Comparison of Efficacy of Glimepiride, Alogliptin, and Alogliptin-Pioglitazone as the Initial Periods of Therapy in Patients with Poorly Controlled Type 2 Diabetes Mellitus: An Open-Label, Multicenter, Randomized, Controlled Study
Article information
Abstract
Background
The choice of an optimal oral hypoglycemic agent in the initial treatment periods for type 2 diabetes mellitus (T2DM) patients remains difficult and deliberate. We compared the efficacy and safety of glimepiride (GLIM), alogliptin (ALO), and alogliptin-pioglitazone (ALO-PIO) in poorly controlled T2DM patients with drug-naïve or metformin failure.
Methods
In this three-arm, multicenter, open-label, randomized, controlled trial, poorly controlled T2DM patients were randomized to receive GLIM (n=35), ALO (n=31), or ALO-PIO (n=33) therapy for 24 weeks. The primary endpoint was change in the mean glycosylated hemoglobin (HbA1c) levels at week 24 from baseline. Secondary endpoints were changes in HbA1c level at week 12 from baseline, fasting plasma glucose (FPG) levels, lipid profiles at weeks 12 and 24, and parameters of glycemic variability, assessed by continuous glucose monitoring for 24 weeks.
Results
At weeks 12 and 24, the ALO-PIO group showed significant reduction in HbA1c levels compared to the ALO group (–0.96%±0.17% vs. –0.37%±0.17% at week 12; –1.13%±0.19% vs. –0.18%±0.2% at week 24). The ALO-PIO therapy caused greater reduction in FPG levels and significant increase in high-density lipoprotein cholesterol levels at weeks 12 and 24 than the ALO therapy. Compared to low-dose GLIM therapy, ALO-PIO therapy showed greater improvement in glycemic variability. The adverse events were similar among the three arms.
Conclusion
ALO-PIO combination therapy during the early period exerts better glycemic control than ALO monotherapy and excellency in glycemic variability than low-dose sulfonylurea therapy in uncontrolled, drug-naïve or metformin failed T2DM patients.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease characterized by progressive deterioration of insulin sensitivity and β-cell function [1]. Effective glycemic control is essential to minimize microvascular and macrovascular complications associated with long-term hyperglycemia [2]. Various antidiabetic drugs with different mechanisms of action are available for T2DM treatment. The pursuit of individualized, tailored therapy has been highlighted; however, the optimal management for hyperglycemia and the choice of an optimal oral hypoglycemic agent (OHA) for T2DM patients continue to be highly challenging.
Dipeptidyl-peptidase IV inhibitors (DPP4is) are effective oral antidiabetic agents that block the inactivation of the incretin hormones, namely, glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide; this affects glucose control through several mechanisms, including the enhancement of glucose-dependent insulin secretion and reduction in postprandial glucagon levels. DPP4is are widely used in treatment owing to their advantages such as glucose-lowering efficacy comparable to that of other OHAs, a low risk of hypoglycemia, and weight gain [3]. Alogliptin (ALO) is a selective DPP4i with excellent drug tolerance [4].
Many currently available guidelines on OHA treatment algorithm recommend metformin monotherapy as the initial choice of OHA; despite this, early initiation of combination therapy has been proposed as an approach to achieve glycemic goals at an earlier stage and to delay the deterioration of glycemic control with possible better preservation of β-cell function [5,6]. With available evidence on this concept, The Vildagliptin Efficacy in combination with metfoRmIn For earlY treatment of type 2 diabetes (VERIFY) trial showed huge benefits of intensive combination therapy of DPP4i and metformin at an early stage over metformin monotherapy in glycemic control and OHA failure [7]. The combination of DPP4i and thiazolidinediones (TZD) was suggested to be advantageous because it addresses both insulin resistance and islet dysfunction in T2DM [8]. Pioglitazone (PIO), a TZD, is a potent insulin sensitizer. It binds specifically to the peroxisome proliferator activated receptor gamma and enhances the sensitivity of the liver, muscle, and adipose tissues to insulin [9]. Early treatment with TZD in prediabetes and T2DM patients led to superior glycemic control, long durability, and high rate of diabetes prevention [10].
Glycemic variability is emerging as an important target for consideration when assessing glycemic control [11]. Glycemic variability was suggested to be associated with a high risk of diabetic macrovascular and microvascular complications, hypoglycemia, mortality rates, and other adverse clinical outcomes [12]. It has also been reported to be correlated with oxidative stress or erythrocyte membrane stability, which emphasizes its contribution to the pathogenesis of diabetic complications [11,13].
Sulfonylureas (SUs) are still one of the most frequently used antidiabetic drugs in various countries, and they act on insulin secretion. However, SUs, particularly the older ones, are linked to a greater prevalence of hypoglycemia and cardiovascular risk [14]. In medical literature, there has not been sufficient research comparing low-dose SUs and ALO or comparing the efficacy of low-dose SUs with that of ALO-PIO. In addition, in real-world clinical practice, many physicians continue to use SU extensively with an expectation of rapid reduction in glucose levels regardless of the guidelines; therefore, there is a need for more objective data, which can be obtained using a suitable study design such as that used in the present study. A suitable treatment option is needed in patients with contraindications or intolerance to metformin.
Based on the pathophysiology of T2DM, there is a need to explore more variable OHA options in place of metformin monotherapy during the initial stage of drug choice. The present study aimed to evaluate the efficacy and safety of glimepiride (GLIM), ALO, and ALO-PIO therapies in patients with poorly controlled T2DM at the initial stage of OHA treatment (patients with drug-naïve or metformin failure). We assessed the glucose-lowering efficacy by measuring the levels of glycosylated hemoglobin (HbA1c), fasting plasma glucose (FPG), lipid profiles, and parameters of glycemic variability using continuous glucose monitoring (CGM).
METHODS
Study design and participants
This study was a three-arm, multicenter, open-label, randomized, controlled trial. It was conducted at nine sites in Korea (ClinicalTrials.gov registration number: NCT04470310).
We enrolled patients who met the following criteria: (1) presence of T2DM; (2) age 19 to 80 years; (3) body mass index (BMI) of >18 kg/m2; (4) who have poor glycemic control (7.5%≤ HbA1c ≤10%); and (5) who are OHA drug-naïve or who have treatment failure for more than 8 weeks with a regimen of 1,000 mg metformin or the maximum tolerance dose of metformin and want to change their medication. Thus, it can be referred to as the initial OHA therapy or dual combination therapy in the early stage of OHA treatment.
The key exclusion criteria were as follows: treatment with an anti-obesity drug or investigational drug or insulin therapy within the past 3 months; systemic corticosteroid treatment or changes in the dosage of thyroid hormones within the previous 6 weeks; C-peptide levels of <0.6 ng/mL; a history of type 1 diabetes mellitus; acute metabolic complications of diabetes within the past 6 months; a history of heart failure (New York Heart Association class III or IV); a history of cardiovascular diseases such as myocardial infarction or angina, or percutaneous transluminal coronary angioplasty, or stoke or transient ischemic attack within 6 months before screening; a history of bladder cancer or active bladder cancer; presence of chronic hepatitis or liver disease (defined as cases in which the alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, or serum total bilirubin level is 2.5 times higher than the upper limit of the normal range); estimated glomerular filtration rate of <50 mL/min/1.73 m2 before screening.
Eligible patients with drug-naïve and with metformin after cessation, were randomly assigned to one of the following treatment groups in a 1:1:1 ratio—the GLIM monotherapy group, the ALO monotherapy group, or the ALO-PIO combination therapy group. GLIM was initiated at a dose of 1 mg/day and increased to a dose of 2 mg/day at week 4, if necessary, on investigators’ decision; the dose was maintained until week 24 (average dose of 1.6 mg at week 24). ALO 25 mg or ALO 25 mg plus PIO 15 mg was taken orally, once-daily, at fixed doses throughout the treatment period for 24 weeks.
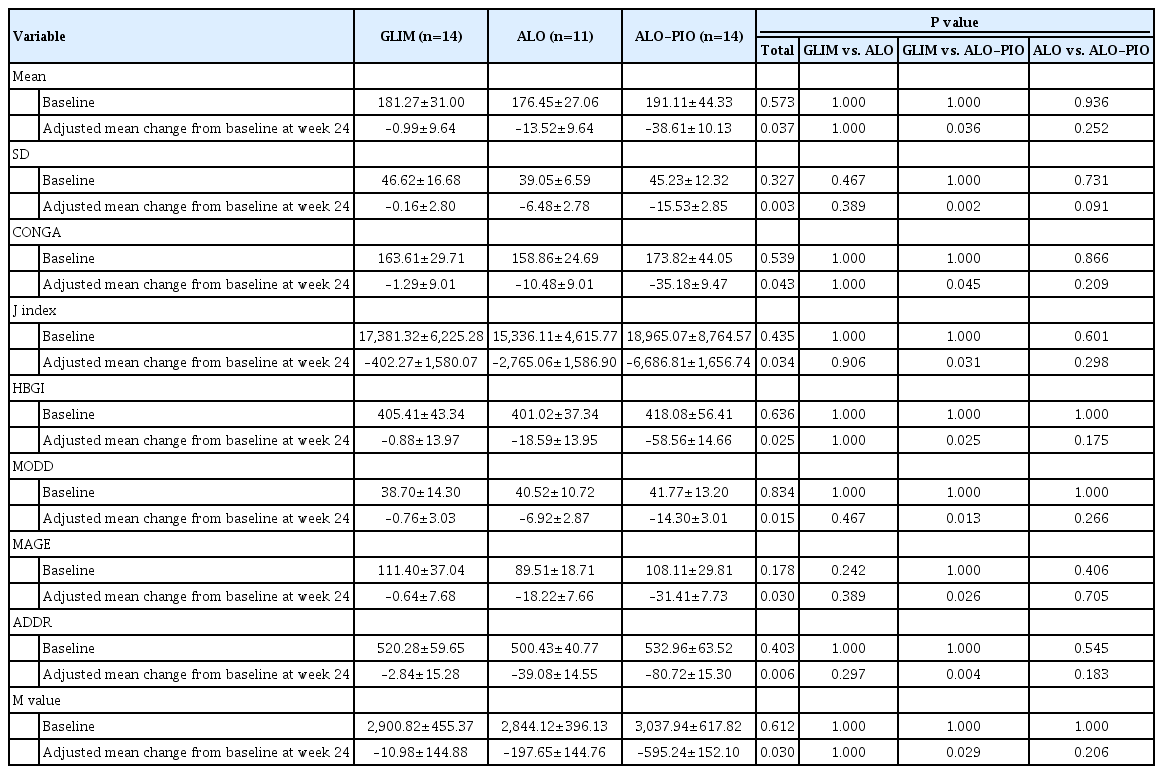
Baseline assessment included demographic characteristics, medical and medication histories, physical examination, FPG, HbA1c, fasting insulin, C-peptide, lipid profile, and laboratory tests for safety (levels of blood creatinine, AST, ALT, gammaglutamyl transpeptidase, and uric acid; complete blood count; and urinalysis). Homeostasis model assessment of insulin resistance (HOMA-IR) ([fasting insulin, μU/mL×fasting glucose, mmol/L]/22.5) was calculated as an index of insulin resistance, and homeostasis model assessment of β-cell function (HOMA-β) ([20×fasting insulin, μU/mL/fasting glucose, mmol/L]–3.5) was calculated as an index of β-cell function. Follow-up visits were scheduled at 4, 12, and 24 weeks after enrollment. At each visit, the body weight, vital signs, FPG levels, and self-monitored blood glucose (SMBG) levels of the subjects and adverse events (AEs) were assessed. HbA1c levels, lipid profile, and laboratory tests for safety were checked at weeks 12 and 24. At baseline and week 24, glycemic variability was measured using a CGM device (iPro2, Medtronic MiniMed, Northridge, CA, USA). Subgroup of patients were selected with additional agreements in randomized manner being performed CGM. CGM was performed for at least 72 hours, and data for the first and last days of wearing the device were excluded from the analysis because of concerns regarding the accuracy of the CGM system during attachment and detachment [15]. Glycemic variability was calculated, using the EasyGV version 9.0 software (Hill NR, University of Oxford, Oxford, UK), for the following parameters: mean, standard deviation (SD), continuous overall net glycemic action (CONGA), J index, High Blood Glucose Index (HBGI), mean of daily differences (MODD), mean amplitude of glucose excursions (MAGE), average daily risk range (ADDR), and M value.
Subjects were withdrawn during the study under one of the following conditions: drug compliance of less than 80%; HbA1c level of >10.0% at week 12; withdrawal of consent by the subject; at the investigators’ discretion, or in certain situations such as significant intercurrent illness or a serious adverse event (SAE) during the trial.
This study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board (IRB) at each participating site, including Seoul National University Bundang Hospital IRB (B-1507-306-005). All patients provided written informed consent before participating in the study.
Outcome measures
The primary efficacy endpoint was the change in the mean HbA1c level at week 24 from baseline. The secondary efficacy endpoints were as follows: changes in HbA1c level at week 12 from baseline and changes in FPG levels at weeks 12 and 24; from baseline changes in the parameters of glycemic variability, including SD, MAGE, MODD, ADDR, and M value, assessed by CGM at week 24 from baseline; changes in lipid profile (i.e., total cholesterol, low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglycerides) at weeks 12 and 24 from baseline.
Safety was assessed by monitoring the overall incidence of AEs, vital signs, laboratory tests, and physical examination. All AEs were recorded and assessed by the investigator for severity and possible relationship with the study medication. Regarding hypoglycemia, any patient who reported an SMBG level of lower than 70 mg/dL with or without symptoms was considered to have a hypoglycemic episode.
Statistical analysis
The target number of subjects to prove the non-inferiority between ALO and ALO-PIO at two-sided significance level of 0.017 (0.05 divided by 3 to correct for the significance level of the three groups) with a power of 90% was 21 per group, assuming a treatment difference of 0.8% and a SD of 0.7%. Considering a drop-out rate of 30% (more than 25% on the expectation), the number of subjects required for this study was estimated to be 30 per group.
Descriptive statistics (mean±SD) were used to describe continuous variables of baseline demographic and biochemical parameters, and counts with percentages were used to describe categorical variables of baseline demographic and biochemical parameters. Changes in the levels at weeks 12 and 24 were expressed as adjusted mean±standard error. The efficacy and safety analyses were based on the full analysis set population consisting of all the patients who received the study drug at least once. The demographic characteristics of the subjects were analyzed using analysis of variance (ANOVA) with Bonferroni test for continuous variables and the chi-square (χ2) test for categorical variables. Efficacy endpoints at weeks 12 and 24 were analyzed using the analysis of covariance (ANCOVA) model with Bonferroni test (baseline value as a covariate) for continuous variables and the Pearson chi-square test adjusted by Bonferroni test for categorical variables. The observed power for the effect size of CGM calculated by general linear model and computed using alpha=0.05 was 63.2%. Safety analyses were performed using Fisher's exact test. Missing data were imputed using the last observation carried forward (LOCF) method. A P value of <0.05 was considered to indicate statistical significance, and SPSS version 25.0 (IBM Corporation, Chicago, IL, USA), was used for performing statistical analyses.
RESULTS
Patient disposition and baseline characteristics
Of 110 patients screened, 99 were randomized to receive the study medication (35 to GLIM, 31 to ALO, and 33 to ALO-PIO), 85 of whom completed the study (Fig. 1). The baseline demographics and clinical characteristics of the study population were similar across the treatment groups (Table 1). The overall mean patient age was 54.99±10.64 years, and the mean BMI was 26.11±3.51 kg/m². The mean duration of T2DM was 4.90±4.34 years, and the mean baseline HbA1c level was 8.17%±0.65%. No significant differences in metabolic parameters, diabetic complications or the use of concomitant medications were observed among the treatment groups at baseline. Mean drug compliance during the 24-week study period was 96.7%±3.8%, 96.0%±7.1%, and 98.0%±2.8% in GLI, ALO, and ALO-PIO group, respectively.
Efficacy
At weeks 12 and 24, the adjusted mean change in HbA1c levels from baseline was significantly greater in the ALO-PIO group than in the ALO group (–0.96%±0.17% vs. –0.37%±0.17% at week 12; –1.13%±0.19% vs. –0.18%±0.2% at week 24) (Table 2 and Fig. 2A). However, there was no significant difference of HbA1c levels between low-dose GLIM group and other groups. The proportion of patients who achieved a target HbA1c level of ≤6.5% at week 24 was greater in the ALO-PIO group than in the ALO group (39.39% vs. 12.9%, P=0.047) (Table 2). When changes in HbA1c level were analyzed by subgroup according to baseline characteristics, a greater reduction in HbA1c level was observed in the ALO-PIO group than in the ALO group, in subjects aged ≥55 years; who were female, who were obese (BMI ≥25 kg/m²); and who had poor baseline glycemic status (HbA1c ≥8%), insulin resistance (HOMA-IR ≥3.8, median value), and low β-cell function (HOMA-β <33.31, median value) (Supplementary Table 1). The ALO-PIO group showed greater reduction in FPG levels than the ALO group at weeks 12 and 24 (Table 2 and Fig. 2B).
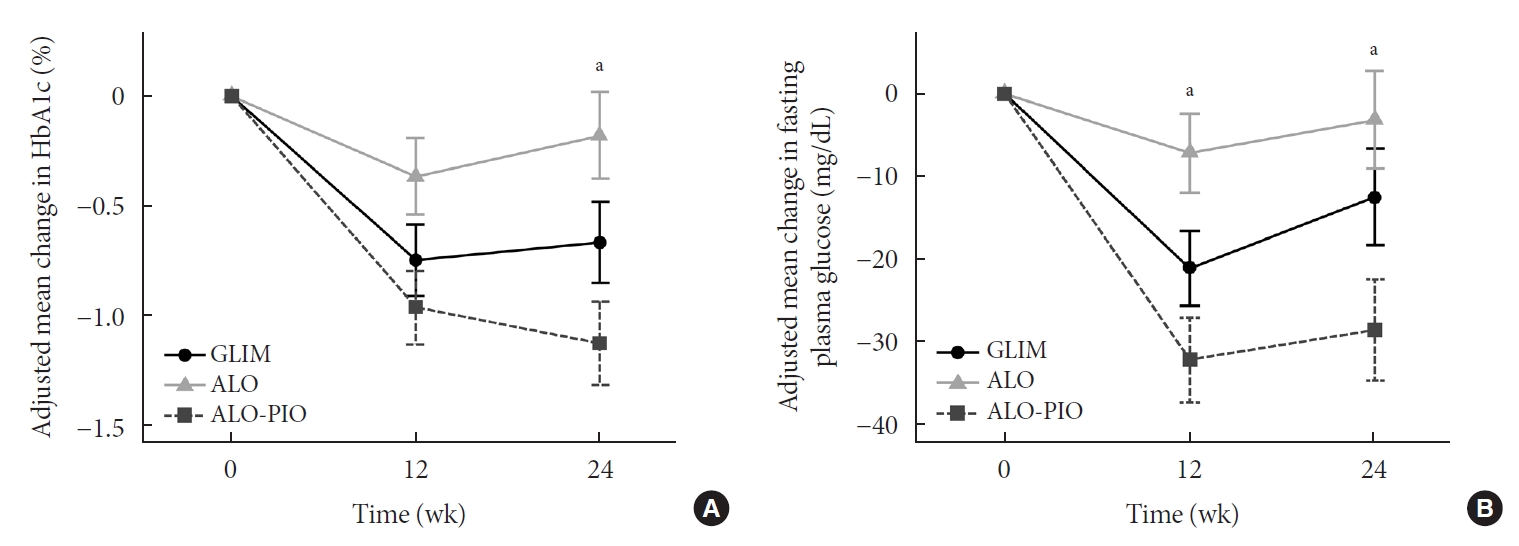
Adjusted mean changes in (A) glycosylated hemoglobin (HbA1c) and (B) fasting plasma glucose from baseline at 12 weeks and 24 weeks during glimepiride (GLIM), alogliptin (ALO), or aloglipitin-pioglitazone (ALO-PIO) combination therapy. Error bar means and standard error of adjusted means. aP value <0.05 in analysis of covariance (ANCOVA).
Among the serum lipid profiles, the ALO-PIO group showed a greater increase in HDL-C levels at weeks 12 and 24 than the ALO group and a greater increase in LDL-C levels at week 12 than the GLIM group. The ALO-PIO group showed greater improvement in the parameters of glycemic variability assessed by CGM, including mean glucose, SD, CONGA, J index, HBGI, MODD, MAGE, ADDR, and M value, than the GLIM group (Table 3). Changes in the other metabolic parameters are summarized in Supplementary Table 2. The ALO-PIO group showed a greater reduction in ALT levels than the GLIM group. No significant differences were observed in changes in body weight, blood pressure, HOMA-IR, or HOMA-β.
Safety
The safety results are summarized in Table 4. Two patients in the GLIM group and one patient in the ALO-PIO group had SAE during the 24-week study period, and one patient in the ALO-PIO group had drug withdrawal owing to SAE (pancreatic cancer). All SAEs were considered to have no causal relationship with the investigational drug. There were no differences in the AE profiles across the three treatment groups. Asymptomatic hypoglycemia occurred in one patient in the ALO-PIO group.
DISCUSSION
In the present study, the ALO-PIO therapy led to a significant reduction in HbA1c and FPG levels and a greater increase in HDL-C levels at weeks 12 and 24 than the ALO therapy. Additionally, the ALO-PIO combination therapy led to a greater improvement in the parameters of glycemic variability assessed by CGM than the GLIM therapy.
As the core pathogenesis of T2DM involves defects in both insulin secretion and insulin action, the DPP4i-TZD combination is a favorable treatment for the pathophysiology of T2DM [16,17]. TZDs are insulin sensitizers that increase peripheral glucose uptake, and DPP4is augment pancreatic insulin secretion and reduce hepatic glucose output through a suppressive effect on pancreatic glucagon secretion [18,19]. This DPP4i-TZD combination is efficacious and advantageous because it has complementary modes of action and improves at least six pathophysiological disturbances, namely, improved insulin resistance in the skeletal muscle, liver, and adipocytes; increased incretin effect; enhanced insulin secretion; and decreased glucagon secretion, with a low risk of hypoglycemia [17]. However, there is scarce evidence from clinical studies on the use of the DPP4i-TZD combination at the initial stage of OHA therapy in patients with T2DM.
Several animal and clinical studies have explored the ALO-PIO combination therapy. In ob/ob mice and db/db mice, combined administration of ALO-PIO significantly improved glycemic control and lipid profiles, increased pancreatic insulin content, maintained islet structure, and preserved islet mass, compared with the administration of ALO or PIO monotherapy [20-22]. A randomized, double-blind, 26-week study compared the ALO-PIO combination therapy (ALO 12.5 mg+PIO 30 mg or ALO 25 mg+PIO 30 mg daily) with each monotherapy (ALO 25 mg/day or PIO 30 mg/day) in 655 patients with T2DM inadequately controlled by diet and exercise [23]. Combination therapy was consistently more effective than ALO or PIO monotherapy irrespective of age, sex, race, ethnicity, or BMI [23].
Our study showed that the ALO-PIO combination therapy had a greater effect on the reduction of HbA1c and FPG levels than the ALO therapy. In our subgroup analysis, there was a significantly greater reduction in HbA1c levels in the ALO-PIO group versus the ALO group, in patients with older age, female sex, obesity, poorer baseline glycemic status, insulin resistance, and low beta cell function. This finding was consistent with that of previous reports wherein TZDs were more effective in obese and insulin resistant patients and in women [24,25].
In the present study, the ALO-PIO group showed greater improvement in various parameters of glycemic variability assessed by CGM, than the GLIM group, although there was no difference in HbA1c level reduction between the two groups. In a study comparing glycemic variability between the DPP4i and GLIM groups, gemigliptin and sitagliptin were more effective than GLIM in reducing glycemic variability as the initial combination therapy with metformin in patients with T2DM [26]. In another randomized study, Kim et al. [27] reported the comparison of vildagliptin and PIO when used in 16-week treatment for glycemic variability in Korean patients with T2DM who had inadequate control with metformin monotherapy, and only the vildagliptin group showed benefits on glycemic variability. In our study, there was no difference in the change in glycemic variability between the ALO and GLIM groups, but ALO-PIO combination therapy was superior over the GLIM group in terms of improvement in the parameters of glycemic variability. We need more evidence for demonstrating the effects of ALO-PIO combination on glycemic variability.
When we compared the GLIM and ALO monotherapy groups, there were no significant differences in efficacy and safety, including HbA1c level reduction, glycemic variability, risk of hypoglycemia, and body weight change. In a randomized controlled study, ALO monotherapy could maintain glycemic control comparable to that with glipizide in patients with T2DM during 1 year of treatment, with a lower risk of hypoglycemia and without weight gain [28]. In a meta-analysis comparing the efficacy and safety of DPP4i and SU as add-on therapies to metformin in patients with T2DM, there was a significantly greater reduction in the HbA1c level from baseline to 12 weeks with SU versus DPP4i (mean difference, 0.21%; 95% confidence interval [CI], 0.06 to 0.35), but there was no significant difference at 52 and 104 weeks (0.06%; 95% CI, −0.03 to 0.15; and 0.02%; 95% CI, −0.13 to 0.18, respectively). SU was associated with weight gain and DPP4i with weight loss at all time points. The incidence of hypoglycemia at 12, 52, and 104 weeks was significantly greater with SU than with DPP4i [29]. With our study design, only low-dose GLIM was allowed to avoid hypoglycemia, considering that all subjects were receiving the initial or initial dual combination therapy. Thus, we can assume that the low-dose SU group and the ALO monotherapy group did not show any difference in the rate of hypoglycemia, weight gain, or rapid glycemic reduction at 12 weeks.
PIO has been shown to improve diabetic dyslipidemia, increase HDL-C levels, reduce plasma triglyceride levels, cause a shift from small dense LDL to larger more buoyant LDL, and have a neutral effect on or slightly increasing LDL-C levels [18,30,31]. Consistent with the findings of previous reports, our study also showed an increase in HDL-C levels in the ALO-PIO combination therapy group compared with that in the ALO group, although we observed only a trend for a decrease in the triglyceride levels. LDL-C levels at week 12 increased in the ALO-PIO group compared with those in the GLIM group. Regarding the other metabolic parameters such as weight, BMI, blood pressure, or liver enzymes, there was no significant difference except for a greater reduction in ALT levels in the ALO-PIO combination therapy group than in the GLIM therapy group in this study. Several studies have reported that TZDs improve aminotransferase and nonalcoholic fatty liver disease (NAFLD) [32,33], and DPP4is improve NAFLD [32,34]. Our results are in line with those of previous reports.
There were no differences in the incidence of AEs across three treatment groups in the present study. There was no event of hypoglycemia in the GLIM group, but one case of asymptomatic hypoglycemia was reported by SMBG ≤70 mg/dL in the ALO-PIO group. Edema was reported in one patient (3.03%) of the ALO-PIO group, which was similar incidence to the previous study (2.9% in ALO 25 mg plus PIO 15 mg group) in Japanese patients with T2DM [35].
Taken together, ALO-PIO dual combination in T2DM, compared ALO and to GLIM monotherapy, had superior effects on HbA1c, FPG, and glycemic variability. This result supports the notion of benefits of early combination therapy as VERIFY study results than monotherapy in the treatment of T2DM.
This study has some limitations. The first limitation is the relatively small number of participants. However, this is due to the stringent study design in prospective, randomized, controlled design with CGM monitoring for having various glycemic variability data. Second, this study was not designed as add-on to metformin, which is different from the current guidelines that recommend metformin as a first-line drug. However, our study suggests that the ALO-PIO combination therapy can be an efficacious and safe option in those who cannot tolerate metformin therapy or need early effective combination therapy in real-world clinical practice.
In conclusion, the ALO-PIO combination therapy during the early period led to greater reduction in HbA1c and FPG levels and increased HDL-C levels in patients with T2DM than the ALO monotherapy. ALO-PIO combination therapy resulted in greater improvement of glycemic variability than GLIM therapy. Initial therapy with the ALO-PIO combination could be an effective and safe early option in T2DM patients who are insulin resistant or cannot tolerate metformin based on pathophysiology. Further large-scale studies with a longer period are required to confirm and validate our findings.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0183.
Changes in HbA1c by subgroup according to baseline characteristics
Changes in metabolic parameters
Notes
CONFLICTS OF INTEREST
In-Kyung Jeong was editor in chief of the Diabetes & Metabolism Journal from 2020 to 2021. Kyu Yeon Hur and Sung Hee Choi were editorial board member of the Diabetes & Metabolism Journal from 2020 to 2021. Jung Hyun Noh was associate editor of the Diabetes & Metabolism Journal from 2020 to 2021. They were not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: H.J.K., S.H.C.
Acquisition, analysis, or interpretation of data: H.J.K, I.K.J., K.Y.H., S.K.K., J.H.N., S.W.C., E.S.K., E.J.R., S.H.C.
Drafting the work or revising: H.J.K., I.K.J., S.H.C.
Final approval of the manuscript: H.J.K, I.K.J., K.Y.H., S.K.K., J.H.N., S.W.C., E.S.K., E.J.R., S.H.C.
FUNDING
The study was funded by the Takeda Pharmaceutical Company limited, Japan and Takeda Pharmaceutical Company limited, Korea with the initiation of investigator proposal of the study. 'Takeda' had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
The authors thank the investigators who participated in the study.