Effects of Rebamipide on Gastrointestinal Symptoms in Patients with Type 2 Diabetes Mellitus
Article information
Abstract
Background
Gastrointestinal (GI) symptoms are common in patients with type 2 diabetes mellitus (T2DM). Rebamipide is an effective gastric cytoprotective agent, but there are few data on its usefulness in T2DM. The aim of this study is to evaluate the improvement of GI symptoms after rebamipide treatment in patients with T2DM.
Methods
Patients with T2DM and atypical GI symptoms were enrolled. They took rebamipide (100 mg thrice daily) for 12 weeks and filled out the diabetes bowel symptom questionnaire (DBSQ) before and after rebamipide treatment. The DBSQ consisted of 10 questions assessing the severity of GI symptoms by a 1 to 6 scoring system. Changes in the DBSQ scores before and after rebamipide treatment were analyzed to evaluate any improvements of GI symptoms.
Results
A total of 107 patients were enrolled, and 84 patients completed the study. The mean age was 65.0±7.8, 26 patients were male (24.8%), the mean duration of T2DM was 14.71±9.12 years, and the mean glycosylated hemoglobin level was 6.97%±0.82%. The total DBSQ score was reduced significantly from 24.9±8.0 to 20.4±7.3 before and after rebamipide treatment (P<0.001). The DBSQ scores associated with reflux symptoms, indigestion, nausea or vomiting, abdominal bloating or distension, peptic ulcer, abdominal pain, and constipation were improved after rebamipide treatment (P<0.05). However, there were no significant changes in symptoms associated with irritable bowel syndrome, diarrhea, and anal incontinence. No severe adverse events were reported throughout the study.
Conclusion
Rebamipide treatment for 12 weeks improved atypical GI symptoms in patients with T2DM.
INTRODUCTION
Gastrointestinal (GI) symptoms are more common in patients with type 2 diabetes mellitus (T2DM) than in the normal population [1]. Yet, the mechanism of GI complications in T2DM is not clearly identified. The duration of diabetes mellitus (DM), autonomic neuropathy, and postprandial hyperglycemia are related with GI symptoms in T2DM. Patients with long-standing T2DM are exposed to chronic hyperglycemia, which induces parasympathetic dysfunction, and develop gastroparesis. Autonomic neuropathy can cause constipation and diarrhea by altering small and large bowel motility. Postprandial hyperglycemia itself impairs gastric emptying [23]. In effect, GI symptoms reduce quality of life in patients with T2DM [4] and increase the medical cost to the public health care system [5]. Therefore, optimal glycemic control and various pharmacologic treatments are widely used to improve GI symptoms [678]. Antireflux agents, such as proton pump inhibitor and histamine H2 blocker, or prokinetics, such as metoclopramide, domperidone, and erythromycin, are applied. However, new therapeutic agents have not been developed over the past decade, despite the adverse effects of pre-existing drugs [7910111213].
Rebamipide was developed for treating peptic ulcers and gastritis. It has various gastric cytoprotective effects [14]. It stimulates gastric endogenous prostaglandin and mucus glycoprotein synthesis. It also stimulates bicarbonate secretion and increases mucosal blood flow. In addition, rebamipide inhibits reactive oxygen species, neutrophil activation, and production of inflammatory cytokines and chemokines. By these actions, rebamipide promotes ulcer healing and prevents ulcer recurrence. In previous studies, because of its anti-inflammatory effects on other tissues, rebamipide was effective for treatment of other diseases, such as stomatitis [15], renal [16], and hepatic damage [17], and for corneal protection [18]. For these reasons, rebamipide is considered as a novel therapeutic option, but there is little current evidence on the effects of rebamipide on GI symptoms in T2DM patients. In this study, we evaluated the improvement of GI symptoms after rebamipide treatment in patients with T2DM.
METHODS
Study patients
This study was conducted on 107 patients with T2DM who visited the Department of Endocrinology and Metabolism in Kyung Hee University Hospital from September 2009 to April 2013. The patients were diagnosed with T2DM and aged between 18 and 80. Every patient complained of ≥1 atypical GI symptom, such as heartburn, dyspepsia, nausea, abdominal distension, irritable bowel syndrome, abdominal pain, constipation, and anal incontinence. Clinicians carefully reviewed the medical records to exclude the patients with organic causes for GI symptoms, such as gastroesophageal reflux disease or peptic ulcer disease. Within the 3 months preceding the study, glycosylated hemoglobin (HbA1c) level was lower than 10%, and the medication associated with GI symptoms was unchanged. Patients who were unable to cooperate or had any other medical disease or medication that could affect the results and safety were excluded. Pregnancy or breastfeeding women were also excluded (Table 1). Finally, a total of 84 patients completed the study (Fig. 1).
Study design
This study was performed as an investigator-initiated clinical trial using a questionnaire on GI symptoms. Each patient visited the outpatient clinic three times during the study. At the first visit, patients filled out the diabetes bowel symptom questionnaire (DBSQ) and started taking rebamipide. Rebamipide (100 mg) was administrated thrice daily. Demographic data collected included age, sex, duration of DM, weight, height, abdominal circumference, blood pressure, alcohol, smoking, medications, and comorbidities. The complete blood cell count and chemical laboratory data including HbA1c, blood urea nitrogen, creatinine, aspartate aminotransferase, and alanine aminotransferase were also collected. On the second visit, 6 weeks from the registration of the study, a physician checked the presence of drug-related adverse events. Clinical changes associated with DM or other underlying diseases, and medication changes, were also evaluated. At the third visit, 12 weeks from the registration of the study, the follow-up assessment of the DBSQ was completed. The presence of any adverse events, clinical changes, and medication changes were checked, as in the second visit. The changes in each DBSQ score and total DBSQ score before and after rebamipide treatment were analyzed. The primary endpoint was the change of GI symptoms represented by changes in DBSQ score. The secondary endpoint was the occurrence of significant drug-related adverse effects.
Diabetes bowel symptom questionnaire
The DBSQ consisted of 10 questions on upper and lower GI symptoms (Supplementary Table 1). The severity of GI symptoms was estimated by a 6-point Likert scale, ranging from "never" to "several times during the week or every day." Question number 1 evaluated the severity of gastroesophageal reflux symptoms by asking the experience of heartburn or regurgitation. Questions numbers 2, 3, 4, and 9 evaluated symptoms associated with gastroparesis: question 2 on postprandial fullness, question 3 on nausea or vomiting, question 4 on abdominal bloating, and question 9 on abdominal pain. Question number 5 evaluated the severity of symptoms associated with peptic ulcers. Question number 6 evaluated the symptoms associated with irritable bowel syndrome: pain relieved by defecation, the pain occurrence in the case of frequent defecation or constipation, and the pain occurrence in the case of very hard or very loose stool. Questions number 7, 8, and 10 evaluated the presence or severity of lower GI symptoms: question 7 on the frequency of diarrhea or loose stool, question 8 on constipation, and question 10 on anal incontinence.
Statistical analysis
The baseline clinical characteristics were described as mean±standard deviation. Paired sample t-test was used to compare DBSQ scores before and after rebamipide treatment. SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. P values <0.05 were considered statistically significant.
Ethics statement
The study protocol was approved by the Institutional Review Board of Kyung Hee University Hospital (KMC IRB 0885-08), and the study was registered in Clinical Research Information Service (KTC0001220). Prior to participation, all patients provided written informed consent.
RESULTS
Baseline clinical characteristics
Table 2 shows the baseline clinical characteristics of the patients. The mean age was 65±7.8 years. The number of female patients (n=79, 75.2%) was higher than males. The mean duration of DM was 14.7±9.1 years, and mean HbA1c level was 7.0%±0.8%. The mean body mass index (BMI) was 25.0±3.0 kg/m2, and mean abdominal circumference was 84.6±9.4 cm. More patients were non-drinkers (n=80, 76.2%) or non-smokers (n=85, 81.0%) than drinkers and smokers, respectively. More than half of the patients were taking sulfonylurea (n=56, 53.3%), metformin (n=55, 52.4%), angiotensin II receptor blocker (n=55, 52.4%), statin (n=54, 51.4%), and clopidogrel (n=68, 64.8%) at the time of enrollment. Eighty-one patients (77.1%) had hypertension, 78 patients (74.3%) had dyslipidemia, 15 patients (14.3%) had ischemic heart disease, and 12 patients (11.4%) had cerebrovascular disease.
DBSQ score
Compared to before rebamipide treatment, the total DBSQ score was significantly reduced, from 24.9±8.0 to 20.4±7.3 (P<0.001) after the treatment. Scores of question numbers 1, 2, 3, 4, 5, 8, and 9 were reduced significantly after rebamipide treatment (P<0.05). In other words, symptoms associated with gastroesophageal reflux, gastroparesis, peptic ulcer, and constipation were improved. However, there were no significant improvements in the scores of questions 6, 7, and 10 that assessed the symptoms associated with irritable bowel syndrome, diarrhea, and anal incontinence (Fig. 2).
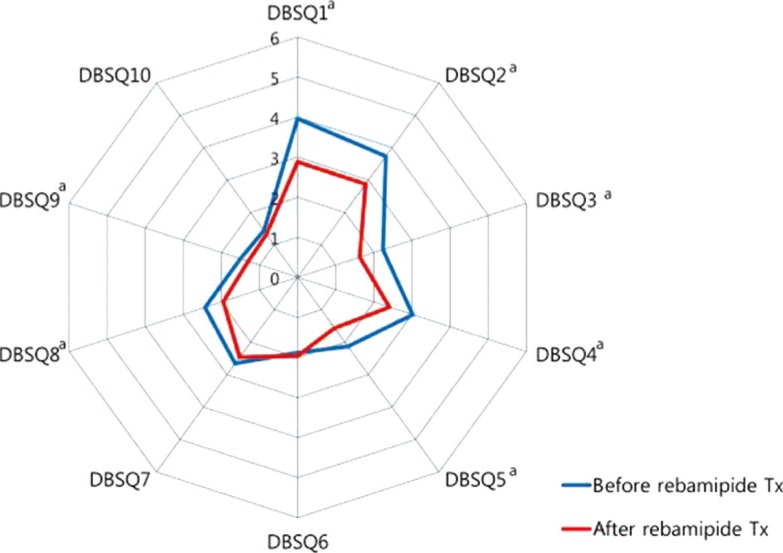
Changes of the diabetes bowel symptom questionnaire (DBSQ) scores before and after rebamipide treatment (Tx). aP<0.05.
Subgroup analyses were conducted according to sex, age, BMI, duration of DM, and HbA1c level. GI symptoms were improved more in women than in men after rebamipide treatment (Fig. 3A). In women, scores of the question numbers 1, 2, 3, 4, 5, 8, and 9 were reduced significantly after rebamipide treatment (P<0.05), whereas none of the scores were reduced significantly in the male subgroup. When the age was divided into three groups (i.e., younger than 60, 60 to 69, and older than 70 years), the biggest improvement of GI symptoms after rebamipide treatment was shown in the 60 to 69 years group (Fig. 3B). Only two individual scores were reduced significantly in the youngest and the oldest group (scores of questions 2 and 6 and questions 1 and 2, respectively), whereas 6 individual scores were reduced significantly in the 60 to 69 years group (scores of the question numbers 1, 2, 3, 4, 5, and 9; P<0.05). Similarly, there were more improvements of GI symptoms after rebamipide treatment in the group with shorter duration of DM (<10 years) (Fig. 3C) and better glycemic control (HbA1c <7%) (Fig. 3D). Scores of seven individual questions were reduced significantly in the shorter duration group (questions 1, 2, 3, 4, 8, and 10), whereas scores of four individual questions were reduced significantly after the rebamipide treatment (questions 1, 2, 3, and 5; P<0.05). Scores of the six individual questions were reduced significantly in the group with better glycemic control (questions 1, 2, 3, 4, and 8), whereas only three individual questions were reduced significantly in the group with poorer glycemic control (questions 1, 2, and 5; P<0.05). However, there was no difference in improvement of GI symptoms after rebamipide treatment with respect to BMI (Fig. 3E).

Comparisons of the diabetes bowel symptom questionnaire (DBSQ) score before and after rebamipide treatment (Tx), with respect to (A) sex, (B) age, (C) duration of mellitus duration (DM), (D) glycosylated hemoglobin (HbA1c) level, and (E) body mass index (BMI). Paired t-test for paired values.
When subgroup analysis was performed according to the administration status of metformin, there was no significant difference of the scores between both groups before and after the rebamipide treatment. It could be inferred from this analysis that the administration of metformin did not influence the change of GI symptoms before and after the rebamipide treatment.
Safety assessment
Adverse events were reported in 12 patients (12.2%) at the second visit and in 20 patients (23.0%) at the third visit. No severe adverse events were reported by any patients throughout the study.
DISCUSSION
Throughout the study, we found significant improvement of atypical GI symptoms after administration of rebamipide for 12 weeks in patients with T2DM. We were able to confirm the improvement of GI symptoms through the DBSQ that consisted of 10 questions reflecting the severity of detailed upper and lower GI symptoms. After treatment with rebamipide, symptoms of gastroesophageal reflux, gastroparesis, peptic ulcer, and constipation were improved, whereas irritable bowel symptoms, diarrhea, and anal incontinence were unimproved. Improvement of symptoms of gastroesophageal reflux and peptic ulcer after rebamipide is due to its ulcer healing effect through promoting prostaglandin production and increasing mucus and bicarbonate production in gastric mucosa. Improvement of gastroparesis may be explained by its upregulation of neuronal nitric oxide [19] because decrease of neuronal nitric oxide synthase expression is related to development of gastroparesis [7]. In contrast, it might be less effective in improving symptoms associated with GI hypermotility, such as irritable bowel syndrome, diarrhea, or anal incontinence.
In our subgroup analyses, the treatment effects of rebamipide were greater in women than in men. The interpretation was limited due to the higher number of female than male patients. However, it was meaningful because, in general, more women experience GI symptoms and are more prone to develop GI complications than men [2021]. Treatment effects of rebamipide were also greater in the groups with shorter duration of DM. This might be partly due to effects of hyperglycemia on GI motility. As the duration of DM increases, the patient may be exposed to chronic hyperglycemia for longer periods, which eventually influences motility of the upper and lower GI tract and increases the prevalence of chronic complications, such as autonomic neuropathy. Likewise, the effect of rebamipide might be greater in younger patients. Yet, in our study, greater effect was found in the group with patients aged 60 to 70 years than younger than 60 years. We believe this is because the number of young patients was relatively small, with the mean age of 65±7.84 years; 18 patients were of age less than 60, 39 were age 60 to 70, and 25 were of age 70 years or older. Therefore, more data are required from a younger population to evaluate the efficacy of rebamipide with respect to age. Greater improvement may be seen in patients with lower HbA1c levels because strict glycemic control is as important as pharmacologic agents in controlling GI symptoms in T2DM. Indeed, in our study, symptom improvement was more prominent in patients with better glycemic control. In our subgroup analyses, improvement of symptoms regarding gastroparesis and constipation was greater in patients with HbAc1 level <7% than in those with HbA1c level ≥7%. BMI did not influence the treatment effect of rebamipide, although it is a known risk factor of GI symptoms [22]. From these results, we concluded that duration of DM, sex, age, HbA1c level, and DM itself have more influence on GI symptoms rather than obesity. In multiple regression analysis, sex and DM duration affected changes of the DBSQ total score.
Our study has great significance because it is the first clinical research to demonstrate GI symptom improvement in T2DM with rebamipide. We statistically analyzed the data from the DBSQ, which is a well-organized questionnaire with detailed questions on upper and lower GI symptoms. Symptoms were subdivided into 10 questions and each question was organized to evaluate severity by assessing the symptoms in six levels, including never, less than once a month, once a month, 2 or 3 times a month, once a week, and several times during the week or every day. These questions were based on elements from questionnaires that have previously been validated [2324]. In this manner, we were able to compare the subjective symptoms of the patients in a relatively objective and systemic way, which gives strength to our research as compared with previous studies.
A number of previous studies reported side effects and limitations of pre-existing medications. Metoclopramide, a dopamine D2-receptor agonist, has been approved for diabetic gastroparesis with its prokinetic and antiemetic properties [25]. Yet, the United States Food and Drug Administration recommends only short-term treatment (4 to 12 weeks) because metoclopramide crosses the blood-brain barrier, producing central nervous system (CNS) side effects, such as anxiety, agitation, somnolence, insomnia, and intractable tardive dyskinesia [1011]. Its use has been even more restricted recently by the European Medicines Agency to a maximum of 5 days. In addition, its long-term efficacy has not been proven [26]. Domperidone, which is another dopamine (D2) antagonist, and erythromycin, a macrolide antibiotic agent, have prokinetic effects that are as potent as metoclopramide. Although domperidone has less severe CNS side effects [27], it might increase the risk of sudden cardiac death [12]. Long-term use of erythromycin is also limited due to development of tachyphylaxis with chronic administration [713] and increased risk of sudden cardiac arrest [28]. Proton pump inhibitors are effective in improving symptoms of reflux disease and peptic ulcer, but its use is limited due to high cost. Due to limitations on the choice and effectiveness of current pharmacologic therapy, demonstrating the efficacy of rebamipide for GI symptom control in DM is of great significance. Moreover, rebamipide is unique among the conventional antireflux and prokinetic agents because, in the present study, it was effective in both gastroesophageal reflux and gastroparesis symptoms.
There are several limitations to our study. First, because it was conducted in a single-center with Korean patients, it is difficult to apply the results to the general population. Second, a highly experienced physician judged the presence of organic causes for GI symptom, but not all patients had gastroscopy, colonoscopy, or image tests, such as sonography and computed tomography checked. In addition, we could not confirm the efficacy and safety with long-term administration of the drug because the study was completed after administration of rebamipide for 12 weeks. Therefore, further investigations with a larger and longer observational period are required to evaluate the treatment effect and safety with long-term use and confirm the efficacy in the general population.
In conclusion, rebamipide treatment for 12 weeks improved atypical GI symptoms, such as gastroesophageal reflux, gastroparesis, peptic ulcer, and constipation in patients with T2DM.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
Supplementary Material
Supplementary Table 1
Diabetes bowel symptom questionnaire





