago, IL, USA). A probability value of less than 0.05 was considered significant.
RESULTS
The prevalence of MetS in this study was 26.2%. All values of MetS components were significantly higher in subjects with MetS than in those without MetS. ApoA-I levels were significantly lower (128.0±20.3 mg/dL vs. 137.4±18.5 mg/dL; P=0.001) and apoB levels were significantly higher (101.7±24.2 mg/dL vs. 88.9±20.4 mg/dL; P<0.001) in the MetS group compared with the non-MetS group. ApoA-II levels were lower (30.5±4.6 mg/dL vs. 31.2±4.6 mg/dL; P=0.261) in the MetS group, but there was no statistical significance (Table 1).
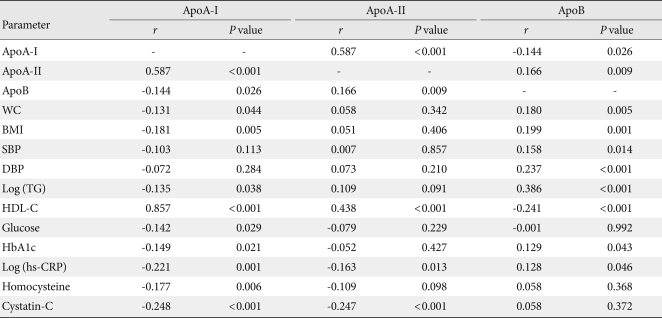
After adjusting for age and sex, apoA-II levels were more positively correlated with apoA-I levels (r=0.587, P<0.001) than apoB levels (r=0.166, P=0.009). When the significant correlation was limited to a coefficient >0.200, apoB levels showed significant positive correlations with diastolic blood pressure (r=0.237, P<0.001) and logTG (r=0.386, P<0.001). ApoB also showed weak positive correlations with waist circumference (r=0.180), BMI (r=0.199), and systolic blood pressure (r=0.158). ApoA-I levels showed weak negative correlations with waist circumference (r=-0.131), BMI (r=-0.181), logTG (r=-0.135), and fasting glucose levels (r=-0.142). ApoAII levels showed significant positive correlation with HDL-C levels (r=0.438, P<0.001), but its correlation was weaker than that of apoA-I levels (r=0.857, P<0.001). ApoB levels showed significant negative correlation with HDL-C levels (r=-0.241, P<0.001). Among the inflammatory markers, apoA-II levels were less negatively correlated with homocysteine and log (hs-CRP) (r=-0.109, P=0.098, and r=-0.163, P=0.013, respectively) than apoA-I levels (r=-0.177, P=0.006, and r=-0.221, P=0.001, respectively) (Table 2).
Partial correlation coefficients adjusted for age and sex for ApoA-I, Apo-AII, ApoB, metabolic risk factors, and other inflammatory markers
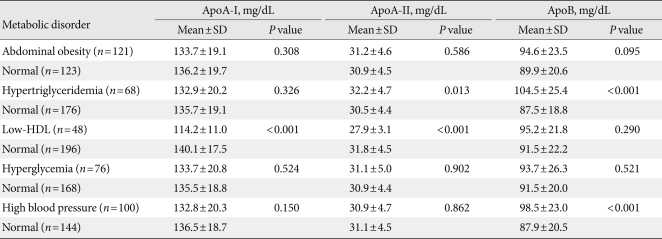
Among the components of MetS, apoA-II levels were significantly higher in subjects with hypertriglyceridemia (32.2±4.7 mg/dL vs. 30.5±4.4 mg/dL; P=0.013), and significantly lower in subjects with low HDL-C levels (27.9±3.1 mg/dL vs. 31.8±4.5 mg/dL; P<0.001). ApoA-I levels were significantly lower in subjects with low HDL-C levels (114.2±11.0 mg/dL vs. 140.1±17.5 mg/dL; P<0.001). ApoB levels were significantly higher in subjects with hypertriglyceridemia (104.5±25.4 mg/dL vs. 87.5±18.8 mg/dL; P<0.001) and high blood pressure (98.5±23.0 mg/dL vs. 87.9±20.5 mg/dL; P<0.001) (Table 3).
Comparison of mean ApoA-I, ApoA-II, and ApoB levels between subjects with and without individual metabolic component disorders
These three markers were divided into quartiles and the prevalence of MetS was evaluated. The prevalence of MetS significantly decreased with an increase in apoA-I quartile (12.3%, 4.1%, 4.9%, and 4.9%; P<0.001). Conversely, MetS significantly increased with an increase in apoB quartile (4.9%, 4.1%, 7.0%, and 10.2%; P=0.007). However, there were no significant differences in MetS prevalence according to the increase in apoA-II quartile (9.0%, 5.7%, 4.9%, and 6.6%; P=0.279) (Fig. 1).
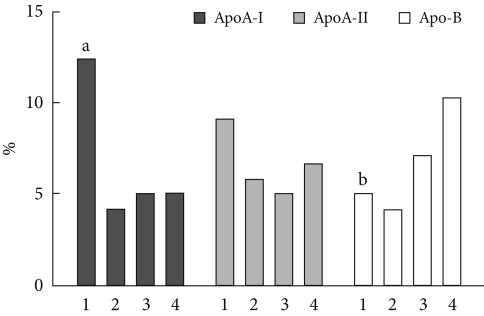
Prevalence of metabolic syndrome according to quartiles of ApoA-I, ApoA-II, and ApoB. Graphic bars are expressed as percent. Differences in prevalence among quartiles of each lipid marker were analysed using the chi-square test. ApoA-I, apolipoprotein A-I; ApoA-II, apolipoprotein A-II; ApoB, apolipoprotein B; 1, 1st quartile; 2, 2nd quartile; 3, 3rd quartile; 4, 4th quartile. aP<0.001; bP=0.007 compared to the 4th quartile.
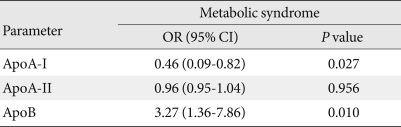
In logistic regression analysis after adjusting for age and sex, the relative risk of the highest quartile of apoA-I compared with the lowest quartile was significantly decreased (odds ratio [OR], 0.46; 95% confidence interval [CI], 0.09 to 0.82; P=0.027), and that of apoB was significantly increased (OR, 3.27; 95% CI, 1.36 to 7.86; P=0.010). However, there was no significant difference in the case of apoA-II (OR, 0.96; 95% CI, 0.95 to 1.04; P=0.956) (Table 4).
DISCUSSION
In the present study, we tried to evaluate the relationship between apoA-II and MetS in comparison with apoA-I (a marker for anti-atherogenic properties) and apoB (a marker for proatherogenic properties) in Korean subjects. ApoA-I levels were found to be significantly lower in the MetS group and they were negatively correlated with components of MetS, such as waist circumference, triglyceride levels, and fasting glucose levels. ApoA-II levels, however, did not differ significantly between the MetS group and non-MetS group and showed no significant correlations with MetS components, except HDLC. Furthermore, apoA-II was less negatively correlated with homocysteine and hs-CRP values, which are also known as markers with pro-atherogenic potential, than was apoA-I with homocysteine and hs-CRP values. With regard to the relationship between homocysteine and apoA-II, Taskinen et al. [17], reported that the greater the increase in homocysteine levels induced by fenofibrate, the smaller the increases in HDL-C and apoA-I levels, but there was a highly significant and positive relationship between fenofibrate-induced changes in homocysteine and apoA-II levels. These findings may suggest that apoA-II is less anti-atherogenic than apoA-I [18,19]. According to another study [9], apoA-II accounts for about 20% of HDL, and mean serum apoA-II concentrations are generally 4 times lower than mean apoA-I levels in normolipidemic subjects. So, this may explain our result of apoA-II being positively correlated with HDL-C levels (r=0.438), while its correlation was also weaker than that of apoA-I levels (r=0.857).
In a study by Birjmohun et al. [9], apoA-II levels showed positive correlation with triglyceride and HDL-C levels in spite of the inverse correlation between triglyceride and HDL-C levels. The authors explained that this result may be due to apoA-II inhibiting lipoprotein lipase and hepatic lipase activities, resulting in less hydrolysis of triglyceride-rich lipoproteins, which leads to increased triglyceride levels. In our study, apoA-II levels were positively correlated with apoB levels (r=0.166), as well as apoA-I levels, and apoA-II levels were significantly higher in the hypertriglyceridemic group. These findings also appear consistent with a previous study related to the pro-atherogenic potentials of apoA-II [10].
Recently, Onat et al. [13] showed that low serum apoA-II concentrations appear to predict incident MetS (relative risk, 3.5) and type 2 diabetes mellitus (relative risk, 4.5) in both sexes at an increment of 1 standard deviation, which is consistent with previous studies [6-9]; however, increased apoA-II values were not associated with prevalent or incident CHD and tended to be marginally atheroprotective only in males. In our study, the prevalence of Mets decreased as the quartiles of apoA-I increased, and increased with increasing quartiles of apoB, but there were no significant differences in MetS percentage between quartiles of apoA-II. Moreover, the relative risk of the highest quartile of apoA-II compared with the lowest quartile was not significantly different in logistic regression analysis after adjusting for age and sex. Birjmohun et al. [9] demonstrated that apoA-II levels are associated with a decreased risk of future CAD in apparently healthy people in a nested case-control study in the prospective EPIC-Norfolk cohort in the United Kingdom, but the authors commented that application of these results to non-white populations must be done with caution. So, given the results of Onat et al. [13] and our study, the possible role of apoA-II in cardiometabolic risk in Asian populations may be marginally atheroprotective or inconclusive.
It is widely known that HDL-C reduces cardiometabolic risk by enhancing the transfer of peripheral free cholesterol to the liver through the process called 'reverse cholesterol transport.' Some studies have reported that HDL containing apoA-I might be more effective in capturing cell-derived cholesterol for cholesterol efflux, the first step of reverse cholesterol transport, than HDL containing both apoA-I and apoA-II [20,21]. Furthermore, the effects of apoA-II on HDL metabolism are more complex and it may have both potential deleterious effects and beneficial effects [22]. The pro-atherogenic properties of apoA-II may inhibit lecithin-cholesterol acyltransferase [23,24], and inhibit hepatic cholesteryl uptake from HDL probably through the class B type 1 scavenger receptor dependent pathway [25,26]. The anti-atherogenic properties of apoA-II may inhibit cholesteryl ester transfer protein activity [27] and increase hepatic lipase activity [28]. Thus, our findings provide further evidence of the complex relationship between apoA-II and cardiovascular risk.
Our study has several limitations. First, it was performed using a cross-sectional design and did not control for potential biases from diet, physical activity, smoking and drinking history, and drug medication status (except for anti-lipid agents). Second, the total number of enrolled subjects was relatively small. Thus, a large-scale, prospective study will be needed to elucidate the exact role of apoA-II in cardiovascular disease.
In summary, apoA-II levels did not show any association with MetS in this study of Korean adults. However, apoA-II may have both anti-atherogenic and pro-atherogenic properties that may be caused by the diverse activities of apoA-II in association with cardiometabolic risk.
ACKNOWLEDGMENTS
This study was supported by the Clinical Research Grant (2010), Pusan National University Yangsan Hospital.
Notes
No potential conflict of interest relevant to this article was reported.