
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Supplementation of Clostridium butyricum Alleviates Vascular Inflammation in Diabetic Mice
- Tian Zhou, Shuo Qiu, Liang Zhang, Yangni Li, Jing Zhang, Donghua Shen, Ping Zhao, Lijun Yuan, Lianbi Zhao, Yunyou Duan, Changyang Xing
- Received April 21, 2023 Accepted July 10, 2023 Published online February 2, 2024
- DOI: https://doi.org/10.4093/dmj.2023.0109 [Epub ahead of print]
- 587 View
- 85 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Background
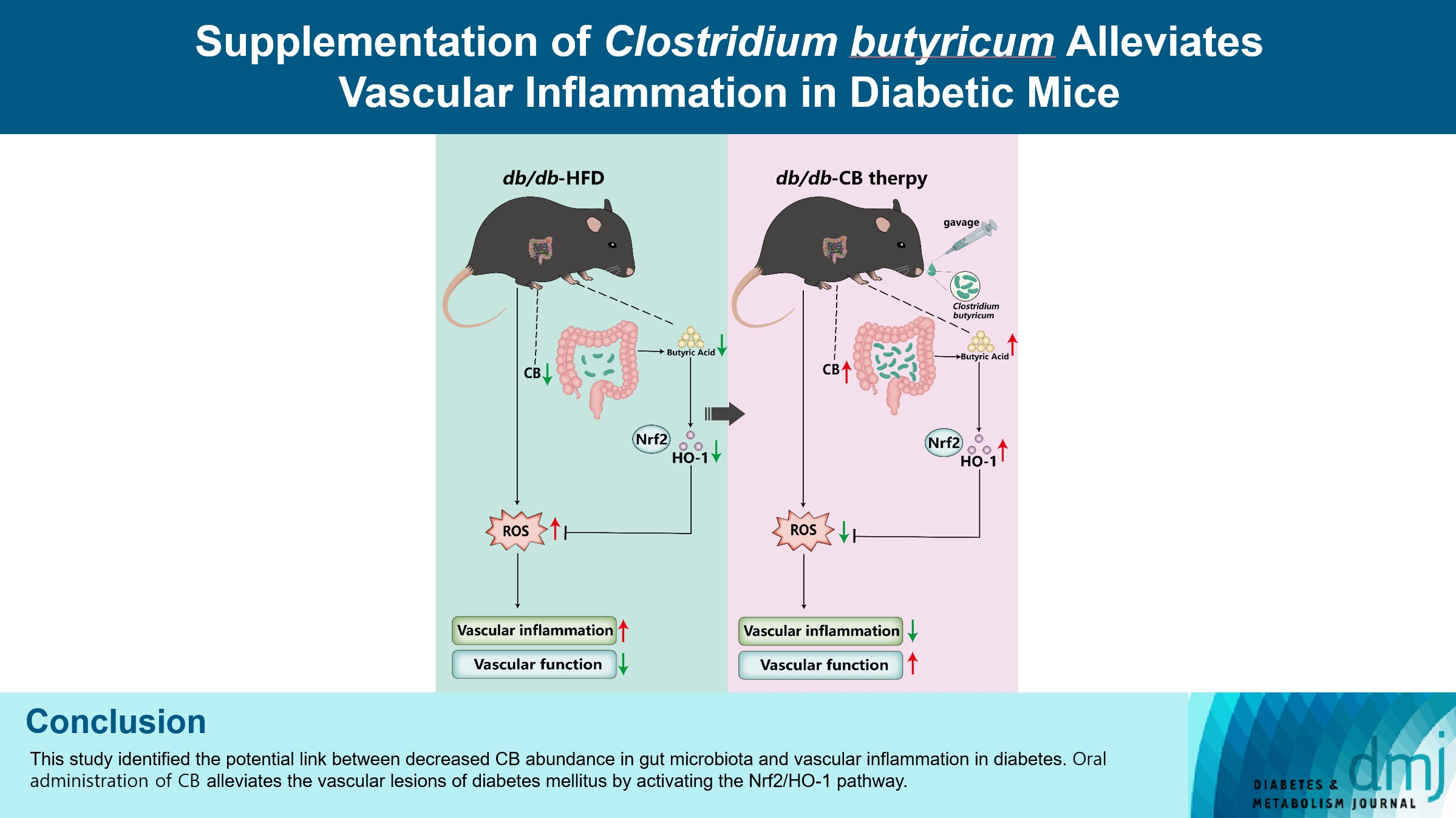
Gut microbiota is closely related to the occurrence and development of diabetes and affects the prognosis of diabetic complications, and the underlying mechanisms are only partially understood. We aimed to explore the possible link between the gut microbiota and vascular inflammation of diabetic mice.
Methods
The db/db diabetic and wild-type (WT) mice were used in this study. We profiled gut microbiota and examined the and vascular function in both db/db group and WT group. Gut microbiota was analyzed by 16s rRNA sequencing. Vascular function was examined by ultrasonographic hemodynamics and histological staining. Clostridium butyricum (CB) was orally administered to diabetic mice by intragastric gavage every 2 days for 2 consecutive months. Reactive oxygen species (ROS) and expression of nuclear factor erythroid-derived 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) were detected by fluorescence microscopy. The mRNA expression of inflammatory cytokines was tested by quantitative polymerase chain reaction.
Results
Compared with WT mice, CB abundance was significantly decreased in the gut of db/db mice, together with compromised vascular function and activated inflammation in the arterial tissue. Meanwhile, ROS in the vascular tissue of db/db mice was also significantly increased. Oral administration of CB restored the protective microbiota, and protected the vascular function in the db/db mice via activating the Nrf2/HO-1 pathway.
Conclusion
This study identified the potential link between decreased CB abundance in gut microbiota and vascular inflammation in diabetes. Therapeutic delivery of CB by gut transplantation alleviates the vascular lesions of diabetes mellitus by activating the Nrf2/HO-1 pathway.
- Basic Research
- Revisiting the Bacterial Phylum Composition in Metabolic Diseases Focused on Host Energy Metabolism
- Yeonmi Lee, Hui-Young Lee
- Diabetes Metab J. 2020;44(5):658-667. Published online July 9, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0220
- 9,018 View
- 131 Download
- 19 Web of Science
- 19 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
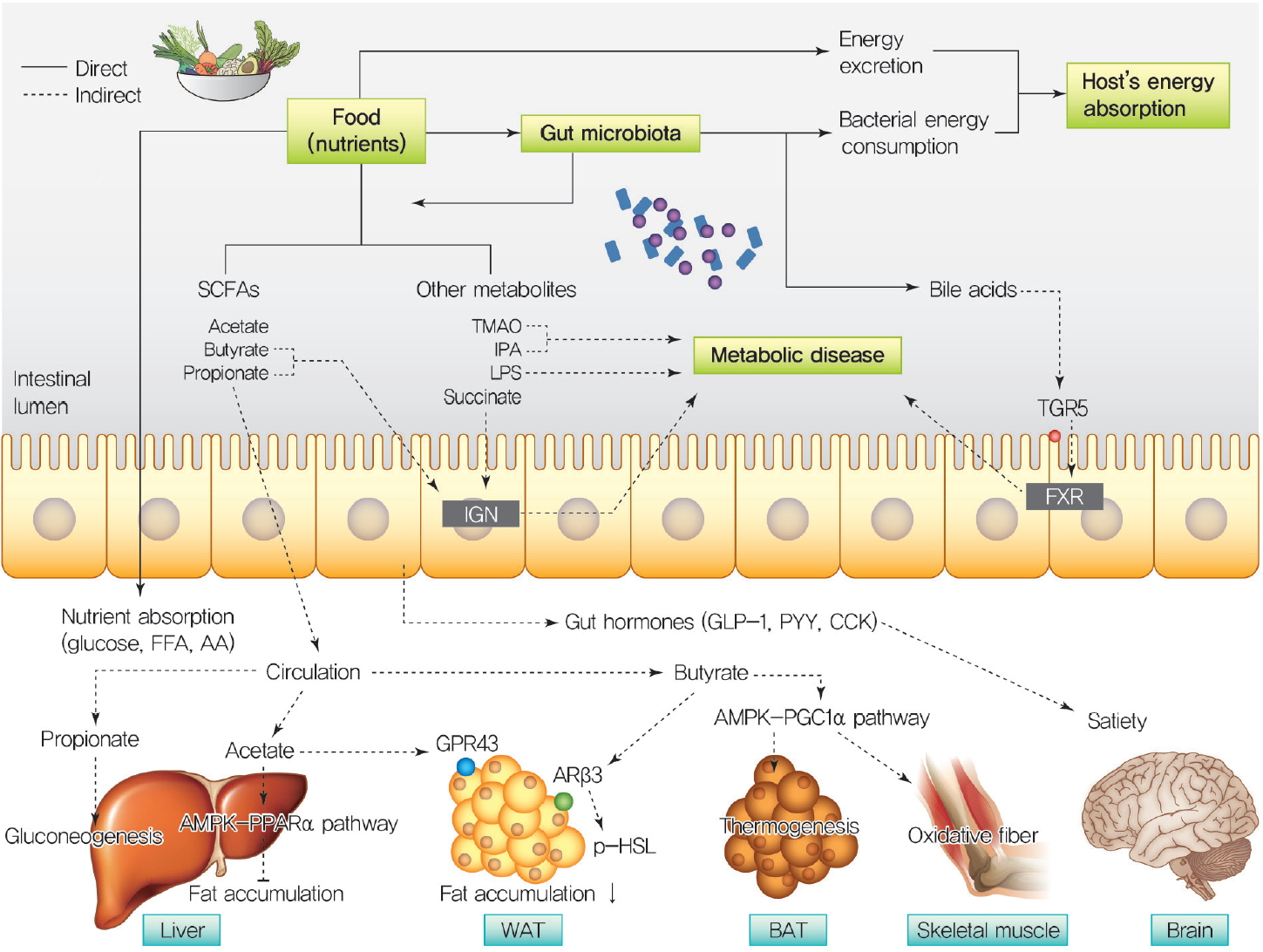
ePub Over a hundred billion bacteria are found in human intestines. This has emerged as an environmental factor in metabolic diseases, such as obesity and related diseases. The majority of these bacteria belong to two dominant phyla,
Bacteroidetes andFirmicutes . Since the ratio ofFirmicutes toBacteroidetes increases in people with obesity and in various animal models, it has been assumed that phylum composition causes the increase in occurrence of metabolic diseases over the past decade. However, this assumption has been challenged by recent studies that have found even an opposite association of phylum composition within metabolic diseases. Moreover, the gut microbiota affects host energy metabolism in various ways including production of metabolites and interaction with host intestinal cells to regulate signaling pathways that affect energy metabolism. However, the direct effect of gut bacteria on host energy intake, such as energy consumption by the bacteria itself and its effects on intestinal energy absorption, has been underestimated. This review aims to discuss whether increased ratio ofFirmicutes toBacteroidetes is associated with the development of metabolic diseases, and whether energy competition between the bacteria and host is a missing part of the mechanism linking gut microbiota to metabolic diseases.-
Citations
Citations to this article as recorded by- Behavior, intestinal health, and growth of small sea cucumbers Apostichopus japonicus in different color morphs
Peng Ding, Yushi Yu, Zihe Zhao, Xiang Li, Xiajing Wang, Huiyan Wang, Xiyuan Huang, Jun Ding, Chong Zhao
Marine Environmental Research.2024; 193: 106300. CrossRef - Traditional Chinese Medicine formula Dai-Zong-Fang alleviating hepatic steatosis in db/db mice via gut microbiota modulation
Li-Wei Zhang, Li-Li Zhu, Xiao-Yun Zhu, Shou-Qiang Fu, Xi-Ming Liu
Frontiers in Pharmacology.2024;[Epub] CrossRef - Repeated inoculation with rumen fluid accelerates the rumen bacterial transition with no benefit on production performance in postpartum Holstein dairy cows
Fanlin Kong, Feiran Wang, Yijia Zhang, Shuo Wang, Wei Wang, Shengli Li
Journal of Animal Science and Biotechnology.2024;[Epub] CrossRef - Identification of oncogenic signatures in the inflammatory colon of C57BL/6 mice fed a high-fat diet
Huawei Zeng, Bryan D. Safratowich, Wen-Hsing Cheng, Michael R. Bukowski
The Journal of Nutritional Biochemistry.2023; 111: 109188. CrossRef - Evaluation of the gut microbiome alterations in healthy rats after dietary exposure to different synthetic ZnO nanoparticles
Xinyi Zhu, Henghui Li, Liuzhu Zhou, Huijun Jiang, Minghui Ji, Jin Chen
Life Sciences.2023; 312: 121250. CrossRef - Microplastic-induced gut microbiota and serum metabolic disruption in Sprague-Dawley rats
Nan Zhao, Meirong Zhao, Hangbiao Jin
Environmental Pollution.2023; 320: 121071. CrossRef - Effects of neutral polysaccharide from Platycodon grandiflorum on high-fat diet-induced obesity via the regulation of gut microbiota and metabolites
Jing Song, Qin liu, Mengqi Hao, Xiaohu Zhai, Juan Chen
Frontiers in Endocrinology.2023;[Epub] CrossRef - Metabolite interactions between host and microbiota during health and disease: Which feeds the other?
Yan Zhang, Rui Chen, DuoDuo Zhang, Shuang Qi, Yan Liu
Biomedicine & Pharmacotherapy.2023; 160: 114295. CrossRef - Connecting Gut Microbial Diversity with Plasma Metabolome and Fecal Bile Acid Changes Induced by the Antibiotics Tobramycin and Colistin Sulfate
Aishwarya Murali, Varun Giri, Franziska Maria Zickgraf, Philipp Ternes, Hunter James Cameron, Saskia Sperber, Volker Haake, Peter Driemert, Hennicke Kamp, Dorothee Funk-Weyer, Shana J. Sturla, Ivonne M.C.M. Rietjens, Bennard van Ravenzwaay
Chemical Research in Toxicology.2023; 36(4): 598. CrossRef - Short-Term Alternate Feeding between Terrestrially Sourced Oil- and Fish Oil-Based Diets Modulates the Intestinal Microecology of Juvenile Turbot
Xiuhua Ma, Yaoyao Kong, Houguo Xu, Qingzhu Bi, Mengqing Liang, Kangsen Mai, Yanjiao Zhang
Biology.2023; 12(5): 650. CrossRef - Effects and action mechanisms of lotus leaf (Nelumbo nucifera) ethanol extract on gut microbes and obesity in high-fat diet-fed rats
Zhang Yanan, Ma Lu, Zhang Lu, Huo Jinhai, Wang Weiming
Frontiers in Nutrition.2023;[Epub] CrossRef - Effects of coffee with different roasting degrees on obesity and related metabolic disorders
Claudia I. Gamboa-Gómez, Laura J. Barragán-Zúñiga, Fernando Guerrero-Romero, Gerardo Martínez-Aguilar, José Luis Gónzalez, Almendra A. Valenzuela-Ramírez, Juan A. Rojas-Contreras, Monica Anese, Maribel Cervantes Flores, Marilisa Alongi
Journal of Functional Foods.2023; 111: 105889. CrossRef - Gut Microbiota and Bacterial Translocation in the Pathogenesis of Liver Fibrosis
Roman Maslennikov, Elena Poluektova, Oxana Zolnikova, Alla Sedova, Anastasia Kurbatova, Yulia Shulpekova, Natyia Dzhakhaya, Svetlana Kardasheva, Maria Nadinskaia, Elena Bueverova, Vladimir Nechaev, Anna Karchevskaya, Vladimir Ivashkin
International Journal of Molecular Sciences.2023; 24(22): 16502. CrossRef - Eugenol, A Major Component of Clove Oil, Attenuates Adiposity, and Modulates Gut Microbiota in High‐Fat Diet‐Fed Mice
Mengjie Li, Yuhan Zhao, Yanan Wang, Ruixuan Geng, Jingjing Fang, Seong‐Gook Kang, Kunlun Huang, Tao Tong
Molecular Nutrition & Food Research.2022;[Epub] CrossRef - Heimao tea polysaccharides ameliorate obesity by enhancing gut microbiota-dependent adipocytes thermogenesis in mice fed with high fat diet
Yu Wang, Ting Li, Yueyue Liu, Chengcheng Yang, Lei Liu, Xiangnan Zhang, Xingbin Yang
Food & Function.2022; 13(24): 13014. CrossRef - The Interplay of Sex Steroids, the Immune Response, and the Intestinal Microbiota
Fernanda Pace, Paula I. Watnick
Trends in Microbiology.2021; 29(9): 849. CrossRef - Heat stress on microbiota composition, barrier integrity, and
nutrient transport in gut, production performance, and its amelioration in farm
animals
Amlan Kumar Patra, Indrajit Kar
Journal of Animal Science and Technology.2021; 63(2): 211. CrossRef - Mechanisms linking gut microbial metabolites to insulin resistance
Hye Rim Jang, Hui-Young Lee
World Journal of Diabetes.2021; 12(6): 730. CrossRef - The impact of gut microbiota metabolites on cellular bioenergetics and cardiometabolic health
Lenka Tomasova, Marian Grman, Karol Ondrias, Marcin Ufnal
Nutrition & Metabolism.2021;[Epub] CrossRef
- Behavior, intestinal health, and growth of small sea cucumbers Apostichopus japonicus in different color morphs
- Basic Research
- Effects of Microbiota on the Treatment of Obesity with the Natural Product Celastrol in Rats
- Weiyue Hu, Lingling Wang, Guizhen Du, Quanquan Guan, Tianyu Dong, Ling Song, Yankai Xia, Xinru Wang
- Diabetes Metab J. 2020;44(5):747-763. Published online May 11, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0124
- 9,331 View
- 136 Download
- 16 Web of Science
- 17 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
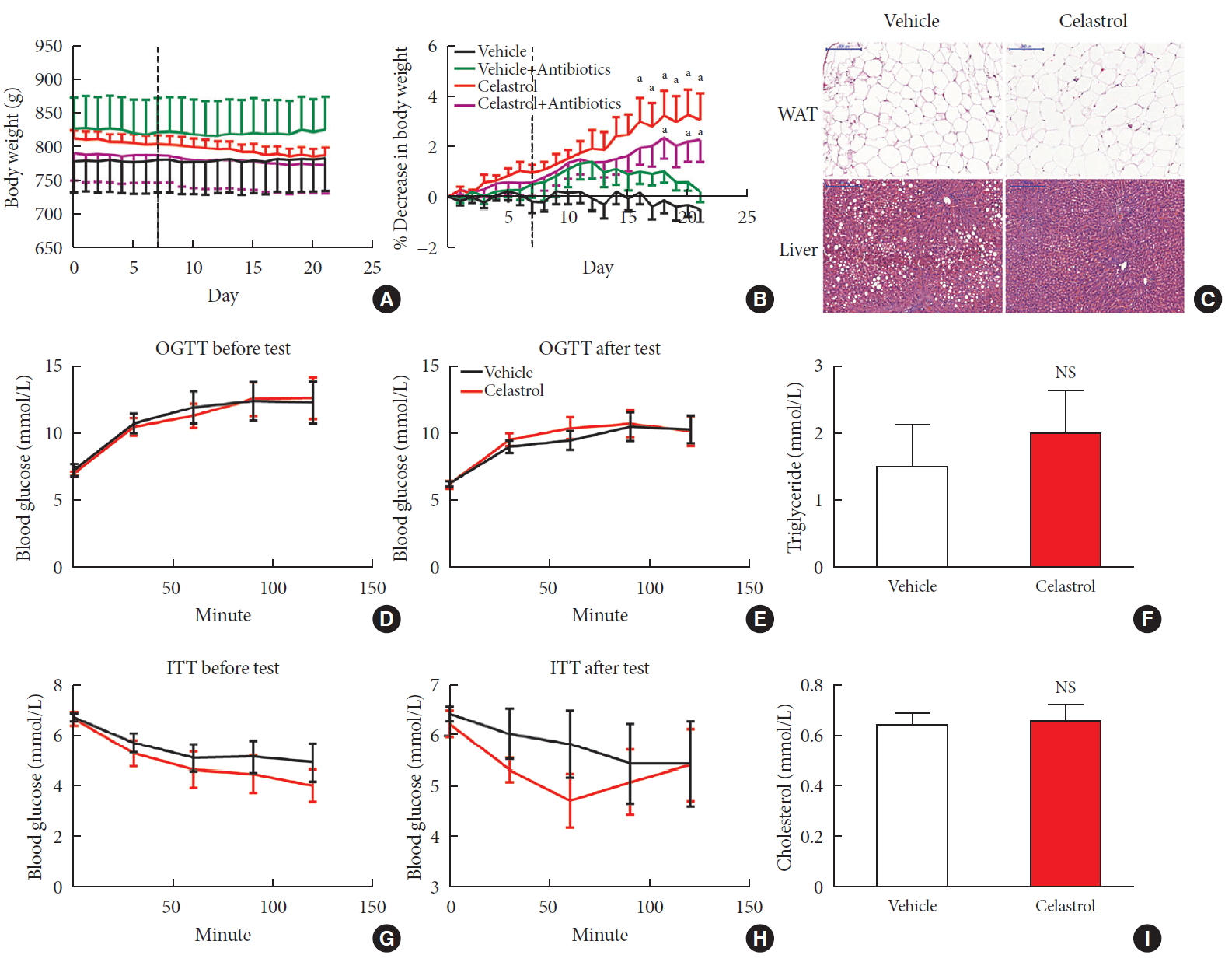
ePub Background Obesity has become one of the most serious issues threatening the health of humankind, and we conducted this study to examine whether and how celastrol protects against obesity.
Methods We fed male Sprague-Dawley rats a high-fat diet and administered celastrol to obese rats for 3 weeks. By recording body weight (BW) and other measures, we identified the effective dose of celastrol for obesity treatment. Feces were collected to perform 16S rRNA sequencing, and hypothalami were extracted for transcriptome sequencing. We then treated leptin knockout rats with celastrol and explored the changes in energy metabolism. Male Institute of Cancer Research (ICR) mice were used to test the acute toxicity of celastrol.
Results We observed that celastrol reduced BW and promoted energy expenditure at a dose of 500 µg/kg BW but that food intake was not changed after administration. The diversity of the gut microbiota was improved, with an increased ratio of
Bacteroidetes toFirmicutes , and the gut microbiota played an important role in the anti-obesity effects of celastrol. Hypothalamic transcriptome analysis showed a significant enrichment of the leptin signaling pathway, and we found that celastrol significantly enhanced energy expenditure, which was mediated by the leptin signaling pathway. Acute lethal toxicity of celastrol was not observed at doses ranging from 0 to 62.5 mg/kg BW.Conclusion Our study revealed that celastrol decreased the BW of obese rats by enhancing energy expenditure but not by suppressing food intake and that this effect was mediated by the improvement of the gut microbiota and the activation of the hypothalamic leptin signaling pathway.
-
Citations
Citations to this article as recorded by- Natural compounds as obesity pharmacotherapies
Xin‐Yuan Zhao, Ji‐Qiu Wang, G. Gregory Neely, Yan‐Chuan Shi, Qiao‐Ping Wang
Phytotherapy Research.2024; 38(2): 797. CrossRef - Celastrol functions as an emerging manager of lipid metabolism: Mechanism and therapeutic potential
Jia Gu, Ya-Ning Shi, Neng Zhu, Hong-Fang Li, Chan-Juan Zhang, Li Qin
Biomedicine & Pharmacotherapy.2023; 164: 114981. CrossRef - Tripterygium hypoglaucum extract ameliorates adjuvant-induced arthritis in mice through the gut microbiota
Jianghui HU, Jimin NI, Junping ZHENG, Yanlei GUO, Yong YANG, Cheng YE, Xiongjie SUN, Hui XIA, Yanju LIU, Hongtao LIU
Chinese Journal of Natural Medicines.2023; 21(10): 730. CrossRef - Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation
Lili Su, Zhifan Hong, Tong Zhou, Yuanyuan Jian, Mei Xu, Xuanping Zhang, Xiaoyan Zhu, Jiayin Wang
Scientific Reports.2022;[Epub] CrossRef - Tripterygium hypoglaucum (Levl.) Hutch: A systematic review of its traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology
Jiangping Wei, Liyun Chen, Sijia Gao, Jirui Wang, Yunhong Wang, Zhiwei Zhang, Yuyu Zhang, Xiaomei Zhang, Yong Yang, Dajian Yang
Pharmacological Research - Modern Chinese Medicine.2022; 3: 100094. CrossRef - Celastrol: An Update on Its Hepatoprotective Properties and the Linked Molecular Mechanisms
Mengzhen Li, Faren Xie, Lu Wang, Guoxue Zhu, Lian-Wen Qi, Shujun Jiang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Celastrol inhibits the proliferation and migration of MCF-7 cells through the leptin-triggered PI3K/AKT pathway
Pingping Chen, Bin Wang, Meng Li, Chunxue Cui, Fei Liu, Yonggang Gao
Computational and Structural Biotechnology Journal.2022; 20: 3173. CrossRef - Investigating Celastrol’s Anti-DCM Targets and Mechanisms via Network Pharmacology and Experimental Validation
Rui Xi, Yongxin Wan, Lihong Yang, Jingying Zhang, Liu Yang, Shuai Yang, Rui Chai, Fengchen Mu, Qiting Sun, Rui Yan, Zhifang Wu, Sijin Li, Zhijun Liao
BioMed Research International.2022; 2022: 1. CrossRef - Celastrol inhibits TXNIP expression to protect pancreatic β cells in diabetic mice
Si-wei Wang, Tian Lan, Fang Zheng, Hui Huang, Hang-fei Chen, Qi Wu, Feng Zhang
Phytomedicine.2022; 104: 154316. CrossRef - Celastrol: A Promising Agent Fighting against Cardiovascular Diseases
Zhexi Li, Jingyi Zhang, Xulei Duan, Guoan Zhao, Min Zhang
Antioxidants.2022; 11(8): 1597. CrossRef - Celastrol: A lead compound that inhibits SARS‐CoV‐2 replication, the activity of viral and human cysteine proteases, and virus‐induced IL‐6 secretion
Carlos A. Fuzo, Ronaldo B. Martins, Thais F. C. Fraga‐Silva, Martin K. Amstalden, Thais Canassa De Leo, Juliano P. Souza, Thais M. Lima, Lucia H. Faccioli, Débora Noma Okamoto, Maria Aparecida Juliano, Suzelei C. França, Luiz Juliano, Vania L. D. Bonato,
Drug Development Research.2022; 83(7): 1623. CrossRef - In vitro activity of celastrol in combination with thymol against carbapenem-resistant Klebsiella pneumoniae isolates
Mahmoud Saad Abdel-Halim, Momen Askoura, Basem Mansour, Galal Yahya, Amira M. El-Ganiny
The Journal of Antibiotics.2022; 75(12): 679. CrossRef - Celastrol alleviates metabolic disturbance in high‐fat diet‐induced obese mice through increasing energy expenditure by ameliorating metabolic inflammation
Xueping Yang, Fan Wu, Lingli Li, Ernest C. Lynch, Linglin Xie, Yan Zhao, Ke Fang, Jingbin Li, Jinlong Luo, Lijun Xu, Xin Zou, Fuer Lu, Guang Chen
Phytotherapy Research.2021; 35(1): 297. CrossRef - Celastrol in metabolic diseases: Progress and application prospects
Shaohua Xu, Yaqian Feng, Weishen He, Wen Xu, Wei Xu, Hongjun Yang, Xianyu Li
Pharmacological Research.2021; 167: 105572. CrossRef - The Anti-Obesity Effect of Traditional Chinese Medicine on Lipid Metabolism
Qijing Fan, Furong Xu, Bin Liang, Xiaoju Zou
Frontiers in Pharmacology.2021;[Epub] CrossRef - Serum Metabolome Mediates the Antiobesity Effect of Celastrol-Induced Gut Microbial Alterations
Shaohua Xu, Liwei Lyu, Huaichang Zhu, Xiaoqiang Huang, Wei Xu, Wen Xu, Yaqian Feng, Yong Fan
Journal of Proteome Research.2021; 20(10): 4840. CrossRef - Interrelated Mechanism by Which the Methide Quinone Celastrol, Obtained from the Roots of Tripterygium wilfordii, Inhibits Main Protease 3CLpro of COVID-19 and Acts as Superoxide Radical Scavenger
Francesco Caruso, Manrose Singh, Stuart Belli, Molly Berinato, Miriam Rossi
International Journal of Molecular Sciences.2020; 21(23): 9266. CrossRef
- Natural compounds as obesity pharmacotherapies
- Basic Research
- Role of Intestinal Microbiota in Metabolism of Voglibose In Vitro and In Vivo
- Mahesh Raj Nepal, Mi Jeong Kang, Geon Ho Kim, Dong Ho Cha, Ju-Hyun Kim, Tae Cheon Jeong
- Diabetes Metab J. 2020;44(6):908-918. Published online April 6, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0147
- 5,667 View
- 114 Download
- 6 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub Background Voglibose, an α-glucosidase inhibitor, inhibits breakdown of complex carbohydrates into simple sugar units in intestine. Studies showed that voglibose metabolism in the liver might be negligible due to its poor intestinal absorption. Numerous microorganisms live in intestine and have several roles in metabolism and detoxification of various xenobiotics. Due to the limited information, the possible metabolism of voglibose by intestinal microbiota was investigated
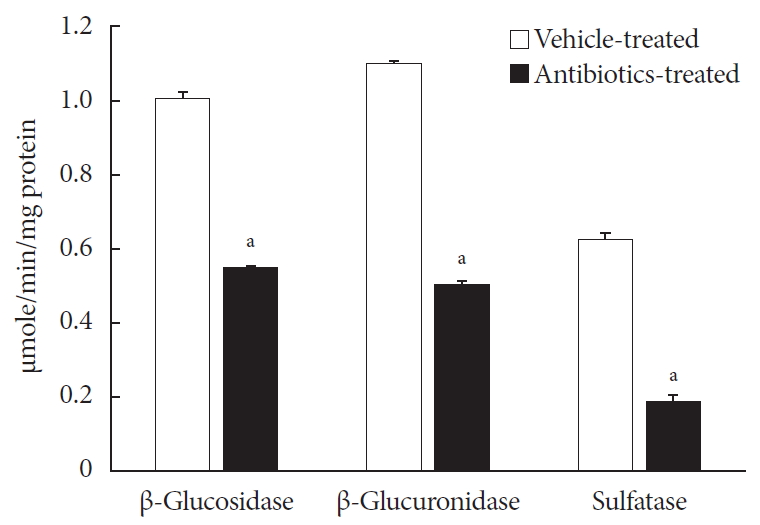
in vitro andin vivo .Methods For the
in vitro study, different concentrations of voglibose were incubated with intestinal contents, prepared from both vehicle- and antibiotics-treated mice, to determine the decreased amount of voglibose over time by using liquid chromatography-mass spectrometry. Similarly,in vivo pharmacodynamic effect of voglibose was determined following the administration of voglibose and starch in vehicle- and antibiotic-pretreated non-diabetic and diabetic mice, by measuring the modulatory effects of voglibose on blood glucose levels.Results The
in vitro results indicated that the remaining voglibose could be significantly decreased when incubated with the intestinal contents from normal mice compared to those from antibiotic-treated mice, which had less enzyme activities. Thein vivo results showed that the antibiotic pretreatment resulted in reduced metabolism of voglibose. This significantly lowered blood glucose levels in antibiotic-pretreated mice compared to the control animals.Conclusion The present results indicate that voglibose would be metabolized by the intestinal microbiota, and that this metabolism might be pharmacodynamically critical in lowering blood glucose levels in mice.
-
Citations
Citations to this article as recorded by- Pharmacomicrobiomics and type 2 diabetes mellitus: A novel perspective towards possible treatment
Liyang Jia, Shiqiong Huang, Boyu Sun, Yongguang Shang, Chunsheng Zhu
Frontiers in Endocrinology.2023;[Epub] CrossRef - Phenolics from endophytic fungi as natural α-glucosidase inhibitors: A comprehensive review
Muhammad Imran Tousif, Saba Tauseef, Sadeer Nabeelah, Jugreet Sharmeen, Gokhan Zengin, Lesetja Legoabe, Muhammad Imran, Mohamad Fawzi Mahomoodally
Journal of Molecular Structure.2023; 1291: 135852. CrossRef - Ligand-targeted fishing of α-glucosidase inhibitors from Tribulus terrestris L. based on chitosan-functionalized multi-walled carbon nanotubes with immobilized α-glucosidase
Xin Meng, Hou Zong, Zhong Zheng, Junpeng Xing, Zhiqiang Liu, Fengrui Song, Shu Liu
Analytical and Bioanalytical Chemistry.2023; 415(14): 2677. CrossRef - Isolation, structure elucidation, and biological activities of sesquiterpenes and phthalides from two edible mushrooms Pleurotus species
Jewel C De Padua, Emi Fukushima-Sakuno, Kotomi Ueno, Thomas Edison E dela Cruz, Atsushi Ishihara
Bioscience, Biotechnology, and Biochemistry.2023; 87(12): 1429. CrossRef - Effects of Oral Glucose-Lowering Agents on Gut Microbiota and Microbial Metabolites
Dongmei Wang, Jieying Liu, Liyuan Zhou, Qian Zhang, Ming Li, Xinhua Xiao
Frontiers in Endocrinology.2022;[Epub] CrossRef - 18:0 Lyso PC, a natural product with potential PPAR-γ agonistic activity, plays hypoglycemic effect with lower liver toxicity and cardiotoxicity in db/db mice
Yiming Ma, Xinyi Du, Dandan Zhao, Kegong Tang, Xiaona Wang, Shaoting Guo, Xiaobei Li, Song Mei, Na Sun, Jiaqi Liu, Chengyu Jiang
Biochemical and Biophysical Research Communications.2021; 579: 168. CrossRef
- Pharmacomicrobiomics and type 2 diabetes mellitus: A novel perspective towards possible treatment
- Basic Research
- Combination of Probiotics and
Salvia miltiorrhiza Polysaccharide Alleviates Hepatic Steatosis via Gut Microbiota Modulation and Insulin Resistance Improvement in High Fat-Induced NAFLD Mice - Wei Wang, Ai-Lei Xu, Zheng-Chao Li, Yi Li, Shun-Fu Xu, Hua-Chao Sang, Fachao Zhi
- Diabetes Metab J. 2020;44(2):336-348. Published online December 3, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0042
- 10,651 View
- 306 Download
- 62 Web of Science
- 63 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Nonalcoholic fatty liver disease (NAFLD) increases the risk of hepatocellular carcinoma, which is currently the leading cause of obesity-related cancer deaths in middle-aged men.
Methods Probiotics with lipid-lowering function were screened from the fecal microbiota of healthy adults. Polysaccharide from different sources was screened for improving insulin resistance. The combination of probiotics and
Salvia miltiorrhiza polysaccharide (LBM) was investigated for alleviating hepatic steatosis.Results First,
Bifidobacterium bifidum V (BbV) andLactobacillus plantarum X (LpX) were obtained from the fecal microbiota of healthy adults. Second, to improve insulin resistance, aSalvia miltiorrhiza Bunge polysaccharide showing good performance in reducing insulin resistance was obtained. The liver total cholesterol (TC) and total triglyceride (TG) levels and the serum levels of free fatty acid, alanine transaminase, aspartate transaminase, low density lipoprotein cholesterol, TG, and TC can be significantly reduced through supplementation with LpX-BbV (LB) in NAFLD mice. Interestingly, the function of the probiotic LB can be enhanced byS. miltiorrhiza Bunge polysaccharide. Furthermore, the gut microbiota was modulated by LpX-BbV+S. miltiorrhiza Bunge polysaccharide (LBM). The lipopolysaccharide concentration of the LBM group was decreased by 73.6% compared to the NAFLD group. Ultimately, the mRNA concentrations of the proinflammatory cytokines (tumor necrosis factor α, interleukin 1β [IL-1β], and IL-6) decreased with LB and LBM treatment.Conclusion The results of this this study indicate that the LBM combination can be used as a therapeutic for ameliorating NAFLD via modulating the gut microbiota and improving insulin resistance.
-
Citations
Citations to this article as recorded by- Bifidobacterium bifidum BGN4 fractions ameliorate palmitic acid-induced hepatocyte ferroptosis by inhibiting SREBP1-CYP2E1 pathway
Guangkui Bu, Gang Chen, Juan Li, Dan Wu, Jiangtao Liao
Journal of Investigative Medicine.2024; 72(1): 67. CrossRef - Docosahexaenoic acid (DHA) alleviates hepatic lipid accumulation by regulating mitochondrial quality control through ERK signaling pathway in grass carp (Ctenopharyngodon idellus)
Chenchen Bian, Shanghong Ji, Caihong Zeng, Jian Sun, Gen Kaneko, Hong Ji
Aquaculture.2024; 579: 740209. CrossRef - Microbial-Based Bioactive Compounds to Alleviate Inflammation in Obesity
Oladayo Emmanuel Apalowo, Grace Adeola Adegoye, Tolulope Mobolaji Obuotor
Current Issues in Molecular Biology.2024; 46(3): 1810. CrossRef - Understanding the role of ursodeoxycholic acid and gut microbiome in non-alcoholic fatty liver disease: current evidence and perspectives
Qingyi Mao, Beibei Lin, Wenluo Zhang, Yu Zhang, Yu Zhang, Qian Cao, Mengque Xu
Frontiers in Pharmacology.2024;[Epub] CrossRef - Smilax China L. polysaccharide prevents HFD induced-NAFLD by regulating hepatic fat metabolism and gut microbiota
Wenkai Zhang, Longhui Yu, Qinru Yang, Jinfeng Zhang, Wenjing Wang, Xinru Hu, Jingen Li, Guodong Zheng
Phytomedicine.2024; 127: 155478. CrossRef - Breaking the barriers: the role of gut homeostasis in Metabolic-Associated Steatotic Liver Disease (MASLD)
Raquel Benedé-Ubieto, Francisco Javier Cubero, Yulia A. Nevzorova
Gut Microbes.2024;[Epub] CrossRef - Prospect of research on anti-atherosclerosis effect of main components of traditional Chinese medicine Yiqi Huoxue Huatan recipe through gut microbiota: A review
Hongtao Huang, Hanjun Zhao, Lv Wenqing, Feiyue Xu, Xiaolong Wang, Yili Yao, Yu Huang
Medicine.2024; 103(5): e37104. CrossRef - Polysaccharides from Eucommia ulmoides Oliv. Leaves Alleviate Acute Alcoholic Liver Injury by Modulating the Microbiota–Gut–Liver Axis in Mice
Yingzhi Li, Huimei Wang, Xueping Leng, Jiaming Gao, Chang Li, Danfei Huang
Foods.2024; 13(7): 1089. CrossRef - Innovative pharmacotherapy for hepatic metabolic and chronic inflammatory diseases in China
Feng Zhang, Jiaming Ju, Hongtao Diao, Jinglun Song, Yu bian, Baofeng Yang
British Journal of Pharmacology.2024;[Epub] CrossRef - Intestinal dysbiosis in nonalcoholic fatty liver disease (NAFLD): focusing on the gut–liver axis
Hui Han, Yi Jiang, Mengyu Wang, Mebratu Melaku, Lei Liu, Yong Zhao, Nadia Everaert, Bao Yi, Hongfu Zhang
Critical Reviews in Food Science and Nutrition.2023; 63(12): 1689. CrossRef - Epigenetic compounds targeting pharmacological target lysine specific demethylase 1 and its impact on immunotherapy, chemotherapy and radiotherapy for treatment of tumor recurrence and resistance
Clement Agboyibor, Jianshu Dong, Clement Yaw Effah, Emmanuel Kwateng Drokow, Maxwell Ampomah-Wireko, Waqar Pervaiz, Augustina Sangmor, Xinli Ma, Jian Li, Hong-Min Liu, Peng Zhang
Biomedicine & Pharmacotherapy.2023; 157: 113934. CrossRef - Salvia miltiorrhiza polysaccharides alleviate florfenicol-induced inflammation and oxidative stress in chick livers by regulating phagosome signaling pathway
Xiao Wang, Wei Liu, Di Zhang, Yulan Jiao, Qianhui Zhao, Ying Liu, Wanyu Shi, Yongzhan Bao
Ecotoxicology and Environmental Safety.2023; 249: 114428. CrossRef - Preparation methods, structural characteristics, and biological activity of polysaccharides from Salvia miltiorrhiza: A review
Yuanyuan Li, Xin Zhang, Yining Li, Pei Yang, Zhiyuan Zhang, Hang Wu, Lihao Zhu, Yuhong Liu
Journal of Ethnopharmacology.2023; 305: 116090. CrossRef - The spleen-strengthening and liver-draining herbal formula treatment of non-alcoholic fatty liver disease by regulation of intestinal flora in clinical trial
Dengcheng Hui, Lu Liu, Nisma Lena Bahaji Azami, Jingru Song, Yanping Huang, Wan Xu, Chao Wu, Dong Xie, Yulang Jiang, Yanqin Bian, Mingyu Sun
Frontiers in Endocrinology.2023;[Epub] CrossRef - The gut microbiota: A new perspective for tertiary prevention of hepatobiliary and gallbladder diseases
Xiaoyu Huang, Yi Yang, Xueli Li, Xiaoya Zhu, Dan Lin, Yueran Ma, Min Zhou, Xiangyi Cui, Bingyu Zhang, Dongmei Dang, Yuhong Lü, Changwu Yue
Frontiers in Nutrition.2023;[Epub] CrossRef - Polysaccharides from Ostrea rivularis rebuild the balance of gut microbiota to ameliorate non-alcoholic fatty liver disease in ApoE−/− mice
Lijun Zhu, Mingmei Xiao, Jigang Luo, Shijie Li, Wenting Liu, Jinchuan Wu, Zhuoyue Song
International Journal of Biological Macromolecules.2023; 235: 123853. CrossRef - Improvement of Inflammation, Diabetes, and Obesity by Forest
Product-Derived Polysaccharides through the Human Intestinal
Microbiota
Seong-woo MYEONG, Yong Ju LEE, Do Hyun KIM, Tae-Jong KIM
Journal of the Korean Wood Science and Technology.2023; 51(5): 358. CrossRef - Recent developments in Salvia miltiorrhiza polysaccharides: Isolation, purification, structural characteristics and biological activities
Lei Luo, Juan Xue, Zheng Shao, Zhang Zhou, Wenqian Tang, Jinxin Liu, Hongfei Hu, Fan Yang
Frontiers in Pharmacology.2023;[Epub] CrossRef - In Vitro Probiotic Properties of Bifidobacterium animalis subsp. lactis SF and Its Alleviating Effect on Non-Alcoholic Fatty Liver Disease
Huihui Lv, Feiyue Tao, Lingling Peng, Shufang Chen, Zhongyue Ren, Jiahui Chen, Bo Yu, Hua Wei, Cuixiang Wan
Nutrients.2023; 15(6): 1355. CrossRef - Ginsenoside Rg1 Ameliorates Pancreatic Injuries via the AMPK/mTOR Pathway in vivo and in vitro
Jin Chen, Guoping Zhu, Wenbo Xiao, Xiaosong Huang, Kewu Wang, Yi Zong
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 779. CrossRef - Managing metabolic diseases: The roles and therapeutic prospects of herb-derived polysaccharides
Xinmei Xu, Lijie Wang, Kun Zhang, Yi Zhang, Gang Fan
Biomedicine & Pharmacotherapy.2023; 161: 114538. CrossRef - Probio-X Relieves Symptoms of Hyperlipidemia by Regulating Patients’ Gut Microbiome, Blood Lipid Metabolism, and Lifestyle Habits
Huan Wang, Cuicui Ma, Yan Li, Lei Zhang, lima A, Chengcong Yang, Feiyan Zhao, Haifeng Han, Dongyang Shang, Fan Yang, Yuying Zhang, Heping Zhang, Zhihong Sun, Ruifang Guo, Yuan Pin Hung
Microbiology Spectrum.2023;[Epub] CrossRef - Sea cucumber sulfated polysaccharides and Lactobacillus gasseri synergistically ameliorate the overweight induced by altered gut microbiota in mice
Zhengqi Liu, Chunqing Ai, Xinping Lin, Xiaoming Guo, Shuang Song, Beiwei Zhu
Food & Function.2023; 14(9): 4106. CrossRef - Supplementation of Lactobacillus plantarum ATCC14917 mitigates non-alcoholic fatty liver disease in high-fat-diet-fed rats
Xingjian Wen, Hejing Liu, Xiaoling Luo, Li Lui, Jiuyu Fan, Yajing Xing, Jia Wang, Xingfang Qiao, Na Li, Guixue Wang
Frontiers in Microbiology.2023;[Epub] CrossRef - Potential herb–drug interactions between anti-COVID-19 drugs and traditional Chinese medicine
Ling Ye, Shicheng Fan, Pengfei Zhao, Chenghua Wu, Menghua Liu, Shuang Hu, Peng Wang, Hongyu Wang, Huichang Bi
Acta Pharmaceutica Sinica B.2023; 13(9): 3598. CrossRef - α‐Lactalbumin Peptide Asp‐Gln‐Trp Ameliorates Hepatic Steatosis and Oxidative Stress in Free Fatty Acids‐Treated HepG2 Cells and High‐Fat Diet‐Induced NAFLD Mice by Activating the PPARα Pathway
Haoran Chen, Yanfeng Ma, Xiaofen Qi, Jianjun Tian, Ying Ma, Tianjiao Niu
Molecular Nutrition & Food Research.2023;[Epub] CrossRef - Lonicerae flos polysaccharides improve nonalcoholic fatty liver disease by activating the adenosine 5′‐monophosphate‐activated protein kinase pathway and reshaping gut microbiota
Chao Han, Zongshuo Li, Ruiying Liu, Zihan Zhao, Yu Wang, Xuli Zuo, Yushi Zhang, Zeyu Geng, Houyu Huang, Xiuzhen Pan, Weidong Li
Journal of the Science of Food and Agriculture.2023; 103(15): 7721. CrossRef - Polysaccharides: The Potential Prebiotics for Metabolic Associated Fatty Liver Disease (MAFLD)
Qin Guo, Yun Li, Xin Dai, Bangmao Wang, Jie Zhang, Hailong Cao
Nutrients.2023; 15(17): 3722. CrossRef - Prebiotics and Probiotics: Therapeutic Tools for Nonalcoholic Fatty Liver Disease
Alejandra Mijangos-Trejo, Natalia Nuño-Lambarri, Varenka Barbero-Becerra, Misael Uribe-Esquivel, Paulina Vidal-Cevallos, Norberto Chávez-Tapia
International Journal of Molecular Sciences.2023; 24(19): 14918. CrossRef - Repair Effect and Mechanism of Electrospinning Nanocomposite Material with Gelatin-Bletilla Striata Gum/Salvia Miltiorrhiza on Orthopedic Refractory Wounds
Geliang Hu, Ming Deng, Yonggang Ma, Jianghua Ming
Journal of Biomedical Nanotechnology.2023; 19(10): 1783. CrossRef -
Characterisation and skin protection activities of polysaccharides from
Schnabelia terniflora
Ying Zhao, Yixian Liu, Huoxiang Zhou, Wei Guo, Weidong Wang, Huiping Chen
Natural Product Research.2023; : 1. CrossRef - Non-alcoholic fatty liver disease risk prediction model and health management strategies for older Chinese adults: a cross-sectional study
Hong Pan, Baocheng Liu, Xin Luo, Xinxin Shen, Jijia Sun, An Zhang
Lipids in Health and Disease.2023;[Epub] CrossRef - Modulatory effects of polysaccharides from plants, marine algae and edible mushrooms on gut microbiota and related health benefits: A review
Henan Zhang, Fuchun Jiang, Jinsong Zhang, Wenhan Wang, Lin Li, Jingkun Yan
International Journal of Biological Macromolecules.2022; 204: 169. CrossRef - Hepatoprotective mechanism of Silybum marianum on nonalcoholic fatty liver disease based on network pharmacology and experimental verification
Guoyan Jiang, Chunhong Sun, Xiaodong Wang, Jie Mei, Chen Li, Honghong Zhan, Yixuan Liao, Yongjun Zhu, Jingxin Mao
Bioengineered.2022; 13(3): 5216. CrossRef - Gut Microbiome in Non-Alcoholic Fatty Liver Disease: From Mechanisms to Therapeutic Role
Haripriya Gupta, Byeong-Hyun Min, Raja Ganesan, Yoseph Asmelash Gebru, Satya Priya Sharma, Eunju Park, Sung-Min Won, Jin-Ju Jeong, Su-Been Lee, Min-Gi Cha, Goo-Hyun Kwon, Min-Kyo Jeong, Ji-Ye Hyun, Jung-A. Eom, Hee-Jin Park, Sang-Jun Yoon, Mi-Ran Choi, Do
Biomedicines.2022; 10(3): 550. CrossRef - Efficacy and Safety of Probiotics Combined With Traditional Chinese Medicine for Ulcerative Colitis: A Systematic Review and Meta-Analysis
Yu Hu, Zhen Ye, Yingqi She, Linzhen Li, Mingquan Wu, Kaihua Qin, Yuzheng Li, Haiqing He, Zhipeng Hu, Maoyi Yang, Fating Lu, Qiaobo Ye
Frontiers in Pharmacology.2022;[Epub] CrossRef - Hepatoprotection of Probiotics Against Non-Alcoholic Fatty Liver Disease in vivo: A Systematic Review
Faezah Sabirin, Siong Meng Lim, Chin Fen Neoh, Kalavathy Ramasamy
Frontiers in Nutrition.2022;[Epub] CrossRef - Salvia miltiorrhiza Bge. (Danshen) in the Treating Non-alcoholic Fatty Liver Disease Based on the Regulator of Metabolic Targets
Jie Liu, Yun Shi, Daiyin Peng, Lei Wang, Nianjun Yu, Guokai Wang, Weidong Chen
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - The Regulatory Roles of Polysaccharides and Ferroptosis-Related Phytochemicals in Liver Diseases
Yijing Ren, Siyue Li, Zixuan Song, Qiuping Luo, Yingying Zhang, Hao Wang
Nutrients.2022; 14(11): 2303. CrossRef - Astaxanthin Alleviates Nonalcoholic Fatty Liver Disease by Regulating the Intestinal Flora and Targeting the AMPK/Nrf2 Signal Axis
Yuhang Li, Juxiong Liu, Bojian Ye, Yueyao Cui, Ruiqi Geng, Shu Liu, Yufei Zhang, Wenjin Guo, Shoupeng Fu
Journal of Agricultural and Food Chemistry.2022; 70(34): 10620. CrossRef - Application of metabolomics in the diagnosis of non-alcoholic fatty liver disease and the treatment of traditional Chinese medicine
Mingmei Shao, Yifei Lu, Hongjiao Xiang, Junmin Wang, Guang Ji, Tao Wu
Frontiers in Pharmacology.2022;[Epub] CrossRef - The chemistry and efficacy benefits of polysaccharides from Atractylodes macrocephala Koidz
Congying Liu, Shengguang Wang, Zedong Xiang, Tong Xu, Mengyuan He, Qing Xue, Huaying Song, Peng Gao, Zhufeng Cong
Frontiers in Pharmacology.2022;[Epub] CrossRef - An Ethnopharmaceutical Study on the Hypolipidemic Formulae in Taiwan Issued by Traditional Chinese Medicine Pharmacies
Min-Han Chi, Jung Chao, Chien-Yu Ko, Shyh-Shyun Huang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Three water soluble polysaccharides with anti-inflammatory activities from Selaginella uncinata (Desv.) Spring
Haochen Hui, Meng Gao, Xuerong Zhao, Lianhong Yin, Lina Xu, Lili Li, Jinyong Peng
International Journal of Biological Macromolecules.2022; 222: 1983. CrossRef - Research Progress on the Therapeutic Effect of Polysaccharides on Non-Alcoholic Fatty Liver Disease through the Regulation of the Gut–Liver Axis
Xiang Chen, Menghan Liu, Jun Tang, Ning Wang, Yibin Feng, Haotian Ma
International Journal of Molecular Sciences.2022; 23(19): 11710. CrossRef - Modulation of gut microbiota by glycyrrhizic acid may contribute to its anti-NAFLD effect in rats fed a high-fat diet
Sai Wang, Xin-Yu Li, Hong-Fang Ji, Liang Shen
Life Sciences.2022; 310: 121110. CrossRef - Structural Alteration of Gut Microbiota During the Amelioration of Chronic Psychological Stress-Aggravated Diabetes-Associated Cognitive Decline by a Traditional Chinese Herbal Formula, ZiBu PiYin Recipe
Wen Zhou, Libin Zhan, Huiying Xu, Lijing Zhang
Journal of Alzheimer's Disease.2022; 90(4): 1465. CrossRef - Bacillus amyloliquefaciens SC06 in the diet improves egg quality of hens by altering intestinal microbiota and the effect is diminished by antimicrobial peptide
Shujie Xu, Fei Wang, Peng Zou, Xiang Li, Qian Jin, Qi Wang, Baikui Wang, Yuanhao Zhou, Li Tang, Dongyou Yu, Weifen Li
Frontiers in Nutrition.2022;[Epub] CrossRef - Effect of stigma maydis polysaccharide on the gut microbiota and transcriptome of VPA induced autism model rats
Xiaolei Yang, Jiyuan Li, Yang Zhou, Ning Zhang, Jicheng Liu
Frontiers in Microbiology.2022;[Epub] CrossRef -
Salvia miltiorrhiza extract may exert an anti-obesity effect in rats with high-fat diet-induced obesity by modulating gut microbiome and lipid metabolism
Zi-Li Ai, Xian Zhang, Wei Ge, You-Bao Zhong, Hai-Yan Wang, Zheng-Yun Zuo, Duan-Yong Liu
World Journal of Gastroenterology.2022; 28(43): 6131. CrossRef - Dysregulated hepatic lipid metabolism and gut microbiota associated with early-stage NAFLD in ASPP2-deficiency mice
Fang Xie, Hang-fei Xu, Jing Zhang, Xiao-ni Liu, Bu-xin Kou, Meng-yin Cai, Jing Wu, Jin-ling Dong, Qing-hua Meng, Yi Wang, Dexi Chen, Yang Zhang
Frontiers in Immunology.2022;[Epub] CrossRef - Structural elucidation and anti-nonalcoholic fatty liver disease activity of Polygonatum cyrtonema Hua polysaccharide
Wei Liu, Taili Shao, Lei Tian, Zhengrui Ren, Lan Gao, Zhiyan Tang, Zheng Fang, Pingchuan Yuan, Chunyan Liu, Jikun Li, Guodong Wang, Jun Han
Food & Function.2022; 13(24): 12883. CrossRef - The protective effects of sulforaphane on high-fat diet-induced metabolic associated fatty liver disease in mice via mediating the FXR/LXRα pathway
Shaotong Ma, Xinyi Pang, Shuhua Tian, Jing Sun, Qiaobin Hu, Xiangfei Li, Yingjian Lu
Food & Function.2022; 13(24): 12966. CrossRef - Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation
Tingting Sang, Chengjie Guo, Dandan Guo, Jianjun Wu, Yujie Wang, Ying Wang, Jiajun Chen, Chaojie Chen, Kaikai Wu, Kun Na, Kang Li, Liu Fang, Cuiling Guo, Xingya Wang
Carbohydrate Polymers.2021; 256: 117594. CrossRef - Annual review of LSD1/KDM1A inhibitors in 2020
Dong-Jun Fu, Jun Li, Bin Yu
European Journal of Medicinal Chemistry.2021; 214: 113254. CrossRef - Diet-Regulating Microbiota and Host Immune System in Liver Disease
Jung A Eom, Goo Hyun Kwon, Na Yeon Kim, Eun Ju Park, Sung Min Won, Jin Ju Jeong, Ganesan Raja, Haripriya Gupta, Yoseph Asmelash Gebru, Satyapriya Sharma, Ye Rin Choi, Hyeong Seop Kim, Sang Jun Yoon, Ji Ye Hyun, Min Kyo Jeong, Hee Jin Park, Byeong Hyun Min
International Journal of Molecular Sciences.2021; 22(12): 6326. CrossRef - Lactobacillus johnsonii BS15 combined with abdominal massage on intestinal permeability in rats with nonalcoholic fatty liver and cell biofilm repair
Wei Zhang, Huanan Li, Na Zhao, Xiongfei Luo, Siwen Liu, an Bao, Yingying Chen, Haiteng Wang, Junshi Wang, Jingui Wang
Bioengineered.2021; 12(1): 6354. CrossRef - Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review
Hang-Yu Li, Dan-Dan Zhou, Ren-You Gan, Si-Yu Huang, Cai-Ning Zhao, Ao Shang, Xiao-Yu Xu, Hua-Bin Li
Nutrients.2021; 13(9): 3211. CrossRef - Correlation Analysis of Huayu Tongmai Decoction Intervention and Prognosis Indexes of Patients with Carotid Atherosclerosis
Guangqing Cheng, Xiaoni Yan, Fengmeng Wang, Chao Chen, Muhammad Wasim Khan
Evidence-Based Complementary and Alternative Medicine.2021; 2021: 1. CrossRef - Targeting the gut microbiota by Asian and Western dietary constituents: a new avenue for diabetes
Abdul Rahman Conteh, Ruixue Huang
Toxicology Research.2020; 9(4): 569. CrossRef - MiR-455 targeting SOCS3 improve liver lipid disorders in diabetic mice
Shu Fang, Jie Feng, Hongbin Zhang, Ping Li, Yudan Zhang, Yanmei Zeng, Yingying Cai, Xiaochun Lin, Yaoming Xue, Meiping Guan
Adipocyte.2020; 9(1): 179. CrossRef - Chinese Medicinal Herbs Targeting the Gut–Liver Axis and Adipose Tissue–Liver Axis for Non-Alcoholic Fatty Liver Disease Treatments: The Ancient Wisdom and Modern Science
Shuwei Zhang, Yui-Tung Wong, Ka-Yu Tang, Hiu-Yee Kwan, Tao Su
Frontiers in Endocrinology.2020;[Epub] CrossRef - Probiyotiklerin Kolon Mikrobiyotasına Etkileri: Güncel Çalışmalar
Çağlar GÖKIRMAKLI, Zeynep SEYDİM
Journal of Biotechnology and Strategic Health Research.2020; 4(3): 212. CrossRef
- Bifidobacterium bifidum BGN4 fractions ameliorate palmitic acid-induced hepatocyte ferroptosis by inhibiting SREBP1-CYP2E1 pathway
- Obesity and Metabolic Syndrome
- Understanding Bile Acid Signaling in Diabetes: From Pathophysiology to Therapeutic Targets
- Jessica M. Ferrell, John Y. L. Chiang
- Diabetes Metab J. 2019;43(3):257-272. Published online June 13, 2019
- DOI: https://doi.org/10.4093/dmj.2019.0043
- 8,760 View
- 244 Download
- 64 Web of Science
- 69 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Diabetes and obesity have reached an epidemic status worldwide. Diabetes increases the risk for cardiovascular disease and non-alcoholic fatty liver disease. Primary bile acids are synthesized in hepatocytes and are transformed to secondary bile acids in the intestine by gut bacteria. Bile acids are nutrient sensors and metabolic integrators that regulate lipid, glucose, and energy homeostasis by activating nuclear farnesoid X receptor and membrane Takeda G protein-coupled receptor 5. Bile acids control gut bacteria overgrowth, species population, and protect the integrity of the intestinal barrier. Gut bacteria, in turn, control circulating bile acid composition and pool size. Dysregulation of bile acid homeostasis and dysbiosis causes diabetes and obesity. Targeting bile acid signaling and the gut microbiome have therapeutic potential for treating diabetes, obesity, and non-alcoholic fatty liver disease.
-
Citations
Citations to this article as recorded by- Gut microbiome-derived secondary bile acids: therapeutic targets for reducing cardiovascular disease in type 2 diabetes?
Sarah A. Johnson, Tiffany L. Weir
The American Journal of Clinical Nutrition.2024; 119(2): 241. CrossRef - Current updates on metabolites and its interlinked pathways as biomarkers for diabetic kidney disease: A systematic review
Soumik Das, V Devi Rajeswari, Ganesh Venkatraman, Ramprasad Elumalai, Sivaraman Dhanasekaran, Gnanasambandan Ramanathan
Translational Research.2024; 265: 71. CrossRef - Variant of the lactase LCT gene explains association between milk intake and incident type 2 diabetes
Kai Luo, Guo-Chong Chen, Yanbo Zhang, Jee-Young Moon, Jiaqian Xing, Brandilyn A. Peters, Mykhaylo Usyk, Zheng Wang, Gang Hu, Jun Li, Elizabeth Selvin, Casey M. Rebholz, Tao Wang, Carmen R. Isasi, Bing Yu, Rob Knight, Eric Boerwinkle, Robert D. Burk, Rober
Nature Metabolism.2024; 6(1): 169. CrossRef - Predictive value of serum TBA for 2-year MACEs in ACS patients undergoing PCI: a prospective cohort study
Wen Wen, Qinze Li, Jianqing She, Xiaofang Bai, Lisha Zhang, Ruifeng Li, Yan Wu, Juan Zhou, Zuyi Yuan
Scientific Reports.2024;[Epub] CrossRef - Collaborative Metabolism: Gut Microbes Play a Key Role in Canine and Feline Bile Acid Metabolism
John C. Rowe, Jenessa A. Winston
Veterinary Sciences.2024; 11(2): 94. CrossRef - The paradigm change from reactive medical services to 3PM in ischemic stroke: a holistic approach utilising tear fluid multi-omics, mitochondria as a vital biosensor and AI-based multi-professional data interpretation
Olga Golubnitschaja, Jiri Polivka, Pavel Potuznik, Martin Pesta, Ivana Stetkarova, Alena Mazurakova, Lenka Lackova, Peter Kubatka, Martina Kropp, Gabriele Thumann, Carl Erb, Holger Fröhlich, Wei Wang, Babak Baban, Marko Kapalla, Niva Shapira, Kneginja Ric
EPMA Journal.2024; 15(1): 1. CrossRef - Investigating the mechanism of cornel iridoid glycosides on type 2 diabetes mellitus using serum and urine metabolites in rats
Yadi Hou, Yanmei Huang, Zihui Shang, Shichao Ma, Tianyi Cui, Ali Chen, Yongxia Cui, Suiqing Chen
Journal of Ethnopharmacology.2024; 328: 118065. CrossRef - Improvement of myocardial injury and gut microbiota disturbance in type 2 diabetic mice by inulin with various degrees of polymerization
Siqiang Jia, Jianpeng Li, Bin Yu, Mengjie Li, Bo Cui
Food Bioscience.2023; 51: 102318. CrossRef - Bile acids and their receptors in regulation of gut health and diseases
Sen Lin, Sutian Wang, Peng Wang, Cuiming Tang, Zhenjiang Wang, Lian Chen, Guoqing Luo, Hong Chen, Yuntao Liu, Bin Feng, De Wu, Douglas G. Burrin, Zhengfeng Fang
Progress in Lipid Research.2023; 89: 101210. CrossRef - Effects of dietary oat supplementation on carcass traits, muscle metabolites, amino acid profiles, and its association with meat quality of Small-tail Han sheep
Li-wei Wang, Shao-feng Su, Jie Zhao, Xiao-long He, Shao-yin Fu, Biao Wang, Yun-fei Wang, Da-qing Wang, Na-na Yun, Xin Chen, Damien P Belobrajdic, Terigele, Xiao-dong Li, Li-li Jiang, Jiang-feng He, Yong-bin Liu
Food Chemistry.2023; 411: 135456. CrossRef - Noni (Morinda citrifolia L.) fruit polysaccharide ameliorated high-fat diet-induced obesity by modulating gut microbiota and improving bile acid metabolism
Wenjing Mo, Jiaqi Zou, Ming Wu, Zijun Peng, Wenjiang He, Wenzhi Li, Xiaoyong Wu
Journal of Functional Foods.2023; 101: 105408. CrossRef - Sodium glucose co-transporter 2 (SGLT2) inhibition via dapagliflozin improves diabetic kidney disease (DKD) over time associatied with increasing effect on the gut microbiota in db/db mice
Jiajia Wu, Yan Chen, Huinan Yang, Leyi Gu, Zhaohui Ni, Shan Mou, Jianxiao Shen, Xiajing Che
Frontiers in Endocrinology.2023;[Epub] CrossRef - Short communication: unique metabolic signature of proliferative retinopathy in the tear fluid of diabetic patients with comorbidities — preliminary data for PPPM validation
Martina Kropp, Eline De Clerck, Trong-Tin Kevin Steve Vo, Gabriele Thumann, Vincenzo Costigliola, Olga Golubnitschaja
EPMA Journal.2023; 14(1): 43. CrossRef - Impact of Vancomycin Treatment and Gut Microbiota on Bile Acid Metabolism and the Development of Non-Alcoholic Steatohepatitis in Mice
Kaichi Kasai, Naoya Igarashi, Yuki Tada, Koudai Kani, Shun Takano, Tsutomu Yanagibashi, Fumitake Usui-Kawanishi, Shiho Fujisaka, Shiro Watanabe, Mayuko Ichimura-Shimizu, Kiyoshi Takatsu, Kazuyuki Tobe, Koichi Tsuneyama, Yukihiro Furusawa, Yoshinori Nagai
International Journal of Molecular Sciences.2023; 24(4): 4050. CrossRef - Mitochondrial Cholesterol Metabolites in a Bile Acid Synthetic Pathway Drive Nonalcoholic Fatty Liver Disease: A Revised “Two-Hit” Hypothesis
Genta Kakiyama, Daniel Rodriguez-Agudo, William M. Pandak
Cells.2023; 12(10): 1434. CrossRef - Bile acid metabolism and signaling: Emerging pharmacological targets of dietary polyphenols
Kevin M. Tveter, Esther Mezhibovsky, Yue Wu, Diana E. Roopchand
Pharmacology & Therapeutics.2023; 248: 108457. CrossRef - Role of liver parameters in diabetes mellitus – a narrative review
Sana Rafaqat, Aqsa Sattar, Amber Khalid, Saira Rafaqat
Endocrine Regulations.2023; 57(1): 200. CrossRef - Bile acids induce IL-1α and drive NLRP3 inflammasome-independent production of IL-1β in murine dendritic cells
Ewa Oleszycka, Eoin C. O’Brien, Michael Freeley, Ed C. Lavelle, Aideen Long
Frontiers in Immunology.2023;[Epub] CrossRef - Machine learning for predicting diabetic metabolism in the Indian population using polar metabolomic and lipidomic features
Nikita Jain, Bhaumik Patel, Manjesh Hanawal, Anurag R. Lila, Saba Memon, Tushar Bandgar, Ashutosh Kumar
Metabolomics.2023;[Epub] CrossRef - The mitochondrial translocator protein (TSPO, 18 kDa): A key multifunctional molecule in liver diseases
Yuchang Li, Liting Chen, Vassilios Papadopoulos
Biochimie.2023;[Epub] CrossRef - Sodium+/taurocholate cotransporting polypeptide as target therapy for liver fibrosis
Ahmad Salhab, Johnny Amer, Yinying Lu, Rifaat Safadi
Gut.2022; 71(7): 1373. CrossRef - Comparative Evaluation of the Effect of Metformin and Insulin on Gut Microbiota and Metabolome Profiles of Type 2 Diabetic Rats Induced by the Combination of Streptozotocin and High-Fat Diet
Nan Hu, Qi Zhang, Hui Wang, Xuping Yang, Yan Jiang, Rong Chen, Liying Wang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Immunomodulatory functions of FXR
Stefano Fiorucci, Angela Zampella, Patrizia Ricci, Eleonora Distrutti, Michele Biagioli
Molecular and Cellular Endocrinology.2022; 551: 111650. CrossRef - Production of New Microbially Conjugated Bile Acids by Human Gut Microbiota
Carlos J. Garcia, Vit Kosek, David Beltrán, Francisco A. Tomás-Barberán, Jana Hajslova
Biomolecules.2022; 12(5): 687. CrossRef - Regulation of the intestinal flora: A potential mechanism of natural medicines in the treatment of type 2 diabetes mellitus
liying he, Fang-Qing Yang, Pan Tang, Ting-Hui Gao, Cai-Xia Yang, Li Tan, Pan Yue, Ya-Nan Hua, Si-Jing Liu, Jin-Lin Guo
Biomedicine & Pharmacotherapy.2022; 151: 113091. CrossRef - Acrylamide induced glucose metabolism disorder in rats involves gut microbiota dysbiosis and changed bile acids metabolism
Zonghao Yue, Yanjuan Chen, Qian Dong, Dan Li, Meng Guo, Li Zhang, Yini Shi, Huiting Wu, Lili Li, Zhongke Sun
Food Research International.2022; 157: 111405. CrossRef - Effects of Herbal Therapy on Intestinal Microbiota and Serum Metabolomics in Different Rat Models of Mongolian Medicine
Guniang Jiu, Riao Dao, Dongxing Wu, Wang Hung, Haburi Jin, Li Li, Xiquan Fu, Chula Sa, Eerdunchaolu, Maulidiani .M
Evidence-Based Complementary and Alternative Medicine.2022; 2022: 1. CrossRef - The role of immune cells in the liver tumor microenvironment: an involvement of gut microbiota-derived factors
Tomonori Kamiya, Naoko Ohtani
International Immunology.2022; 34(9): 467. CrossRef - Reduced Cytokine Tumour Necrosis Factor by Pharmacological Intervention in a Preclinical Study
Armin Mooranian, Jacqueline Chester, Edan Johnston, Corina Mihaela Ionescu, Daniel Walker, Melissa Jones, Susbin Raj Wagle, Bozica Kovacevic, Thomas Foster, Momir Mikov, Hani Al-Salami
Biomolecules.2022; 12(7): 877. CrossRef - Crisis of the Asian gut: associations among diet, microbiota, and metabolic diseases
Phatthanaphong THERDTATHA, Akari SHINODA, Jiro NAKAYAMA
Bioscience of Microbiota, Food and Health.2022; 41(3): 83. CrossRef - Valorization of avocado seeds with antioxidant capacity using pressurized hot water extraction
Eng Shi Ong, Janelle Low, Joseph Choon Wee Tan, Su Yi Foo, Chen Huei Leo
Scientific Reports.2022;[Epub] CrossRef - The fungicide prothioconazole and its metabolite prothioconazole-desthio disturbed the liver-gut axis in mice
Lingyu Hu, Xiaofang Wang, Zhiwei Bao, Qihao Xu, Mingrong Qian, Yuanxiang Jin
Chemosphere.2022; 307: 136141. CrossRef - Gut Microbiota and Bile Acids Mediate the Clinical Benefits of YH1 in Male Patients with Type 2 Diabetes Mellitus: A Pilot Observational Study
Yueh-Hsiang Huang, Yi-Hong Wu, Hsiang-Yu Tang, Szu-Tah Chen, Chih-Ching Wang, Wan-Jing Ho, Yi-Hsuan Lin, Geng-Hao Liu, Pei-Yeh Lin, Chi-Jen Lo, Yuan-Ming Yeh, Mei-Ling Cheng
Pharmaceutics.2022; 14(9): 1857. CrossRef - Gut–Liver Axis and Non-Alcoholic Fatty Liver Disease: A Vicious Circle of Dysfunctions Orchestrated by the Gut Microbiome
Salvatore Pezzino, Maria Sofia, Gloria Faletra, Chiara Mazzone, Giorgia Litrico, Gaetano La Greca, Saverio Latteri
Biology.2022; 11(11): 1622. CrossRef - The Role of Bile Acids in Cardiovascular Diseases: from Mechanisms to Clinical Implications
Shuwen Zhang, Junteng Zhou, Wenchao Wu, Ye Zhu, Xiaojing Liu
Aging and disease.2022;[Epub] CrossRef - Role of bile acids in overweight and obese children and adolescents
Cosimo Giannini, Concetta Mastromauro, Serena Scapaticci, Cristina Gentile, Francesco Chiarelli
Frontiers in Endocrinology.2022;[Epub] CrossRef - Bile acids and microbes in metabolic disease
Dhiraj Kumar Sah, Archana Arjunan, Sun Young Park, Young Do Jung
World Journal of Gastroenterology.2022; 28(48): 6846. CrossRef - The Fungicide Prothioconazole and its Metabolite Prothioconazole-Desthio Disturbed the Liver-Gut Axis in Mice
Yuanxiang Jin, Lingyu Hu, Xiaofang Wang, Zhiwei Bao, Qihao Xu, Mingrong Qian
SSRN Electronic Journal .2022;[Epub] CrossRef - The Critical Effect of Bile Acids in Atherosclerosis
Shangwen Qi, Xu Luo, Shuangfang Liu, Bishi Ling, Hua Jin
Journal of Cardiovascular Pharmacology.2022; 80(4): 562. CrossRef - Role of sirtuin-1 (SIRT1) in hypoxic injury in pancreatic β-cells
Ye-Jee Lee, Esder Lee, Young-Hye You, Yu-Bae Ahn, Ki-Ho Song, Ji-Won Kim, Seung-Hyun Ko
Journal of Drug Targeting.2021; 29(1): 88. CrossRef - Impact of gut microbiota: How it could play roles beyond the digestive system on development of cardiovascular and renal diseases
Kanmani Suganya, Taekwon Son, Kyu-Won Kim, Byung-Soo Koo
Microbial Pathogenesis.2021; 152: 104583. CrossRef - Determination of bile acids from human gallbladder by 1H‐MRS—Protocol optimization and estimation of reproducibility
Peter Vermathen, Gaëlle Diserens, Dino Kröll, Philipp Nett, Guido Stirnimann, Reiner Wiest
NMR in Biomedicine.2021;[Epub] CrossRef - The gut microbiome-bile acid axis in hepatocarcinogenesis
Liwei Wu, Jiao Feng, Jingjing Li, Qiang Yu, Jie Ji, Jianye Wu, Weiqi Dai, Chuanyong Guo
Biomedicine & Pharmacotherapy.2021; 133: 111036. CrossRef - Gut microbiome and bile acids in obesity-related diseases
Rumei Li, Sergio Andreu-Sánchez, Folkert Kuipers, Jingyuan Fu
Best Practice & Research Clinical Endocrinology & Metabolism.2021; 35(3): 101493. CrossRef - Gut Microbiome of Indonesian Adults Associated with Obesity and Type 2 Diabetes: A Cross-Sectional Study in an Asian City, Yogyakarta
Phatthanaphong Therdtatha, Yayi Song, Masaru Tanaka, Mariyatun Mariyatun, Maisaroh Almunifah, Nancy Eka Putri Manurung, Siska Indriarsih, Yi Lu, Koji Nagata, Katsuya Fukami, Tetsuo Ikeda, Yuan-Kun Lee, Endang Sutriswati Rahayu, Jiro Nakayama
Microorganisms.2021; 9(5): 897. CrossRef - Microbial Ecosystem in Diabetes Mellitus: Consideration of the Gastrointestinal System
Awgichew Shewasinad Yehualashet, Berhan Begashaw Yikna
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 1841. CrossRef - Role of bile acids in inflammatory liver diseases
Ioannis Evangelakos, Joerg Heeren, Esther Verkade, Folkert Kuipers
Seminars in Immunopathology.2021; 43(4): 577. CrossRef - Plasma Bile Acid Profile in Patients with and without Type 2 Diabetes
Alessandro Mantovani, Andrea Dalbeni, Denise Peserico, Filippo Cattazzo, Michele Bevilacqua, Gian Luca Salvagno, Giuseppe Lippi, Giovanni Targher, Elisa Danese, Cristiano Fava
Metabolites.2021; 11(7): 453. CrossRef - Maternal cecal microbiota transfer rescues early-life antibiotic-induced enhancement of type 1 diabetes in mice
Xue-Song Zhang, Yue Sandra Yin, Jincheng Wang, Thomas Battaglia, Kimberly Krautkramer, Wei Vivian Li, Jackie Li, Mark Brown, Meifan Zhang, Michelle H. Badri, Abigail J.S. Armstrong, Christopher M. Strauch, Zeneng Wang, Ina Nemet, Nicole Altomare, Joseph C
Cell Host & Microbe.2021; 29(8): 1249. CrossRef - Pregnane X receptor exacerbates nonalcoholic fatty liver disease accompanied by obesity- and inflammation-prone gut microbiome signature
Sarah Kim, Sora Choi, Moumita Dutta, Jeffrey O. Asubonteng, Marianne Polunas, Michael Goedken, Frank J. Gonzalez, Julia Yue Cui, Maxwell A. Gyamfi
Biochemical Pharmacology.2021; 193: 114698. CrossRef - Gut microbiota as a target for prevention and treatment of type 2 diabetes: Mechanisms and dietary natural products
Fan Xia, Lu-Ping Wen, Bing-Chen Ge, Yu-Xin Li, Fang-Ping Li, Ben-Jie Zhou
World Journal of Diabetes.2021; 12(8): 1146. CrossRef - Association between circulating bile acid alterations and nonalcoholic steatohepatitis independent of obesity and diabetes mellitus
Youngae Jung, Bo Kyung Koo, Seo Young Jang, Dain Kim, Heeyeon Lee, Dong Hyeon Lee, Sae Kyung Joo, Yong Jin Jung, Jeong Hwan Park, Taekyeong Yoo, Murim Choi, Min Kyung Lee, Sang Won Kang, Mee Soo Chang, Won Kim, Geum‐Sook Hwang
Liver International.2021; 41(12): 2892. CrossRef - Bile acid activated receptors: Integrating immune and metabolic regulation in non-alcoholic fatty liver disease
Michele Biagioli, Stefano Fiorucci
Liver Research.2021; 5(3): 119. CrossRef - Synthesis of 12β-Methyl-18-nor-bile Acids
Andreas Luxenburger, Lawrence D. Harris, Elizabeth M. Ure, Roselis A. Landaeta Aponte, Anthony D. Woolhouse, Scott A. Cameron, Chris D. Ling, Ross O. Piltz, Andrew R. Lewis, Graeme J. Gainsford, Alex Weymouth-Wilson, Richard H. Furneaux
ACS Omega.2021; 6(38): 25019. CrossRef - Serum metabolomic biomarkers of perceptual speed in cognitively normal and mildly impaired subjects with fasting state stratification
Kamil Borkowski, Ameer Y. Taha, Theresa L. Pedersen, Philip L. De Jager, David A. Bennett, Matthias Arnold, Rima Kaddurah-Daouk, John W. Newman
Scientific Reports.2021;[Epub] CrossRef - Myocardial Infarction and Coronary Artery Disease in Menopausal Women With Type 2 Diabetes Mellitus Negatively Correlate With Total Serum Bile Acids
Xunxun Feng, Guangyao Zhai, Jiaqi Yang, Yang Liu, Yujie Zhou, Qianyun Guo
Frontiers in Endocrinology.2021;[Epub] CrossRef - An Integrated Bile Acids Profile Determination by UHPLC-MS/MS to Identify the Effect of Bile Acids Supplement in High Plant Protein Diet on Common Carp (Cyprinus carpio)
Xian Wei, Ting Yao, Fatou Ndoye Fall, Min Xue, Xiaofang Liang, Jie Wang, Wenlong Du, Xu Gu
Foods.2021; 10(10): 2465. CrossRef - Fatty liver index and development of cardiovascular disease in Koreans without pre-existing myocardial infarction and ischemic stroke: a large population-based study
Jun Hyung Kim, Jin Sil Moon, Seok Joon Byun, Jun Hyeok Lee, Dae Ryong Kang, Ki Chul Sung, Jang Young Kim, Ji Hye Huh
Cardiovascular Diabetology.2020;[Epub] CrossRef - Methionine restriction alleviates high-fat diet-induced obesity: Involvement of diurnal metabolism of lipids and bile acids
Luanfeng Wang, Bo Ren, Qian Zhang, Chuanqi Chu, Zhenting Zhao, Jianbin Wu, Weiyang Zhao, Zhigang Liu, Xuebo Liu
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2020; 1866(11): 165908. CrossRef - Bile Acids: Key Regulators and Novel Treatment Targets for Type 2 Diabetes
Yingjie Wu, An Zhou, Li Tang, Yuanyuan Lei, Bo Tang, Linjing Zhang
Journal of Diabetes Research.2020; 2020: 1. CrossRef - Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy
John Y. L. Chiang, Jessica M. Ferrell
American Journal of Physiology-Gastrointestinal and Liver Physiology.2020; 318(3): G554. CrossRef - Imidacloprid disturbed the gut barrier function and interfered with bile acids metabolism in mice
Guiling Yang, Xianling Yuan, Cuiyuan Jin, Dou Wang, Yanhua Wang, Wenyu Miao, Yuanxiang Jin
Environmental Pollution.2020; 266: 115290. CrossRef - Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis
John Y.L. Chiang, Jessica M. Ferrell
Liver Research.2020; 4(2): 47. CrossRef -
Diabetes and Metabolism Journal in 2020: Good to Great
In-Kyung Jeong
Diabetes & Metabolism Journal.2020; 44(1): 1. CrossRef - Healthy dietary patterns to reduce obesity-related metabolic disease: polyphenol-microbiome interactions unifying health effects across geography
Camilla Diotallevi, Francesca Fava, Marco Gobbetti, Kieran Tuohy
Current Opinion in Clinical Nutrition & Metabolic Care.2020; 23(6): 437. CrossRef - Metabolic profiling of pre-gestational and gestational diabetes mellitus identifies novel predictors of pre-term delivery
Ilhame Diboun, Manjunath Ramanjaneya, Yasser Majeed, Lina Ahmed, Mohammed Bashir, Alexandra E. Butler, Abdul Badi Abou-Samra, Stephen L. Atkin, Nayef A. Mazloum, Mohamed A. Elrayess
Journal of Translational Medicine.2020;[Epub] CrossRef - Quantification of common and planar bile acids in tissues and cultured cells
Stephanie J. Shiffka, Jace W. Jones, Linhao Li, Ann M. Farese, Thomas J. MacVittie, Hongbing Wang, Peter W. Swaan, Maureen A. Kane
Journal of Lipid Research.2020; 61(11): 1524. CrossRef - Influência da microbiota intestinal na Doença Hepática Gordurosa Não Alcoólica
Isadora Barbosa de Almeida, Wermerson Assunção Barroso, Caroline Amélia Gonçalves
Revista Científica Multidisciplinar Núcleo do Conhecimento.2020; : 14. CrossRef - Ultra-Early and Early Changes in Bile Acids and Insulin after Sleeve Gastrectomy among Obese Patients
Adriana Florinela Cӑtoi, Alina Elena Pârvu, Aurel Mironiuc, Horațiu Silaghi, Ioana Delia Pop, Andra Diana Andreicuț
Medicina.2019; 55(12): 757. CrossRef
- Gut microbiome-derived secondary bile acids: therapeutic targets for reducing cardiovascular disease in type 2 diabetes?

 KDA
KDA
 First
First Prev
Prev





