Diabetes and the Risk of Infection: A National Cohort Study
Article information
Abstract
Background
Several studies have shown that people with diabetes are vulnerable to infection. This study compared the risk of infection-related hospitalizations, intensive care unit (ICU) admission, and deaths between the person with diabetes and the general population in South Korea.
Methods
We conducted a cohort study of 66,426 diabetes and 132,852 age-sex-region-matched non-diabetes controls from the general population using a sample of data from the National Health Insurance Service-National Sample Cohort. The cohort was followed up for 9 years. Infections were classified into 17 separate categories. We used Poisson regression, with adjustment for household income and other comorbidities, to estimate incidence rate ratios (IRRs) in order to compare of infection-related hospitalizations, ICU admissions, and deaths.
Results
Compared to non-diabetes controls, diabetes group had a greater risk of almost all the types of infections considered, with the adjusted IRRs (aIRRs) for infection-related hospitalizations being the highest for hepatic abscess (aIRR, 10.17; 95% confidence interval [CI], 7.04 to 14.67), central nervous system (CNS) infections (aIRR, 8.72; 95% CI, 6.64 to 11.45), and skin and soft tissue infections other than cellulitis (SSTIs) (aIRR, 3.52; 95% CI, 3.20 to 3.88). Diabetes group also had a greater risk of ICU admission and death due to SSTIs (aIRR, 11.75; 95% CI, 7.32 to 18.86), CNS infections (aIRR, 5.25; 95% CI, 3.53 to 7.79), and bone and joint infections (aIRR, 4.78; 95% CI, 3.09 to 7.39).
Conclusion
In South Korea, people with diabetes has a considerably higher incidence of infection-related hospitalizations and deaths than the general population.
INTRODUCTION
Diabetes mellitus is an important public health issue, affecting an estimated 424.9 million people worldwide, of whom one-third are older than 65 years [1]. In South Korea, the prevalence of diabetes has increased in parallel with an increased incidence of obesity [2]. In 2016, the prevalence of diabetes among adults aged 30 years and older was 14.4% [34]. Diabetes is known to lead to chronic diseases, including chronic kidney disease (CKD) and retinopathy, and diabetes have an increased risk of infection [56]. Among diabetes, poor glycemic control increases the risk of infection, diabetes neuropathy, and impaired innate and adaptive immune responses [78]. Population-based cohort studies conducted in Europe and the United States have found that the incidence of infectious diseases and hospitalization was higher in diabetes than in the general population [691011].
However, in South Korea, information about the risk of infection among person with diabetes is limited. Considering the increasing prevalence of diabetes in South Korea, hospitalizations and deaths due to infection are likely to increase. In this study, we compared the incidence of infection-related hospitalizations, intensive care unit (ICU) admissions, and deaths between the person with diabetes and the general population in South Korea.
METHODS
Data source and study design
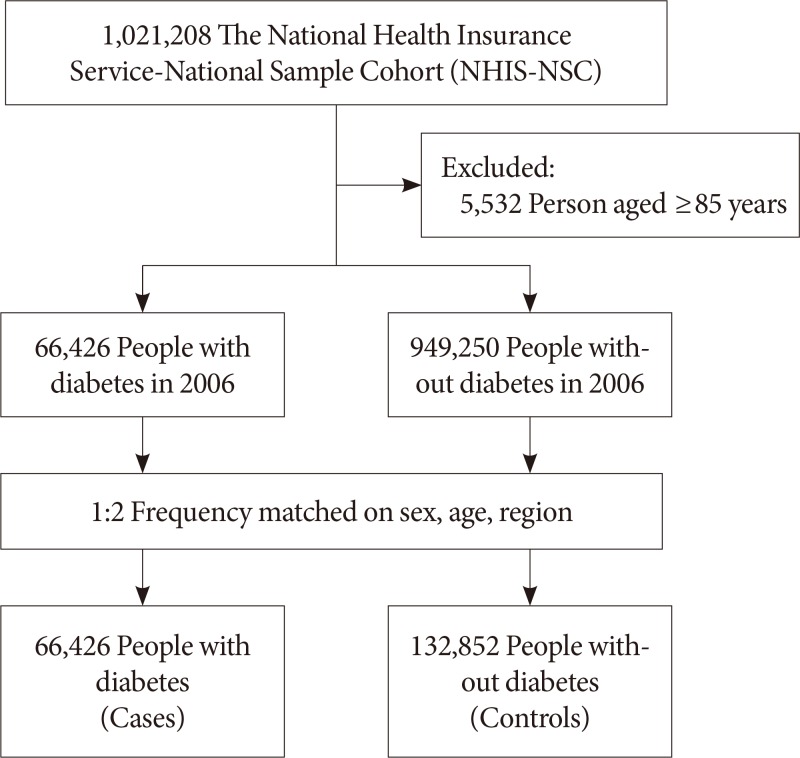
The National Health Insurance Service-National Sample Cohort (NHIS-NSC) was established in 2006, as a nationally representative randomly selected sample of 2.2% of the entire South Korean population. This cohort was followed up for 9 years, until the end of 2015. A detailed description of the study design and methods has been published previously [12]. We conducted a retrospective matched cohort study among people with diabetes who were aged under 85 years in 2006. Person in the cohort were classified as having diabetes if they met any of the following criteria: a diagnosis of diabetes according to the 10th edition of the International Classification of Diseases (ICD-10) codes: E10–E14 more than twice or having been prescribed oral glucose-lowering medications (Anatomical Therapeutic Chemical [ATC] code: A10B) for more than 30 days; receiving a prescription for insulin (ATC code: A10A) as an outpatient. For each person with diabetes, we selected two controls without diabetes between January 1, 2002, and December 31, 2006, from the general population and matched them individually to each case by age, sex, and area of residence (Fig. 1). All participants were followed up from January 1, 2007, until the date of the death or December 31, 2015, whichever occurred earliest. During follow-up, the controls who were diagnosed with diabetes or treated for diabetes were censored on the date of diagnosis or treatment. However, we could not distinguish type 1 diabetes mellitus from type 2 diabetes mellitus.
Classification of infections
Infections during 2007 to 2015 were classified into 17 different groups using ICD-10 codes for hospital admissions and cause of death (Supplementary Table 1). With the exception of bone and joint infection, within each group, any code repeated within a 90-day period was considered to be a single event, and codes repeated more than 90 days apart were considered to be distinct events. Bone and joint infections were considered to be a single event if the codes were repeated within a year, and distinct events if the codes were repeated more than a year apart. The total number of infection events was counted for each participant. For each of the types of infection, two types of outcome were defined: (1) any infection that resulted in a hospital admission and (2) any infection that resulted in death and/or an ICU admission.
Covariates
In order to minimize the effects of confounding, statistical analyses were adjusted for household income and the presence of hypertension, dyslipidemia, cardiovascular disease (CVD) (including ischemic heart disease, heart failure, cerebral infarction, and peripheral arterial disease), and CKD, between January 1, 2002, and December 31, 2006. Supplementary Table 2 provides a list of the confounding variables, with the corresponding ICD-10 or ATC codes.
Statistical analysis
The baseline characteristics of person with the diabetes were compared to those of the non-diabetes controls using chi-square tests for categorical variables and t-tests for continuous variables. The incidence rates were calculated based on the total number of incident infections during the study period, divided by the total number of person-years at risk. To determine the independent risk of diabetes associated with each type of infection, incidence rate ratios (IRRs) and their 95% confidence intervals (CIs) were calculated using Poisson regression models, with adjustment for the household income, hypertension, dyslipidemia, CVD, and CKD. We also performed subgroup analyses stratified by age, sex, the presence of CVD, the presence of CKD, and the use of insulin. All analyses were done using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was considered at P<0.05.
Ethical considerations
The study protocol was reviewed and approved by the Ajou University Institutional Review Board (IRB) (IRB No. AJIRB-MED-EXP-18-547). The IRB waived the requirement for written informed consent because all personal identifiers were removed from the dataset prior to analysis.
RESULTS
Demographic characteristics
A total of 66,426 person with diabetes and 132,852 non-diabetes controls were followed for a mean of 7.8 years. Table 1 summarizes baseline characteristics of the study population. Participants with diabetes had a mean±standard deviation age of 58.2±13.3 years, 50.4% were women, and 36.6% had been diagnosed with diabetes more than 4 years before the inception of the cohort. Of the person with diabetes, 19.0% were using insulin. Person with diabetes were more likely than non-diabetes controls to have an income in the top 30% (30.1% vs. 26.2%). The prevalence of comorbidities, including hypertension, dyslipidemia, CVD, and CKD, was also significantly higher in person with diabetes than in non-diabetes controls.
Incident rate of infections among diabetes and non-diabetes controls
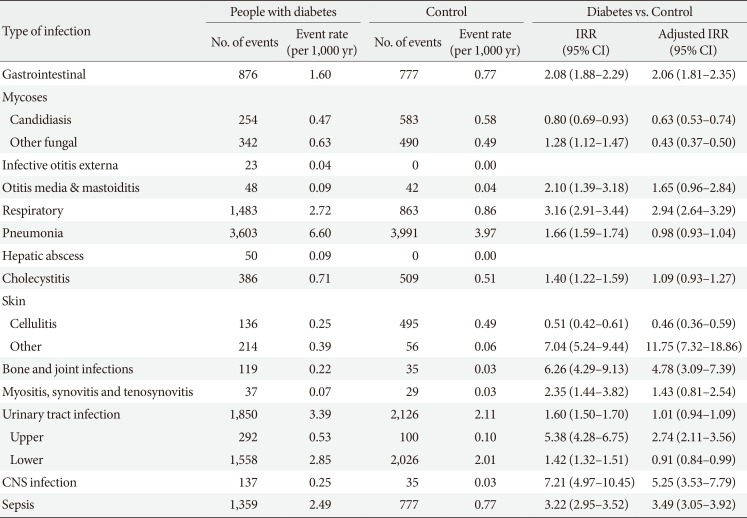
During the 9-year follow-up period, there were total events of 85,808 hospitalizations due to infections in the person with diabetes, an incidence rate of 1.29 admissions per a person. Of the diabetes group, respiratory infection (35.84 event per 1,000 person-years) and pneumonia (25.16 event per 1,000 person-years) were the most common causes of hospitalization, and urinary tract infection (UTI) (24.12 event per 1,000 person-years) was the second most common cause. The person with diabetes had a higher IRR (2.01; 95% CI, 1.99 to 2.03) related hospitalizations for all infection types combined. Table 2 shows the incidence rates of infection-related hospitalizations, and the IRRs for the diabetes group and non-diabetes controls according to the infection type. All types of infection, with the exception of infective otitis externa, and all hospitalizations related to the different types of infection were more common in diabetes group than in non-diabetes controls. The person with diabetes had a highest increased risk of central nervous system (CNS) infections (IRR, 6.38; 95% CI, 5.17 to 7.87), gastrointestinal (GI) infections (IRR, 2.71; 95% CI, 2.61 to 2.80), and skin and soft tissue infections (SSTIs) other than cellulitis (IRR, 2.52; 95% CI, 2.36 to 2.70). Even after adjusting for differences in income levels and comorbidities, diabetes group continued to have a higher incidence of infection-related hospitalizations. IRR after adjustment was still high for CNS infections (adjusted IRR [aIRR], 8.72; 95% CI, 6.64 to 11.45), SSTIs except cellulitis (aIRR, 3.52; 95% CI, 3.20 to 3.88), and hepatic abscess (aIRR, 10.17; 95% CI, 7.04 to 14.67). The incidence of hospitalizations due to sepsis was more than twice as high in diabetes group than in the general population (IRR, 2.21; 95% CI, 2.07 to 2.36) (Table 2).
In the stratified analyses, the IRRs for GI infection, pneumonia, UTI, and sepsis were higher in diabetes on insulin and diabetes with CVD. The IRRs for pneumonia, UTI, and sepsis were higher among diabetes with CKD. UTI and sepsis were more incident in women younger than 65 years, but GI infection was higher in person with diabetes older than 65 years and male with diabetes (Fig. 2).

Adjusted incidence rate ratios (IRRs) of infection-related hospitalizations in diabetes and matched non-diabetes controls, stratified by age, sex, comorbidities, and the use of insulin. The IRRs were estimated using Poisson models. CI, confidence interval.
With respect to the attributable mortality and ICU admission-related infections between the two groups, IRRs were higher in diabetes group in 11 of the 17 categories of infectious disease types (Table 3). The IRRs of CNS infection (IRR, 7.21; 95% CI, 4.97 to 10.45), bone and joint infections (IRR, 6.26; 95% CI, 4.29 to 9.13), and SSTIs except cellulitis (IRR, 7.04; 95% CI, 5.24 to 9.44) were especially higher in diabetes group. Sepsis IRR was also significantly higher in diabetes group (IRR, 3.22; 95% CI, 2.95 to 3.52). This result was the same after adjusting for household income and comorbidities. The highest aIRRs of mortality and ICU hospitalization-related infections were observed for SSTIs except for cellulitis (aIRR, 11.75; 95% CI, 7.32 to 18.86), CNS infections (aIRR, 5.25; 95% CI, 3.53 to 7.79), and bone and joint infections (aIRR, 4.78; 95% CI, 3.09 to 7.39) (Table 3). Among person with diabetes, the most incidence of ICU hospitalization and death was due to pneumonia and UTI. However, the risk ratio of ICU hospitalization and death due to pneumonia and lower UTI in diabetes group was not higher in comparison with the general population control.
DISCUSSION
This is the first large cohort study in South Korea to compare the incidence of infection-related hospitalizations and infection-related mortality between diabetes group and age-sex-region-matched non-diabetes controls and to provide detailed estimates of hospital resource and mortality for infections among person with diabetes in the South Korea.
Previous studies have provided strong evidence of an association between diabetes mellitus and infection. Laboratory studies have shown that diabetes increased susceptibility to infection is related to neutrophil dysfunction (including impaired chemotaxis, phagocytic abilities, and lower microbicidal activities) [13141516] or to disturbances in the adaptive immune response [1718]. Alternatively, the colonization rate of pathogenic strains of microbes may be increased in the presence of hyperglycemia [1920]. Moreover, recent population-based studies provide evidence of an increased risk of infection in person with diabetes [62122]. In a large retrospective cohort study conducted in England (n=102,493 patients with diabetes mellitus vs. n=203,518 matched control subjects), IRRs for any infection-related hospitalizations were 3.71 (95% CI, 3.27 to 4.21) in those with type 1 diabetes mellitus and 1.88 (95% CI, 1.83 to 1.92) in those with type 2 diabetes mellitus. The researchers estimated that 6% of infection-related hospitalizations and 12% of infection-related deaths were attributable to diabetes. In a matched cohort study using Canadian Primary Care Sentinel Surveillance Network, patients with diabetes (n=1,779 diabetes patients vs. n=11,066 matched control subjects) had an increased risk of any infection (adjusted odds ratio, 1.21; 95% CI, 1.07 to 1.37). In a large Danish national cohort study with type 2 diabetes mellitus (n=155,158) and age-sex-residence-matched controls without diabetes (n=774,017), the adjusted rate ratio of community-based antibiotic prescriptions was 1.24 (95% CI, 1.23 to 1.25) and adjusted rate ratio of hospital-treated infections was 1.49 (95% CI, 1.47 to 1.52) for a median follow-up of 2.8 years [10]. In a retrospective analysis of the socioeconomic impact of inpatient infection management in the USA, the person with diabetes were more than twice as likely to be hospitalized for infection management than those without diabetes [9].
In this study, diabetes had a risk of infection-related hospitalizations for all infection types combined and for 16 of the 17 specific types of infection. This increased risk persisted even after adjusting for potential confounders such as the presence of CVD and CKD. Therefore, further studies are needed to investigate the increasing incidence and severity of infectious diseases caused by diabetes in South Korea and the resulting socioeconomic impact. The mechanism and risk factors that cause the infectious disease associated with diabetes require further investigation. We believe our results could stimulate such research.
Many studies and reports have reported increased incidence of UTIs, SSTIs, bone and joint infections, and sepsis related to diabetes [6923]. This study also found that the person with diabetes had an increased incidence of UTIs, SSTIs, sepsis, CNS infections, and bone and joint infections and a significantly higher severity of infections. The high incidence rate of hepatic abscess observed in this study reflects the high incidence in Northeast Asia. Previous studies in China and South Korea have identified diabetes as a risk factor for hepatic abscess [242526]. Hyperglycemia may promote the activation of nuclear factor κB signaling that has a regulatory effect on the pathogenesis of liver abscesses caused by Klebsiella pneumoniae [27]. The increased susceptibility of diabetes to Klebsiella infections may also affect the incidence of liver abscess. The high incidence of CNS infections may be associated with rhino-orbital-cerebral mucormycosis, malignant otitis media, and skin infections in diabetes. Diabetes may increase the risk of CNS infections, given that it is a risk factor for Listeria and Klebsiella infections [28]. However, there has been limited research in this area; therefore, further research is need. Diabetes also had a relatively high incidence of SSTIs and bone and joint infections. These types of infection may occur as results of peripheral neuropathy, autonomic neuropathy, and vascular insufficiency due to diabetic complications. The presence of diabetes foot is also a risk factor for these types of infection. In a recent retrospective study of SSTIs, diabetes was an important risk factor associated with readmission to hospital and death [29]. Therefore, it is necessary to study how to minimize the risks associated with each type of infection based on this study. The aIRR of infective otitis externa was 0.76 (95% CI, 0.64 to 0.90) in Table 2 and this means that hospitalization due to infective otitis externa is less, not decrease in incidence rates. Infective otitis externa is a disease mainly based on outpatient treatment, so the result may be as shown, and diabetes is not a risk factor in infective otitis externa occurrence. In Table 3, ICU admission or death due to infective otitis externa is zero, reflecting the low severity of the disease. In addition, we analyzed otitis media and mastoiditis separately, and it is estimated that incidence rate is low due to separation of malignant otitis media with high severity and associated with diabetes. Therefore, infective otitis externa may need to be analyzed again through data including outpatient prescriptions.
On stratified analyses for pneumonia, UTIs, and GI infections, which showed significantly higher risk ratios in person with diabetes, we observed significantly high risk ratios in diabetes person with CVD or insulin users. Person with diabetes using insulin may have worse glycemic control than those receiving oral medications or, may have type 1 diabetes mellitus. A previous study showed that poor glycemic control was associated with increased incidence of infectious disease [30]. Therefore, it can be interpreted as an increase in risk associated with hyperglycemia, but well-designed studies are needed for further investigation. Pneumonia is one of the most common infectious diseases in diabetes patients as well as the most common cause of ICU admission and death. However, compared to the general population, person with diabetes showed an increased incidence of infectious diseases; however, the increase in mortality and severity of infectious diseases was not significant. Other studies involving patients with diabetes have reported increases in incidence but not in mortality of infectious diseases [31]. The relationships between CVD and infectious diseases and between diabetes and pneumonia must be studied in depth.
The study has several limitations. Firstly, the NHIS-NSC database was a sample cohort and contained only a limited number identified variables. Therefore, it was difficult to determine precise risk factors due to a lack of information on factors such as body mass index and smoking history. The malignancy of the comorbidity was also lost. In particular, the relationship between diabetes mellitus and hyperglycemia could not be studied. Secondly, the dataset did not distinguish between type 1 and type 2 diabetes mellitus. Taking other studies into consideration, the incidence and risk factors for different types of infection may differ in type 1 and type 2 diabetes mellitus, and determining risk factors may require a different approach. However, as type 1 diabetes mellitus affects about 0.02% of the Korean population [32], our data can be attributed to people with type 2 diabetes mellitus. Thirdly, the incidence of infectious diseases is likely to have been underestimated due to the exclusion of certain infections such as tuberculosis and other national surveillance infectious diseases. In particular, South Korea has an intermediate incidence of tuberculosis. Subsequent studies on tuberculosis will be necessary to determine the association between diabetes and tuberculosis. Despite these limitations, the important strengths of our study are that it is population-based study with a large sample size and a long follow-up time and that it investigated a wide range of infectious diseases.
In conclusion, we found that the overall risk of infection-related hospitalizations is higher in the person with diabetes than in the general population, and that diabetes also have a higher risk of more serious infectious diseases and a higher infectious disease-related mortality rate than the general population. Improved diabetes control could help to reduce the incidence of infectious diseases among person with diabetes. There is also a need to address policy and program issues related to the prevention of infections in diabetes, such as developing recommendations for vaccination of person with diabetes. Furthermore, it is important to find ways to reduce the incidence of infectious diseases by studying the mechanisms of interaction between diabetes and infectious diseases and the risk factors for infectious diseases in diabetes.
ACKNOWLEDGMENTS
This study used National Health Insurance Service (NHIS) data (NHIS-2019-2-104) made by NHIS.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS:
Conception or design: E.J.K., D.J.K., Y.H.C.
Acquisition, analysis, or interpretation of data: K.H.H., Y.H.C.
Drafting the work or revising: E.J.K.
Final approval of the manuscript: K.H.H., D.J.K., Y.H.C.
References
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2019.0071.
Supplementary Table 1
ICD-10 diagnostic codes for hospital admissions and causes of infectious disease-related deaths
Supplementary Table 2
Confounding variables with corresponding ICD-10 codes and ATC codes