A Cross-Sectional Study of the Phenotypes of Obesity and Insulin Resistance in Adults with Down Syndrome
Article information
Abstract
Background
Despite the confluence of multiple cardiovascular risk factors, subclinical atherosclerotic damage and cardiovascular events remain extremely rare in adults with Down syndrome (DS). We aim to determine the prevalence of obesity and metabolic disorders in an adult cohort with DS and to compare our findings with adults without DS.
Methods
Cross-sectional study of 51 consecutively selected adults with DS living in the community and 51 healthy controls in an outpatient clinic of a tertiary care hospital in Madrid, Spain. Epidemiological data (age and gender), anthropometric data (body mass index and waist-to-height ratio), coexisting clinical conditions, and laboratory data (fasting glucose, insulin, glycated hemoglobin, creatinine, thyroid hormones, vitamins, and lipid profile) were measured and compared between the groups.
Results
Adults with DS were significantly younger and more often men with a higher prevalence of overweight and obesity than controls. Their waist-to-height ratio was higher, and they more frequently had abdominal obesity. The results of an analysis adjusted for age and gender revealed no differences in fasting insulin levels, homeostatic model assessment indexes, or lipid profile between adults with DS and controls.
Conclusion
Adults with DS presented a high prevalence of overweight and obesity. However, we found no differences in lipid profile, prevalence of insulin resistance, or metabolic syndrome between adults with DS and controls.
INTRODUCTION
Atherosclerosis is the main pathophysiological substrate of cardiovascular disease (CVD), which is the leading cause of morbidity and mortality in Western countries [1]. The prevalence of CVD increases not only with age but also with the presence of multiple concomitant risk factors, such as arterial hypertension, diabetes mellitus, dyslipidemia, obesity, and sedentary lifestyle [2]. When present together, high blood pressure and metabolic risk factors interact to produce the metabolic syndrome, which leads to a greater total CVD risk than the sum of its individual components [3]. Obesity and insulin resistance play a central role in the pathogenesis of metabolic syndrome [4]. The prevalence of obesity is high in all age groups and continues to rise in most Western countries. Of particular concern is the rising prevalence of weight-related disorders in children and adolescents, which can increase their risk of CVD in later life [5,6]. The economic burden of CVD, including healthcare and nonhealthcare-related costs, is increasing in most developed countries, thus highlighting the importance of these diseases in our milieu [7].
Since the 1980s, the improved survival of children with Down syndrome (DS) has resulted in dramatically improved life expectancy for a growing population of adults [8]. A population-based study in the United States revealed an increase in median age at death of adults with DS from 25 years in 1983 to 49 years in 1997 [9]. This "new" population of adults with DS raises unique clinical issues, which differ from those of the pediatric population with DS or the general population [10]. Obesity and dyslipidemia appear to be more prevalent in both children and adults with DS [11,12,13]. Murdoch et al. [14] found no significant differences in mean total cholesterol and triglyceride levels between adults with and without DS; however, other studies have observed a more atherogenic lipid profile in individuals with DS than in the general population [11,13]. Furthermore, a higher incidence of type 1 diabetes mellitus has also been observed in individuals with DS [15].
Despite the confluence of multiple risk factors for CVD, subclinical atherosclerotic damage, and cardiovascular events remain extremely rare in adults with DS [16,17,18]. In 1977, DS was reported to be an "atheroma-free model"; atheroma was completely absent at autopsy in five institutionalized adults with DS, while atheroma was present in institutionalized adults without DS [14]. Subsequent studies both supported [16] and contradicted this finding [19,20,21]. Based on carotid intima-media thickness or pulse wave velocity, recent studies found no increase in subclinical vascular damage in adults with DS compared to healthy controls, even though both groups had a similarly elevated risk of CVD [17,18]. However, most previous studies were cross-sectional evaluations of single CVD risk factors in small samples and insufficiently powered to generate any hypothesis for this relevant clinical discrepancy, which could have implications for protection from atherosclerosis in the general population. We present a cross-sectional study with a comprehensive assessment of multiple CVD risk factors, focusing on carbohydrate metabolism, lipids, and adiposity in a cohort of adults with DS. Our working hypothesis was that adults with DS would have a higher prevalence of obesity and subsequent metabolic disorders than adults without DS. Our objectives were to study the prevalence of weight-related and metabolic disorders (states of glycemic dysregulation such as impaired fasting glucose, diabetes mellitus, dyslipidemia, and metabolic syndrome) in adults with DS and to describe any differences with a cohort of healthy controls.
METHODS
Study design
Ours was a cross-sectional study of 51 adults with DS and 51 control participants. The former were consecutively selected from all patients at the Adult Down Syndrome Outpatient Clinic of the Department of Internal Medicine, Hospital Universitario de La Princesa in Madrid, Spain. The recruitment period ran from January 2012 to March 2013. The study was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice Guidelines, and the local Institutional Review Board approved the study protocol. All participants and family members were extensively informed by two of three investigators (DRA, PP, and RC) before the initial evaluation and provided their written informed consent before undergoing any study procedure. All data were treated in the strictest confidence, according to the most recent Spanish legislation on data protection.
Patients
The inclusion criteria were age over 18 years and presence of DS documented by karyotype. All adults with DS included in the study had a full trisomy 21 (we found no adults with Robertsonian translocations or mosaics). Control participants were race-matched healthy individuals. Family members and legal guardians of adults with DS were invited to participate voluntarily in this study. Siblings of adults with DS were used as controls in 44 cases (44/51, 86%); as for the remainder, two were parents of adults with DS and five were unrelated healthy individuals. The exclusion criteria were as follows: severe congenital heart disease not treated with surgery, severe sensory impairment, inability to provide consent, pregnancy, and a known diagnosis of diabetes mellitus or metabolic syndrome.
Measurements
The variables collected in all study participants were as follows.
Epidemiological variables
Age and gender.
Anthropometric variables
Height, weight, body mass index (BMI), waist-to-height ratio, and total body fat percentage. Height was measured to the nearest 0.5 cm using a stadiometer, and weight was measured on a calibrated balance scale to the nearest 0.5 kg. Body weight categories (normal weight, overweight, and obesity) were defined using internationally accepted BMI cutoff values [22]. Waist-to-height ratio was calculated, and a cutoff value of 0.5 was considered diagnostic of abdominal obesity [23,24]. We favored the waist-to-height ratio to assess abdominal obesity, since this index is superior to waist circumference for the diagnosis of abdominal obesity in the general population and is presumed to more truly reflect fat distribution in individuals with a short stature [24].
Clinical variables
Clinical variables were obtained through systematic review of medical records, as follows: family history of early CVD events, presence of arterial hypertension, dyslipidemia, diabetes mellitus, smoking, other coexisting conditions (thyroid disorders, obstructive sleep apnea, Alzheimer's disease), relevant medications (including antihypertensive agents, lipid-lowering agents, antidiabetic drugs, anxiolytics, antidepressants, antipsychotics, antiepileptics, nonsteroidal anti-inflammatory drugs, levothyroxine, glucocorticoids, vitamin B or D supplements, and oral contraceptives/estrogen replacement therapy), office blood pressure, resting heart rate, and fat/fruit/fiber intake. Blood pressure was determined using a validated oscillometric device (OMRON M-6 comfort or OMRON 711 models; OMRON Healthcare, Vernon Hills, IL, USA). Office blood pressure was taken with the participant seated after a minimum 3- to 5-minute rest. Three consecutive measurements were made, with a 1- to 2-minute interval between them, and their mean was considered the final office blood pressure, in accordance with the European Society of Hypertension guidelines [25]. Fat/fruit/fiber consumption was assessed using Block screening questionnaires [26].
Blood testing
All participants underwent a fasting blood test after a minimum 10-hour overnight fast. Fasting glucose, fasting insulin, glycated hemoglobin, creatinine, thyroid hormones (thyroid stimulating hormone [TSH] and free thyroxine [FT4]), vitamins (25-OH-vitamin D, vitamin B12, and folic acid), total cholesterol, high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol, and triglyceride levels were determined in a Roche/Hitachi modular-D analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Homeostatic model assessment-insulin resistance (HOMA-IR) values were estimated as follows: [glucose (mg/dL)×insulin (mcU/mL)]/405. HOMA-β cell function (HOMA-β) values were calculated as follows: [360×insulin (mcU/mL)]/[glucose (mg/dL)-63] [27]. We used a HOMA-IR cutoff value of 3.8 (90th percentile), owing to its high specificity for the diagnosis of IR [28]. The definitions used for diagnosis of impaired fasting glucose or diabetes mellitus were those of the most recent American Diabetes Association criteria [29]. Dyslipidemia and metabolic syndrome were defined according to modified National Cholesterol Education Program-Adult Treatment Panel III criteria [4].
Statistical analysis
All data were processed using SPSS version 20.0 (IBM Co., Armonk, NY, USA). We compared the prevalence of obesity and overweight, impaired fasting glucose, diabetes, dyslipidemia, and metabolic syndrome between adults with DS and controls, as well as the distribution of the values for blood sugar, adiposity (weight, BMI, waist circumference, and total body fat percentage), and lipids. Qualitative results are presented as absolute frequencies (percentages), whereas quantitative results are presented as means±standard deviations. A chi-square test (with Yates correction when applicable) was used to evaluate statistical significance in the comparison of categorical variables. All analyses were adjusted for age and gender. A multiple logistic regression analysis with all significant variables from the univariate comparison between groups (which included age, gender, BMI, waist-to-height ratio, abdominal fat percentage, TSH levels, and daily fruit and fiber intake), was performed in order to identify possible determinants of IR in adults with DS.
RESULTS
The study population comprised 102 participants (51 adults with DS and 51 controls). All participants were included in the analysis of clinical and anthropometric variables. Three adults with DS and 18 controls refused to provide a blood sample. Therefore, the laboratory results correspond to 48 adults with DS and 33 controls.
Anthropometric differences in adults with DS
The baseline characteristics of the participants are presented in Table 1. Adults with DS were significantly younger (36±11 years vs. 42.5±12.5 years; difference in means -6.5 years; 95% confidence interval [CI], -11.1 to -1.9; P<0.01), more frequently men (31 men with DS [61%] vs. 20 controls [39%]; P<0.05), and showed a higher prevalence of overweight and obesity than controls (normal weight, 13 adults with DS [25.5%] vs. 31 controls [61%]; overweight, 19 adults with DS [37%] vs. 13 controls [25.5%]; obesity, 19 adults with DS [37%] vs. 7 controls [14%]; P<0.001 for the distribution). Adults with DS had a higher waist-to-height ratio (0.62±0.09 vs. 0.54±0.08, difference in means 0.08; 95% CI, 0.05 to 0.11; P<0.001) and were more prevalently classified as having abdominal obesity with this index (44 adults with DS [86%] vs. 35 controls [68%]; P<0.05). These differences remained unchanged after adjusting the analysis for age and gender.
Clinical differences in adults with DS
Coexisting conditions and dietary habits are also listed in Table 1. None of the adults with DS presented arterial hypertension or had ever smoked (P<0.01 for both comparisons with controls). Two adults with DS were newly diagnosed with diabetes mellitus during the study; and a new diagnosis of metabolic syndrome was reached in five adults with DS. Although most participants in both groups presented a high daily fat intake, regular use of olive oil in cooking accounted for most of the excess observed in both groups. Thyroid-related disorders were more frequent in adults with DS, and their distribution in the study groups is depicted in Fig. 1. Out of 34 adults with DS and a prior diagnosis of hypothyroidism, 14 presented subclinical hypothyroidism (average TSH, 6.2±3.3 mU/L), and three more presented iatrogenic subclinical hyperthyroidism at evaluation. Only one participant had low FT4 levels. A prior diagnosis of hypothyroidism was detected in eight controls, of whom three presented subclinical hypothyroidism at the time of the evaluation. Average TSH levels were similar in adults with DS and controls (Table 2).

Distribution of thyroid disorders in the study sample. (A) Prevalence of thyroid disorders in adults with Down syndrome (n=51). (B) Prevalence of thyroid disorders in control subjects (n=51).
Regarding the use of medication, adults with DS more often received antidepressants (14 adults with DS vs. 2 controls; P<0.01), neuroleptic drugs (5 adults with DS vs. 0 controls; P<0.05), vitamin D supplements (21 adults with DS vs. 0 controls; P<0.001), and levothyroxine (26 adults with DS vs. 5 controls; P<0.001). All antidepressants were selective serotonin uptake inhibitors, and all were prescribed at doses equivalent to sertraline 100 mg once daily or lower. Antihypertensive agents were more frequently taken by controls (0 adults with DS vs. 6 controls; P<0.05). Statins were taken by six adults with DS and three controls (P=not significant). No other relevant differences in medication were found between the groups.
Evaluation of biochemical parameters of insulin resistance and lipid metabolism
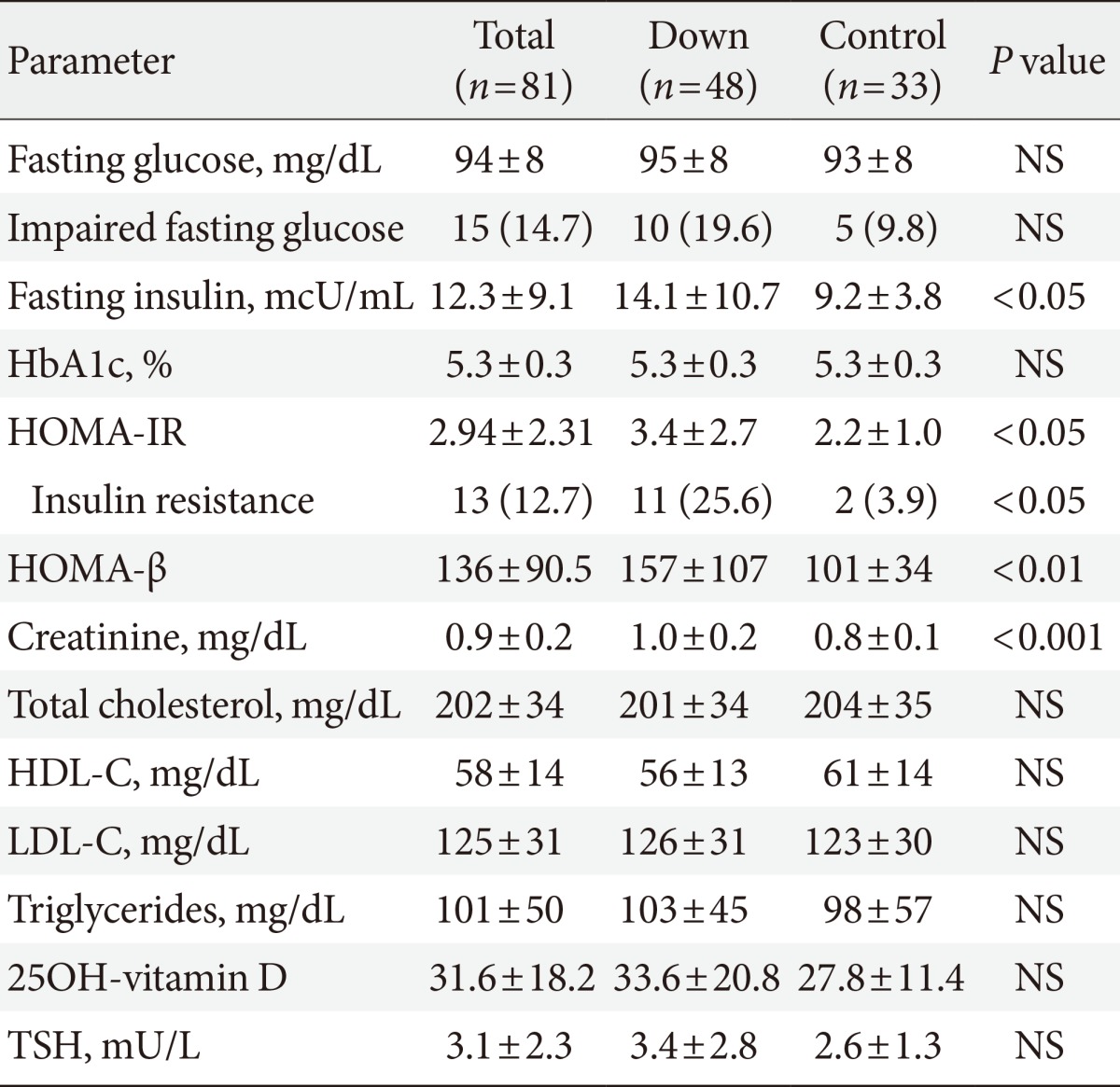
Mean laboratory values are reported in Table 2. Adults with DS had higher fasting insulin levels and a higher prevalence of impaired fasting glucose, although this difference did not reach statistical significance. Both HOMA indexes were also higher in adults with DS, who presented a higher prevalence of insulin resistance than controls, according to HOMA-IR values. However, this statistically significant difference in HOMA indexes did not remain after adjusting the analysis for age and gender (HOMA-IR preadjustment P<0.05, postadjustment P=0.22 [95% CI, -0.5 to 2.2]; and HOMA-β preadjustment P<0.01, postadjustment P=0.1 [95% CI, -9.1 to 95]). A multiple logistic regression analysis was performed to identify possible determinants of IR in adults with DS. All variables with a statistically significant difference between groups were selected for the final analysis, which included age, gender, BMI, waist-to-height ratio, abdominal fat percentage, TSH levels, and daily fruit and fiber intake. However, none of the variables presented a significant correlation with the degree of IR in this population (Table 3).
DISCUSSION
Abdominal obesity was highly prevalent among adults with DS in our study. Eight out of 10 adults with DS in our sample had a waist-to-height ratio greater than 0.5, and over two-thirds presented overweight or obesity according to their BMI. However, impaired glucose tolerance and insulin resistance were not more frequent among individuals with DS, and no relevant differences in lipid profile were found between the groups.
The proportion of weight-related disorders in our sample is somewhat lower than previously reported. Prasher [12] and Fujiura et al. [30] observed higher rates of obesity among adults with DS (47% of women and 48% of men and 43% of women and 25% of men respectively). Rubin et al. [31] found a higher rate of overweight individuals in this population (56% of women and 45% of men), whereas Melville et al. [11] reported a lower prevalence of obesity. However, direct comparisons with prior series are cumbersome owing to the use of different diagnostic BMI cutoff points. To our knowledge, this is the first report of weight-related disorders in adults with DS living in the community in a Mediterranean setting. In our study, adults with DS ate more daily fruit and vegetables and dietary fiber than controls. Regular use of olive oil in cooking accounted for the excess in dietary fat observed in both groups. These differences could have partially accounted for the lower prevalence of overweight and obesity compared with cohorts in which a Mediterranean diet was not followed [32].
Surprisingly, differences were not observed in insulin resistance parameters or in the lipid profile between groups, even though adults with DS presented higher rates of subclinical hypothyroidism and more regular use of medications which are known to predispose to metabolic disorders, such as selective serotonin uptake inhibitors or neuroleptics [33,34]. These findings contrast with those of prior studies, where lower HDL-C and higher triglyceride levels were consistently documented in children and adults with DS [12,13]. Nevertheless, when carefully evaluated, the mean differences in lipid levels observed in those studies, although statistically significant, were not clinically relevant. Furthermore, despite a higher prevalence of abdominal obesity, previous studies were unable to demonstrate a predominance of metabolic syndrome in adults with DS when compared to persons with other types of intellectual disability or healthy controls [35]. Moreover, the role of subclinical hypothyroidism in lipid metabolism when TSH levels are only moderately elevated (as in our cohort) remains open to debate [36]. Since TSH levels were not related to insulin resistance in our multivariate analysis, we believe that these factors did not play a major role in explaining present results. Moreover, other drugs, such as antipsychotics, were prescribed at a low dose. We agree with Adelekan et al. [13] that a possible explanation for these surprising findings could be the peculiar balance between leptin and adiponectin in individuals with DS. Corsi et al. [37] described relative leptin resistance and higher levels of adiponectin in individuals with DS. Surprisingly, Corsi et al. [37] found that leptin levels decreased with age, whereas adiponectin levels were higher in adults and elderly persons with DS. This pathogenic pathway has yet to be explored. The possible effect of a Mediterranean diet in our population could also explain their improved lipid profile [38].
Our study has several strengths. Biological siblings were used as controls, thus reducing the effect of potential genetic and environmental factors. We collected relevant metabolic and anthropometric variables and performed a multivariate analysis to correct our results for possible confounders. Our inclusion criteria were broad, enabling our results to be generalized to most adults with DS. Our study is also subject to a number of limitations. The loss of data related to the refusal to give a blood sample, especially in the control group, left a small sample for laboratory analysis, thus minimizing potential differences in insulin resistance indexes or in the lipid profile. Furthermore, families willing to participate in our study may have siblings with more complex medical needs.
In this study, the prevalence of weight-related disorders (abdominal obesity, overweight, and global obesity) was very high in adults with DS. However, the lipid profile and the prevalence of insulin resistance and metabolic syndrome in this group was very similar to that of controls. The effect of the Mediterranean diet and the role of the leptin-adiponectin pathway warrant further investigation.
ACKNOWLEDGMENTS
DRA and PP contributed equally to the completion of the study and to drafting the manuscript. DRA, PP, and RC participated in the study design, recruited the participants, reviewed the medical records, and performed the study procedures. DRA and PP performed the statistical analysis and drafted the manuscript. CS and FM conceived the study, helped with its design, and coordinated the recruitment of participants. CS collaborated in the final draft of the manuscript. All authors have read and approved the final version of the manuscript. The authors would like to thank Mr. T. O'Boyle for his kind review of the final draft of the manuscript and Mr. F. Rodriguez for his help with the statistical analysis.
Notes
No potential conflict of interest relevant to this article was reported.