
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Ahead-of print > Article
-
Original ArticleBasic Research Supplementation of Clostridium butyricum Alleviates Vascular Inflammation in Diabetic Mice
-
Tian Zhou1*
 , Shuo Qiu1*
, Shuo Qiu1* , Liang Zhang1*
, Liang Zhang1* , Yangni Li2, Jing Zhang1,3, Donghua Shen4, Ping Zhao1, Lijun Yuan1, Lianbi Zhao1
, Yangni Li2, Jing Zhang1,3, Donghua Shen4, Ping Zhao1, Lijun Yuan1, Lianbi Zhao1 , Yunyou Duan1
, Yunyou Duan1 , Changyang Xing1,2
, Changyang Xing1,2
-
DOI: https://doi.org/10.4093/dmj.2023.0109
Published online: February 2, 2024
- 624 Views
- 85 Download
1Department of Ultrasound Diagnostics, Tangdu Hospital, Air Force Medical University, Xi’an, China
2Department of Aerospace Medicine, Air Force Medical University, Xi’an, China
3Department of Biochemistry and Molecular Biology, Air Force Medical University, Xi’an, China
4Department of Ultrasound Diagnostics, The PLA Rocket Force Characteristic Medical Center, Beijing, China
-
Corresponding authors: Changyang Xing
 Department of Ultrasound Diagnostics, Tangdu Hospital, Air Force Medical University, Xinsi road No. 569th, Xi’an 710038, China E-mail: xingcy712@163.com
Department of Ultrasound Diagnostics, Tangdu Hospital, Air Force Medical University, Xinsi road No. 569th, Xi’an 710038, China E-mail: xingcy712@163.com -
Yunyou Duan
 Department of Ultrasound Diagnostics, Tangdu Hospital, Air Force Medical University, Xinsi road No. 569th, Xi’an 710038, China E-mail: duanyy@fmmu.edu.cn
Department of Ultrasound Diagnostics, Tangdu Hospital, Air Force Medical University, Xinsi road No. 569th, Xi’an 710038, China E-mail: duanyy@fmmu.edu.cn -
Lianbi Zhao
 Department of Ultrasound Diagnostics, Tangdu Hospital, Air Force Medical University, Xinsi road No. 569th, Xi’an 710038, China E-mail: libby0710@126.com
Department of Ultrasound Diagnostics, Tangdu Hospital, Air Force Medical University, Xinsi road No. 569th, Xi’an 710038, China E-mail: libby0710@126.com - *Tian Zhou, Shuo Qiu, and Liang Zhang contributed equally to this study as first authors.
Copyright © 2024 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Gut microbiota is closely related to the occurrence and development of diabetes and affects the prognosis of diabetic complications, and the underlying mechanisms are only partially understood. We aimed to explore the possible link between the gut microbiota and vascular inflammation of diabetic mice.
-
Methods
- The db/db diabetic and wild-type (WT) mice were used in this study. We profiled gut microbiota and examined the and vascular function in both db/db group and WT group. Gut microbiota was analyzed by 16s rRNA sequencing. Vascular function was examined by ultrasonographic hemodynamics and histological staining. Clostridium butyricum (CB) was orally administered to diabetic mice by intragastric gavage every 2 days for 2 consecutive months. Reactive oxygen species (ROS) and expression of nuclear factor erythroid-derived 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) were detected by fluorescence microscopy. The mRNA expression of inflammatory cytokines was tested by quantitative polymerase chain reaction.
-
Results
- Compared with WT mice, CB abundance was significantly decreased in the gut of db/db mice, together with compromised vascular function and activated inflammation in the arterial tissue. Meanwhile, ROS in the vascular tissue of db/db mice was also significantly increased. Oral administration of CB restored the protective microbiota, and protected the vascular function in the db/db mice via activating the Nrf2/HO-1 pathway.
-
Conclusion
- This study identified the potential link between decreased CB abundance in gut microbiota and vascular inflammation in diabetes. Therapeutic delivery of CB by gut transplantation alleviates the vascular lesions of diabetes mellitus by activating the Nrf2/HO-1 pathway.
- • Hyperglycemic and high-fat diets decreased Clostridium butyricum (CB).
- • Reduced butyrate levels led to diabetic vascular inflammation and dysfunction.
- • Administrating CB improved diabetic vascular lesion by activating Nrf2/HO-1 pathway.
- • CB probiotics offer potential for protecting against diabetic vascular complications.
Highlights
- Vasculopathy is one of the most common complications of diabetes mellitus (DM), which is also the main cause of casualties in diabetic patients [1]. The hyperglycemia, insulin resistance and hyperinsulinemia are the main mechanisms of DM and progression to diabetic vascular complications [2,3]. In the conditions of hyperglycemia and insulin resistance, persistent hyperglycemia leads to the formation of sugar-derived adducts referred as advanced glycation end-products (AGEs), which predisposes vessel walls to lipid deposition and triggers inflammatory responses [4]. AGEs are inducers of oxidative stress and inflammation through driving the production of reactive oxygen species (ROS) and free radicals, interrupting the secretion of nitric oxide, leading to endothelial dysfunction and ultimately accelerating the formation of atherosclerosis [5].
- Currently, increasing evidence indicates that the pathogenesis of type 2 diabetes mellitus (T2DM) is closely related to the gut microbiota of the host in addition to obesity, genetic, and environmental factors [6]. The gut microbiota is referred as “microbial organs” in the human body, which participates in the body’s energy metabolism [7]. The gut microbiota is in a relatively upstream position in the progression of pre-diabetes, diabetes to diabetic complications, and mediates insulin resistance [8,9], oxidative stress [10] and chronic inflammation [11] mechanisms throughout the process. It is speculated that the intestinal microbiota may be closely related to the vascular complications of diabetes, and even the hyperglycemia and glucose variability in T2DM may be the result of gut microbiota imbalance [12-14]. However, the composition and function of the gut microbiota related with diabetic macroangiopathy have not been systematically studied, which may help us understand the occurrence and development diabetic vasculopathy complications, and contribute to the finding of new and early intervention targets.
- In present study, we found that Clostridium butyricum (CB) and its metabolites were significantly reduced in the intestinal microbiota of diabetic mice, enhanced vascular oxidative stress and inflammatory response, and aggravated the damage of endothelial function. Therefore, in vitro and in vivo experiments were performed to evaluate the effects of CB intervention on vascular endothelial cells in mouse models of T2DM. In order to further explore the association between the intestinal microbiota and the vascular damage of T2DM, pulse wave velocity (PWV) was determined to evaluate the vascular stiffness by ultrasound. The anti-inflammatory and oxidative stress effects of CB were also investigated.
INTRODUCTION
- Bacterial preparation
- CB bio-53296 (ATCC19398, Beijing baioubowei Biotechnology Co. Ltd., Beijing, China) was grown on solid medium in agar-supplemented reinforced clostridial medium (RCM) broth (Hopebio, Qingdao, China) for 48 hours in an anaerobic chamber (5% CO2) at 37°C, and cultured under RCM broth in anaerobic tubes sealed at 286 rpm at 37°C in an incubator shaker for 24 hours. Then, centrifuged and resuspended in sterile phosphate-buffered saline (PBS), and the final experimental concentration was 1.0×1010 colony forming unit/kg per 2 days.
- Experimental animals and design
- Male 5-week (Lepr) knockout (KO)/KO mice (db/db) and (Lepr) wild-type (WT)/WT mice purchased from GemPharmatech Limited Company (Nanjing, China) were fed in separate cages. Mice were maintained under standardized conditions at 20% humidity, and a temperature of 22°C to 24°C, and fed either a normal chow or high-fat diet with free access to water on a 12-hour light/12-hour dark cycle.
- For CB treatment, the experimental group (diabetic [DB]-CB) mice were gavaged every 2 days with a suspension of CB (bio53296, ATCC19398, Beijing Baioubowei Biotechnology Co. Ltd.) freshly prepared as previously described, once a day for 60 days. The DB-PBS group mice were gavaged with PBS instead. The WT mice were used as the control group. All mice at indicated time were subjected to PWV measurement as described below at the indicated times. At the end of the experiment, all animals were sacrificed and the tissues were isolated for further analysis. All animal experiments were carried out in accordance with the suspension guidelines of the Animal Care and Use Committee of Air Force Medical University (TD-201903-02).
- PWV measurement
- Animal PWV was performed by experienced ultrasound technicians using Vevo 2100 imaging system (FUJIFILM VisualSonics, Toronto, ON, Canada). The mice were thoroughly depilated in advance, and were fixed with tape on the heated mouse plate with electrocardiogram connected. During pre-anesthesia, the inhalation concentration of isoflurane was 3% to 4%, and then maintained at 1.5% to 2.5% during the experiment. The Doppler velocity spectra were obtained at the ascending and abdominal aorta. PWV was calculated as the distance between abdominal aorta and ascending aorta signals detected divided by the time difference between two pulse arrivals relative to the R-wave of the electrocardiogram [15,16].
- Histology
- At the end of the experiment, the aortic tissues of diabetic mice were fixed in 4% paraformaldehyde for 24 hours, followed by embedding in paraffin sectioned at 10 μm thickness. All cross sections were immediately stained with hematoxylin and eosin, Masson trichrome for histological analysis and observed via light microscopy.
- 16S rRNA sequencing and analysis
- The abdominal cavity was exposed immediately after euthanasia, and the feces of each mouse (300 to 500 mg per mouse) were collected and stored immediately at −80°C. The microbiota was analyzed by 16S rRNA sequencing as previously described [17] by Bioprofile (Shanghai, China). In brief, total DNA was extracted from feces, DNA was quantified by Nanodrop (Thermo Scientific, Waltham, MA, USA). Then, after quantitative polymerase chain reaction (qPCR) amplification of the sample target fragment, the library was quantified on the QuantiFluor (Promega, Madison, WI, USA) fluorescence quantitative system using the Quant-iT PicoGreen dsDNA Assay Kit. The processed sequences defined at a 97% similarity threshold were assigned to operational taxonomic units (OTUs). Sequence denoising or OTU clustering was performed according to the QIIME2 dada2 analysis procedure or the analysis procedure of Vsearch software (Open and Free Tool by Robert C. Edgar, USA).
- Short-chain fatty acids
- Short-chain fatty acid (SCFA) composition was analyzed from fecal samples following the previously described protocol [18] by Bioprofile (Shanghai, China). Take 50 mg of sample, add 50 μL 15% phosphoric acid (Sinoreagent, Shanghai, China), add 125 μg/mL internal standard (isocaproic acid, sigma, >98%) solution 100 μL and diethyl ether (Sinoreagent) 400 μL homogenize 1 minute, centrifuged at 12,000 rpm for 10 minutes at 4°C, and the supernatant was taken. Supernatants were analyzed by Thermo TRACE 1310-ISQ LT gas chromatography–mass spectrometry (GC-MS, Thermo Scientific).
- Immunofluorescence
- Immunofluorescence was used to examine and quantify oxidant stress (via nuclear factor erythroid-derived 2-related factor 2 [Nrf2] and heme oxygenase-1 [HO-1]) in the arterial walls of diabetic mice. Arterial vascular tissue was collected and processed for paraffin embedding as previously described [19]. Paraffin sections were deparaffinized, serum blocked at room temperature, and incubated overnight at 4°C with anti-Nrf2 (GB113808, Servicebio, Wuhan, China) and anti-HO-1 (GB12104, Servicebio), respectively, followed by fluorescence labeled secondary antibody. After these processes, the sections were counterstained with 4’,6-diamidino-2-phenylindole (DAPI). All sections were visualized and images were captured with a fluorescent microscope (ECLIPSE C1, NIKON, Tokyo, Japan). Regions of interest positive for protein markers were selected from each image and measured via Image ProPlus 6.0 (Media cybernetics, Rockville, MD, USA).
- Measurement of reactive oxygen species
- Superoxide levels were measured using the fluorescent dye dihydroethidium (DHE) (D7008, 1:500, Sigma, St. Louis, MO, USA) as previously described [20]. DHE, a cell-permeable fluorescent probe for assessing ROS levels, was oxidized by superoxide to ethidium bromide, which subsequently generates red fluorescence with DNA and was trapped within the nuclei of cells. Arterial vascular tissue was collected and processed for embedded in optimal cutting temperature compound at −80°C. Tissue sections were incubated with DHE (2 μmol/L) at 37°C for 30minutes. DHE oxidation products were extracted with acetonitrile. After the sections were washed three times, the images were observed and captured with a fluorescence microscope (ECLIPSE C1), and the fluorescence intensity was analyzed with Image ProPlus 6.0.
- Cell culture, high glucose, and butyrate treatment
- Mouse aortic vascular smooth muscle cell (VSMC) line (MIC-iCell-c004 icell) was obtained from iCell Bioscience Inc., (Shanghai, China) and cultured in PriMed-iCELL-004 culture medium (iCell Bioscience Inc.) with 10% fetal bovine serum (FBS, Gemini, Life Technologies, Carlsbad, A, USA) and 1% antibiotic penicillin-streptomycin (Hyclone, Logan, UT, USA). Human umbilical vein endothelial cell (HUVEC) and RAW264.7 cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), cultured in Dulbecco’s Modified Essential Medium (DMEM) culture medium (Hyclone) and RPMI 1640 Medium (Hyclone) respectively, both supplemented with 10% FBS and 1% antibiotic. The cultures were maintained in a humidified atmosphere containing 5% CO2 at 37°C until reaching 80% confluence and then passaged.
- In order to investigate the effects of blood glucose on the vessels, all cells were stimulated with media with different sugar concentrations according to previous research methods [21,22]. After cells were passaged to passages 2–3, they were further incubated with normal glucose (NG; 5.5 mM) and high glucose (HG; 25 mM) for 24 hours. For butyrate treatment, in order to investigate the protective effect of sodium butyrate (SB) on the vascular endothelium, cells were cultured in HG (25 mM) for 24 hours, and then treated by addition of 5 mM SB (B5887, Sigma) for 24hours, as previously described [23,24]. All experiments were repeated three times. Cells were collected for subsequent experiments.
- Western blot analysis
- The protein samples from different groups of mouse aortic tissues were obtained by tissue grinding in radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime, Haimen, China). Protein concentrations were determined using bicinchoninic acid (BCA) Protein Assay Kit (Thermo Scientific). Then, the protein samples were separated via 10% or 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Immobilon P, Millipore, Burlington, MA, USA). Membranes were blocked with 8% milk in Tris-buffered saline containing 0.1% Tween-20 for 1 hour at room temperature. After blocking, membranes were incubated with Nrf2 (BF8017, Affinity Biosciences, Cincinnati, OH, USA) and HO-1 (AF5393, Affinity Biosciences) overnight at 4°C, followed by secondary antibodies for 1 hour at room temperature. β-Actin was used as an internal control. The bands were visualized using the enhanced chemiluminescent (ECL) Prime Western Blotting Detection Reagent (GE, Buckinghamshire, UK). Quantitative analysis of the Western blotting bands by ImageJ v1.8.0 software.
- qPCR analysis of the mRNA expression
- The mRNA expression of interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), Nrf2, and HO-1 was detected using real-rime quantitative reverse transcription (RT-qPCR). Total RNA was extracted from all cells or mouse aortic tissues using the TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Then, reverse transcription was done with the PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Beijing, China). RT-qPCR analysis was performed using LightCycler Instrument 96 (Roche Diagnostic, Mannheim, Germany) and LightCycler Software 1.5 according to manufacturer’s protocol. The relative mRNA expressions were calculated using 2–ΔΔCt method, and β-actin was used as the internal control.
- Statistical analysis
- All data are expressed as the mean±standard error of the mean. Statistical significance was analyzed by GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) using the Student’s t-test for two group comparison or analysis of variance (ANOVA) for more than three groups. The between-group post hoc analyses were performed for the significant results of ANOVA analysis with Tukey’s method. Differences with P<0.05 were considered statistically significant.
METHODS
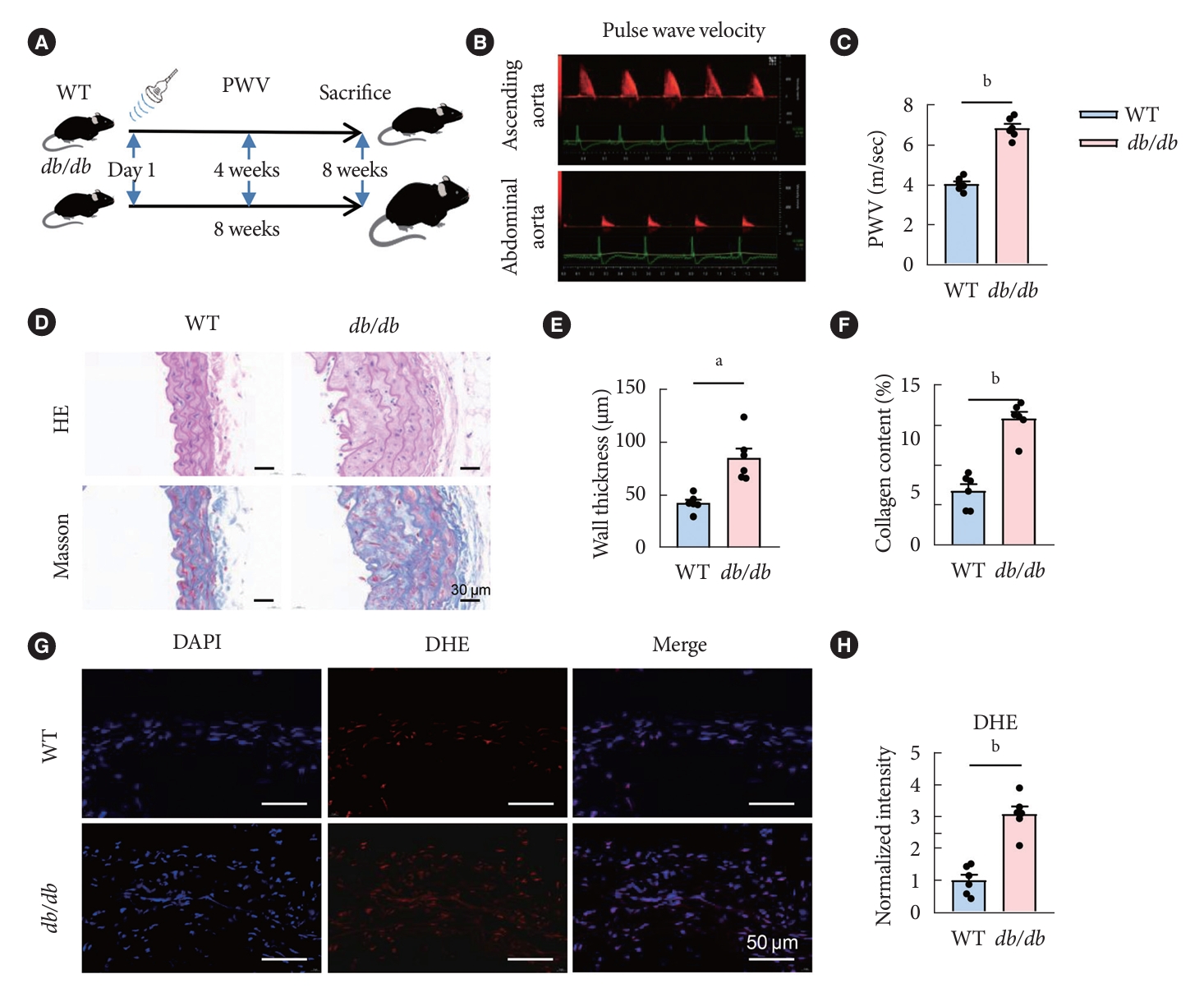
- Aggravated vascular oxidative stress damage and arterial stiffness in the db/db mice
- To investigate histological changes of vascular injury in macrovascular complications during diabetes progression of diabetes, we fed the db/db mice with a high-fat diet and the control group with a normal diet for 8 weeks, and the arterial stiffness was then assessed by PWV at day 1, 4, and 8 weeks of age (Fig. 1A and B). The aorta PWV in db/db mice showed a significant higher PWV in comparison with the control (Fig. 1C). All mice were euthanized at the end of the 8th week, and then tissues were taken for histological staining. The artery cross sections were stained with hematoxylin-eosin (HE) and Masson trichrome. In db/db mice, the vascular wall thickness was markedly increased, the smooth muscle fibers arrangement was disorderly, and the structure was blurred. Compared with the control group, vessels from db/db group mice exhibited an enlarged inter-smooth muscle cell space and subendothelial space filled with extracellular matrix and cellular debris. And quantitative analysis of Masson’s staining revealed a significant increase in collagen deposition of db/db mice compared with control (Fig. 1D-F). Moreover, to detect ROS in the ex vivo aorta tissues of db/db and control mice, DHE staining was performed. The relative quantitative results showed that that the fluorescence intensity of ROS signal in the db/db group was significantly stronger than that of the control group (Fig. 1G and H).
- Changes in gut microbiota in diabetic mice
- The diversity of microbial communities was assessed using the alpha diversity index, which includes Chao1 index, Shannon index, and Faith’s phylogenetic diversity (PD) index. Chao1 indices characterize richness, Shannon indices characterize diversity, and Faith’s PD index characterize evolution-based diversity. The alpha diversity index of the db/db group was significantly lower than those in the control group (Fig. 2A). As seen from the principal component analysis, at the class and order level, the relative abundance of Clostridia and Clostridiales in db/db mice was significantly reduced as a proportion of the total species compared with WT mice (Fig. 2B and C). And the relative abundance of Clostridium _vadinBB60_group was also reduced as a proportion of the total species at the family and genus level in the fecal bacterial community of db/db mice (Fig. 2D and E). In addition, at the level of class, order, family, and genus, the total OTUs of Clostridiales in the db/db mice were significantly decreased compared to WT mice (Fig. 2F).
- CB supplementation improves vascular function via gut microbiota remodeling and SCFAs change in diabetic mice
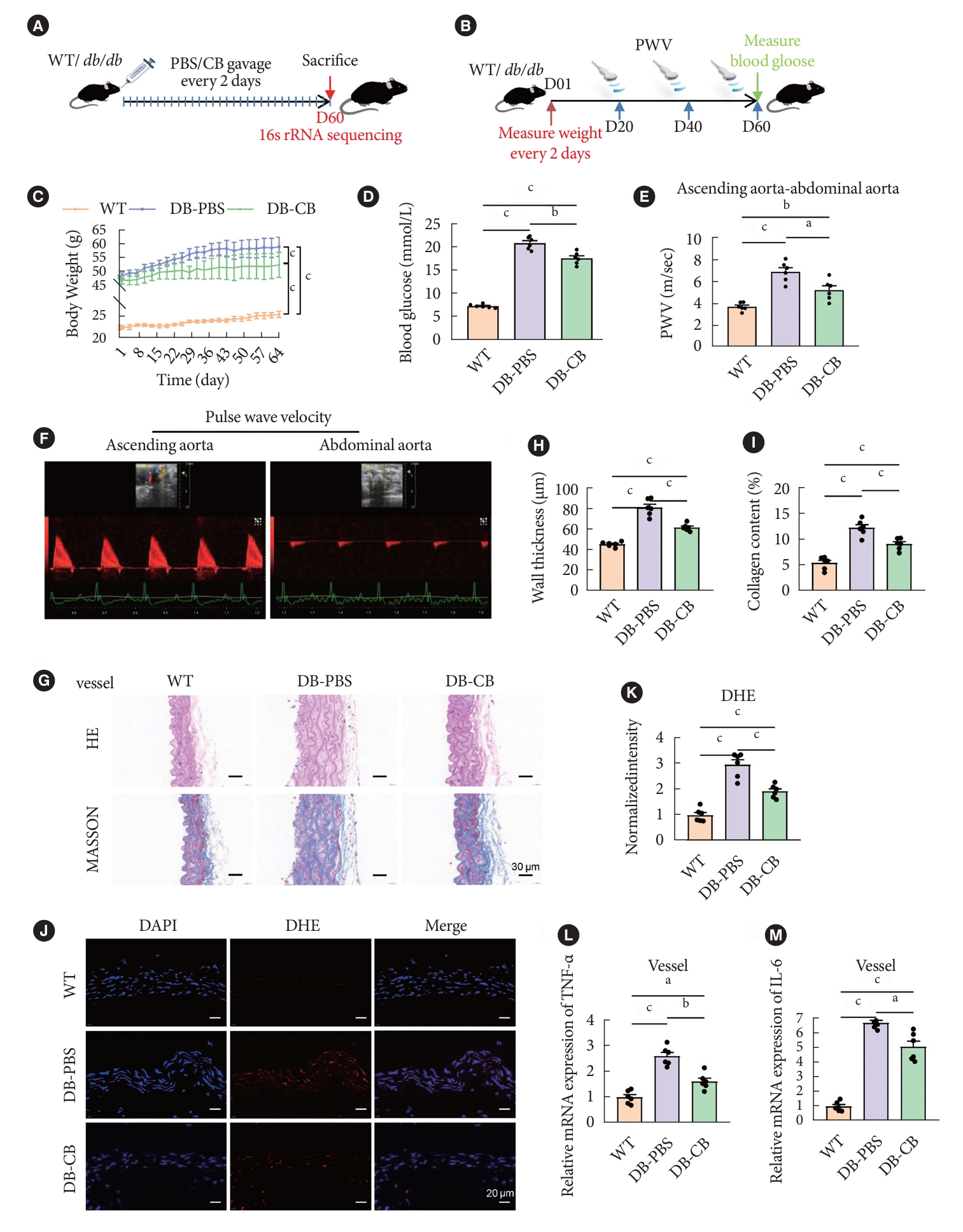
- To explore the potential effect of CB on vascular injury in diabetic mouse models, we administered 200 µL of CB suspension every other day for 60 days (Fig. 3A). During this period, the body weight and the blood glucose level of the mice was measured. And vascular PWV detection was performed on mice on day 20, 40, and 60, respectively (Fig. 3B). Mice in the CB-treated group gained weight significantly slower than the db/db mice in the PBS-treated group (Fig. 3C). After the treatment with CB, the blood glucose level was significantly decreased compared with DB-PBS group (Fig. 3D). The aorta PWVs were different across the groups, which suggested alterations in vascular stiffness. PWVs were significantly increased in untreated DB-PBS group mice compared to age-matched WT mice, and CB-treated DB mice have significantly lower PWV than non-treated DB mice. The mean PWVs in WT, DB-CB, and DB-PBS group was 3.69, 5.22, and 6.88 m/sec, respectively. The CB-treated DB mice group decreased the PWV value by 24.21% (Fig. 3E and F).
- We next explored whether CB supplementation would be beneficial to prevent inflammatory changes in blood vessels in diabetic mice; All mice were euthanized at day 60, and then tissues were taken for histological staining. HE staining showed that the vascular wall thickness was markedly increased in diabetic mice, however, compared with the DB-PBS group, it was significantly decreased after oral gavage of CB in DB-CB group (P<0.01) (Fig. 3G and H). Consistently, there was increased collagen deposition in vascular wall in DB-PBS mice group as revealed by Masson’s trichrome staining. And in the DB-CB group, there was a significant improvement in smooth muscle fiber sparseness, disorder, and collagen deposition compared with the DB-PBS group (P<0.01) (Fig. 3G and I). To detect ROS in the aorta tissues of db/db and WT mice, DHE staining was performed. The results showed that the fluorescence intensity of the ROS signal in the DB-PBS group was significantly stronger than that in the WT group, while the diabetic group treated with CB was significantly lower than the DB-PBS group (n=6, P<0.01) (Fig. 3J and K). After 60 days, the inflammatory response was improved, and the qPCR of arterial tissue results showed that the mRNA expression of inflammatory cytokines (IL-6 and TNF-α) were found to be significantly reduced in DB-CB group mice after CB treatment compared with those in the untreated DB-PBS group mice (n=6, P<0.01) (Fig. 3L and M).
- To investigate changes in gut microbiota after microbiota transplantation of CB gavage in diabetic mice, the gut microbiota was analyzed by 16sRNA sequencing in 60-day CB gavagetreated db/db mice and control mice (Fig. 3A). The diversity of the microbial community significantly decreased in the DB-PBS group, while the microbial community diversity of the DB-CB group was not significantly different from the WT group (Fig. 4A). Beta diversity also significantly differed between habitat types (Fig. 4B). We constructed a Venn diagram to determine the degree of OTU overlap between samples, which showed that the overlap between the DB-CB group and the WT group was 2.29%, and the overlap between the DB-PBS group and the WT group was 1.61% (Fig. 4C). Linear discriminant analysis (LDA) effect size (LEfSe) analysis indicating significant differences in bacterial taxa (LDA score >2.0; alpha value P<0.05). At the genus level, LEfSe analysis identified LDA scores above 2.0 for each group of genera. LEfSe identified genera that were differentially enriched in OTUs between the DB-PBS group and the other two groups, such as: Clostridiales_vadinBB60_group (increased in WT group; LDA score=3.23, P=0.025; increased in DB-CB group; LDA score=2.98, P=0.040). In addition, Clostridiales_vadinBB60_group were significantly deficient in DB-PBS group (Fig. 4D). At the level of order and family taxonomy, the total OTUs of Clostridiales in DB-CB group were significantly increased compared to DB-PBS group, which was not gavaged with CB (Fig. 4E and F). A notable decrease in butyric acid content in diabetic mice feces compared to WT group, while treated with CB by oral gavage (DB-CB group) showed increased levels (P<0.01) of butyric acid in comparison with DB-PBS group (Fig. 4G).
- The function of CB supplementation correlates with Nrf2 and HO-1 restoration
- Studies have confirmed that oxidative stress would be inhibited through activation the Nrf2/HO-1 signaling pathway. To further explored the relationship between CB intestinal intervention and oxidative stress in diabetic mice, we performed immunofluorescence staining and Western blot quantitative detection the level of Nrf2 and HO-1 proteins in vascular tissues of the mice model. The results showed that the fluorescence intensity of Nrf2 and HO-1 signals both in DB-CB group and DB-PBS group was significantly stronger than that in WT group. However, the fluorescence intensity of Nrf2 and HO-1 signals in DB-PBS group was significantly weaker than that in DB-CB group with CB-treated (n=6, P<0.01) (Fig. 5A-D). Moreover, representative Western blot results and relative quantitation of Western blot are shown that the increased levels of Nrf2 and HO-1 proteins in the DB-CB group compared with the DB-PBS group (n=6, P<0.05) (Fig. 5E-G).
- SB represses the inflammatory reactions caused by HG, and elevates the levels of Nrf2 and HO-1
- It is generally believed that inflammation play pivotal role in the progression of diabetic vascular complications [25]. Therefore, we investigated the effects of SB on inflammatory cytokines in HUVEC, RAW264.7 and VSMC cells. RT-qPCR results showed that in HUVEC and RAW264.7 cells experiments respectively, the mRNA expression levels of IL-6 and TNF-α in HG group were significantly higher than those in NG group, whereas the HG+SB group treated with SB significantly decreased the mRNA expression levels of IL-6 and TNF-α (P<0.05) (Fig. 6A, B, E, and F). Moreover, in HUVEC, RAW264.7 and VSMC cell experiments, compared with NG group, the mRNA expression levels of Nrf2 and HO-1 both in HG group and HG+SB group were significantly increased. And the mRNA expression levels of Nrf2 and HO-1 in HG+SB group were significantly higher than those in HG group (P<0.05) (Fig. 6C, D, and G-J).
RESULTS
- Vasculopathy is a severe complication of diabetes due to uncontrolled HG, and a major cause of morbidity and mortality [1]. It has been reported that fecal microbiota transplantation is an effective treatment for T2DM by reverse insulin resistance and islet damage [26]. However, whether there is a potential link between the gut microbiota and vascular complications in diabetic patients is yet to be clarified. Therefore, to explore the relationship between the gut microbiota and vascular complications of diabetes, using a db/db mouse model, we demonstrated inflammatory and oxidative stress damage in diabetic vessels. It was further revealed that the alteration of gut microbiota, especially the reduction of butyric acid bacteria, might be responsible for the vascular inflammation and oxidative stress in diabetic mice. Oral supplementation of CB partially retarded weight gain, vascular dysfunction and oxidative stress in diabetic mice. Our findings, for the first time, indicate that CB may attenuate vascular inflammatory and oxidative stress of DM effectively via activating the Nrf2/HO-1 pathway. Regulation of gut microbiota could be helpful for the management of diabetic vascular complications.
- An increasing number of studies have confirmed that excessive inflammatory response and oxidative stress often occur in the progression of hyperglycemia and insulin resistance-induced metabolic disorders [27]. Theoretically, persistent hyperglycemia can cause the vascular wall to more easily “capture” lipids, leading to disorders of glucose and lipid metabolism, promoting the production of ROS and free radicals, triggering oxidative stress and inflammatory responses, and aggravating vascular endothelial dysfunction [28]. In this study, using diabetic mouse model fed with a high-fat diet, we confirmed that diabetic mice had severe vascular inflammation and oxidative stress damage, and further found that altered in intestinal flora of diabetic mice, especially decreased CB.
- Studies have shown that the composition and function of the gut microbiota play a crucial role in obesity and metabolic disease [29]. The microbial metabolites, SCFAs, are the “secret weapons” of intestinal bacteria. Butyric acid, acetic acid, and propionic acid are all involved in the regulation of energy metabolism. Moreover, several effective T2DM therapies, such as metformin and berberine, have been reported to be able to restore the abundance of SCFA-producing bacteria [30]. CB is one of the main sources of butyric acid. In a mouse model of obesity, oral butyrate reduces food intake by stimulating the vagus nerve to induce a constant feeling of satiety [31]. It has also been shown that hypoglycemic effects are achieved by increasing the number of butyrate-producing bacteria in the gut of a T2DM mouse model, resulting in the production of butyrate and the secretion of glucagon-like peptide-1 and insulin [32]. CB played an important role in diabetic vasculopathy not only through butyrate, but also through immune and other mechanisms. CB interacts with a variety of systemic immune cells to influence systemic inflammation [33,34], which then influences the development of vascular complications involved in diabetes. Thus, in vivo experiments, our study confirmed that the intestinal CB in the diabetic mouse model was reduced compared with normal mice. Intragastrically transplanted CB significantly reduced blood glucose, delayed weight gain, and improved vascular inflammation and oxidative stress in diabetic mice. In addition, other than the CB strain used in present study (ATCC19398), the CB CGMCC0313.1 may also be a promising CB strain in the protection for diabetic vascular injury duo to its therapeutic benefit in improving metabolic diseases reported previously [35,36]. The gene and function differences between these two strains worths further investigations.
- Oxidative stress has an important role in the pathogenesis of diabetes and its complications [37,38]. Nrf2 is a redox-sensitive transcription factor that is generally silent [39]. Activation of the Nrf2 signaling pathway can induce upregulation of antioxidant enzyme gene expression and thus protect organs damaged by oxidative stress induced by hyperglycemia in diabetes [40]. And the activation of Nrf2 signaling ameliorates DM by protecting pancreatic beta cells and suppressing gluconeogenesis-related gene expression [41], suggesting that the Nrf2 system is a critical target for preventing the onset of DM. Additionally, activated Nrf2 enters the cell nucleus and exerts antioxidant effects through its interaction with downstream antioxidant genes such as HO-1 [42]. According to the aforementioned results, the high fasting blood glucose levels in diabetic rats were significantly reduced upon oral administration of CB. Therefore, it is confirmed by present study that CB treatment activates the Nrf2 system by increasing the level of butyrate in the blood, thus achieving a reduction in the ROS content of arteries, decreasing vascular oxidative stress and improving vascular function. To further explore the mechanism, using an experimental model of HG-induced HUVEC, RAW264.7 and VSMC cells, it was found that the elevation of inflammatory cytokine levels by HG were significantly reduced after SB treatment. And SB treatment also increased the levels of Nrf2 and HO-1 in HUVEC, RAW264.7 and VSMC cells. Based on the above results, we speculate that the mechanism of SB in regulating oxidative damage during the development of diabetic vascular complications may be related to the Nrf2/HO-1 pathway. However, our study found that CB intervention acted on diabetic vasculopathy not only by increasing butyrate in the blood, but also by improving the diversity of the intestinal flora, increasing the content of probiotic bacteria in the intestinal flora, improving the disordered intestinal flora in diabetic mice, and eventually stopping the progression of diabetic vascular complications. Moreover, the genes and receptor mRNA expression could significantly influence the effects of butyrate. Multiple genes and receptors, and their complex interactions could all involved in the functioning of butyrate as shown by previous studies [35,36]. Metagenome sequencing may help answer this question.
- In this study, we first found the association between microbiota and diabetes vascular complications, and further developed the corresponding therapeutic strategy by microbiota transplantation. The supplementation of CB could increase the metabolite butyrate levels, regulate gut microbiota diversity, and thus reduce the blood glucose, decelerate weight gain and improve the vascular function through the alleviation of vascular inflammatory and oxidative stress by Nrf2/HO-1 pathway in diabetic mice. It would be of great clinical interest to explore complementary reagents of CB probiotics for diabetes vascular protection.
DISCUSSION
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: T.Z., L.Z., Y.D., C.X.
Acquisition, analysis, or interpretation of data: T.Z., S.Q., L.Z., Y.L., J.Z., D.S., P.Z., L.Y., L.Z., Y.D., C.X.
Drafting the work or revising: T.Z., S.Q., Y.D., C.X.
Final approval of the manuscript: T.Z., S.Q., L.Z., Y.L., J.Z., D. S., P.Z., L.Y., L.Z., Y.D., C.X.
-
FUNDING
This study was supported by grants from National Natural Science Foundation of China (No. 81901751 and 81901861) and Innovative Talent Promotion Program of Shaanxi Province (2022KJXX-106). Changyang Xing was also supported by the Special Fund for Aerospace Medical Research.
NOTES
-
Acknowledgements
- We are grateful for the technical help from Guodong Yang. We would also express our thanks for all the lab members for critical reading of the manuscript.




- 1. Pearson-Stuttard J, Cheng YJ, Bennett J, Vamos EP, Zhou B, Valabhji J, et al. Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol 2022;10:46-57.ArticlePubMedPMC
- 2. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol 2014;10:293-302.ArticlePubMedPDF
- 3. Paneni F, Costantino S, Cosentino F. Insulin resistance, diabetes, and cardiovascular risk. Curr Atheroscler Rep 2014;16:419.ArticlePubMedPDF
- 4. Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021;119:154766.ArticlePubMed
- 5. James DE, Stockli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nat Rev Mol Cell Biol 2021;22:751-71.ArticlePubMedPDF
- 6. Tai N, Wong FS, Wen L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev Endocr Metab Disord 2015;16:55-65.ArticlePubMedPMCPDF
- 7. Ruan W, Engevik MA, Spinler JK, Versalovic J. Healthy human gastrointestinal microbiome: composition and function after a decade of exploration. Dig Dis Sci 2020;65:695-705.ArticlePubMedPDF
- 8. Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 2016;12:144-53.ArticlePubMedPDF
- 9. Defronzo RA. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773-95.PubMedPMC
- 10. Rodriguez ML, Perez S, Mena-Molla S, Desco MC, Ortega AL. Oxidative stress and microvascular alterations in diabetic retinopathy: future therapies. Oxid Med Cell Longev 2019;2019:4940825.PubMedPMC
- 11. Li F, Wang M, Wang J, Li R, Zhang Y. Alterations to the gut microbiota and their correlation with inflammatory factors in chronic kidney disease. Front Cell Infect Microbiol 2019;9:206.ArticlePubMedPMC
- 12. Li X, Wang Y, Zhou J, Wang Z, Wang Y, Zheng J, et al. Mixed nuts with high nutrient density improve insulin resistance in mice by gut microbiota remodeling. Food Funct 2022;13:9904-17.ArticlePubMed
- 13. Hao J, Zhang Y, Wu T, Liu R, Sui W, Zhu J, et al. The antidiabetic effects of Bifidobacterium longum subsp. longum BL21 through regulating gut microbiota structure in type 2 diabetic mice. Food Funct 2022;13:9947-58.ArticlePubMed
- 14. Zhao D, Zhu H, Gao F, Qian Z, Mao W, Yin Y, et al. Antidiabetic effects of selenium-enriched Bifidobacterium longum DD98 in type 2 diabetes model of mice. Food Funct 2020;11:6528-41.ArticlePubMed
- 15. Butlin M, Tan I, Spronck B, Avolio AP. Measuring arterial stiffness in animal experimental studies. Arterioscler Thromb Vasc Biol 2020;40:1068-77.ArticlePubMedPMC
- 16. Wang C, Xing C, Li Z, Liu Y, Li Q, Wang Y, et al. Bioinspired therapeutic platform based on extracellular vesicles for prevention of arterial wall remodeling in hypertension. Bioact Mater 2021;8:494-504.ArticlePubMedPMC
- 17. Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015;519:92-6.ArticlePubMedPMCPDF
- 18. Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012;488:621-6.ArticlePubMedPMCPDF
- 19. Maddahi A, Edvinsson L. Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J Neuroinflammation 2010;7:14.ArticlePubMedPMC
- 20. Hervera A, De Virgiliis F, Palmisano I, Zhou L, Tantardini E, Kong G, et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat Cell Biol 2018;20:307-19.ArticlePubMedPDF
- 21. Zhou J, Zhang L, Zheng B, Zhang L, Qin Y, Zhang X, et al. Salvia miltiorrhiza bunge exerts anti-oxidative effects through inhibiting KLF10 expression in vascular smooth muscle cells exposed to high glucose. J Ethnopharmacol 2020;262:113208.ArticlePubMed
- 22. Hamzah N, Safuan S, Wan Ishak WR. Potential effect of polyphenolic-rich fractions of corn silk on protecting endothelial cells against high glucose damage using in vitro and in vivo approaches. Molecules 2021;26:3665.ArticlePubMedPMC
- 23. Xu YH, Gao CL, Guo HL, Zhang WQ, Huang W, Tang SS, et al. Sodium butyrate supplementation ameliorates diabetic inflammation in db/db mice. J Endocrinol 2018;238:231-44.ArticlePubMed
- 24. Yang T, Yang H, Heng C, Wang H, Chen S, Hu Y, et al. Amelioration of non-alcoholic fatty liver disease by sodium butyrate is linked to the modulation of intestinal tight junctions in db/db mice. Food Funct 2020;11:10675-89.ArticlePubMed
- 25. Mthiyane FT, Dludla PV, Ziqubu K, Mthembu SX, Muvhulawa N, Hlengwa N, et al. A review on the antidiabetic properties of Moringa oleifera extracts: focusing on oxidative stress and inflammation as main therapeutic targets. Front Pharmacol 2022;13:940572.ArticlePubMedPMC
- 26. Wang H, Lu Y, Yan Y, Tian S, Zheng D, Leng D, et al. Promising treatment for type 2 diabetes: fecal microbiota transplantation reverses insulin resistance and impaired islets. Front Cell Infect Microbiol 2020;9:455.ArticlePubMedPMC
- 27. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25:1822-32.ArticlePubMedPMCPDF
- 28. Beckman JA, Paneni F, Cosentino F, Creager MA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur Heart J 2013;34:2444-52.ArticlePubMed
- 29. Machate DJ, Figueiredo PS, Marcelino G, Guimaraes RC, Hiane PA, Bogo D, et al. Fatty acid diets: regulation of gut microbiota composition and obesity and its related metabolic dysbiosis. Int J Mol Sci 2020;21:4093.ArticlePubMedPMC
- 30. Xu J, Lian F, Zhao L, Zhao Y, Chen X, Zhang X, et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J 2015;9:552-62.ArticlePubMedPDF
- 31. Avagliano C, De Caro C, Cuozzo M, Liguori FM, La Rana G, Micheli L, et al. Phaseolus vulgaris extract ameliorates high-fat diet-induced colonic barrier dysfunction and inflammation in mice by regulating peroxisome proliferator-activated receptor expression and butyrate levels. Front Pharmacol 2022;13:930832.ArticlePubMedPMC
- 32. Yang YN, Wang QC, Xu W, Yu J, Zhang H, Wu C. The berberine-enriched gut commensal Blautia producta ameliorates high-fat diet (HFD)-induced hyperlipidemia and stimulates liver LDLR expression. Biomed Pharmacother 2022;155:113749.ArticlePubMed
- 33. Wang FY, Liu JM, Luo HH, Liu AH, Jiang Y. Potential protective effects of Clostridium butyricum on experimental gastric ulcers in mice. World J Gastroenterol 2015;21:8340-51.ArticlePubMedPMC
- 34. Liu J, Fu Y, Zhang H, Wang J, Zhu J, Wang Y, et al. The hepatoprotective effect of the probiotic Clostridium butyricum against carbon tetrachloride-induced acute liver damage in mice. Food Funct 2017;8:4042-52.ArticlePubMed
- 35. Jia L, Li D, Feng N, Shamoon M, Sun Z, Ding L, et al. Anti-diabetic effects of Clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in type 2 diabetic mice. Sci Rep 2017;7:7046.ArticlePubMedPMCPDF
- 36. Jia L, Shan K, Pan LL, Feng N, Lv Z, Sun Y, et al. Clostridium butyricum CGMCC0313.1 protects against autoimmune diabetes by modulating intestinal immune homeostasis and inducing pancreatic regulatory T cells. Front Immunol 2017;8:1345.ArticlePubMedPMC
- 37. Malik A, Morya RK, Saha S, Singh PK, Bhadada SK, Rana SV. Oxidative stress and inflammatory markers in type 2 diabetic patients. Eur J Clin Invest 2020;50:e13238.ArticlePubMedPDF
- 38. Li H, Shi Y, Wang X, Li P, Zhang S, Wu T, et al. Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-kB pathways in diabetic cardiomyopathy. Chem Biol Interact 2019;310:108754.PubMed
- 39. Ichikawa T, Li J, Meyer CJ, Janicki JS, Hannink M, Cui T. Dihydro-CDDO-trifluoroethyl amide (dh404), a novel Nrf2 activator, suppresses oxidative stress in cardiomyocytes. PLoS One 2009;4:e8391.ArticlePubMedPMC
- 40. Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 2003;43:233-60.ArticlePubMed
- 41. Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, et al. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol 2013;33:2996-3010.ArticlePubMedPMCPDF
- 42. Di Marco E, Jha JC, Sharma A, Wilkinson-Berka JL, Jandeleit-Dahm KA, de Haan JB. Are reactive oxygen species still the basis for diabetic complications? Clin Sci (Lond) 2015;129:199-216.ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations


 KDA
KDA
 PubReader
PubReader ePub Link
ePub Link Cite
Cite










