A 4-Week, Two-Center, Open-Label, Single-Arm Study to Evaluate the Safety and Efficacy of EOPatch in Well-Controlled Type 1 Diabetes Mellitus
Article information
Abstract
This study evaluated the safety and efficacy of tubeless patch pump called EOPatch in patients with well-controlled type 1 diabetes mellitus (T1DM). This 4-week, two-center, open-label, single-arm study enrolled 10 adult patients diagnosed with T1DM with glycosylated hemoglobin less than 7.5%. The co-primary end points were patch pump usage time for one attachment and number of serious adverse events related to the patch pump. The secondary end points were total amount of insulin injected per patch and changes in glycemic parameters including continuous glucose monitoring data compared to those at study entry. The median usage time per patch was 84.00 hours (interquartile range, 64.50 to 92.50). Serious adverse events did not occur during the trial. Four weeks later, time in range 70 to 180 mg/dL was significantly improved (70.71%±17.14 % vs. 82.96%±9.14%, P=0.01). The times spent below range (<54 mg/dL) and above range (>180 mg/dL) also improved (All P<0.05). Four-week treatment with a tubeless patch pump was safe and led to clinical improvement in glycemic control.
INTRODUCTION
Multiple daily injection (MDI) therapy and continuous subcutaneous insulin infusion (CSII) with an external pump are standard methods of intensive insulin therapy for diabetes. CSII increases patient satisfaction and is effective for glycemic control, prevention of hypoglycemia, and decreasing cardiovascular mortality compared to MDI [1–4]. An insulin infusion set (IIS) delivers insulin from a pump to subcutaneous tissue and consists of a plastic tube connected to a cannula or needle [5]. Problems related to IIS include kinking, dislocation or leakage of cannula, reservoir leakage, a loose connection between the reservoir and cannula, or occlusion [6]. IIS failure is important because it is the most common problem in patients using insulin pumps, and defects can cause serious adverse effects such as diabetic ketoacidosis (DKA). Patch pumps generally are small and attach directly to the skin without tubing to reduce IIS-related problems [7]. They also are easy to train patients to use, maintain insulin at similar temperatures inside the patch pump, and show low variation in insulin delivery according to position [8].
A Korean company, EOFLOW (Seongnam, Korea), has developed the EOPatch, a disposable patch type insulin pump that was approved by the National Institute of Food and Drug Safety Evaluation (NIFDS) in Korea in December 2017 (Product license No. 17-959). A new model that was upgraded with an interlinking blood glucose meter, alarm, and usage time was developed and approved by the NIFDS in June 2020.
We investigate patch pump usage time for one attachment, serious adverse events, and glycemic outcomes in patients with well-controlled T1DM.
METHODS
Study design and procedures
We performed this study at Samsung Medical Center (SMC) and Asan Medical Center (AMC), Korea, from March 2020 through July 2020. The study flow is outlined in Supplementary Fig. 1, and specific training programs are described in the Supplementary Material. Participants used Dexcom G5 to check glucose and advanced diabetes manager (ADM) to control insulin dose and infusion rate (Supplementary Figs. 2 and 3). Bluetooth-connected EOBridge (Supplementary Fig. 4), which is an application for Android and iOS, allows participants and guardians to monitor insulin dose and patch pump operation. In the 4th week, investigators collected the ADM and assessed exact insulin doses and usage times. We also conducted patch pump satisfaction survey (Appendix 1).
Patients
We enrolled adults (19 years of age or older) who were diagnosed with T1DM more than 6 months prior, had used MDI or an insulin pump for more than 3 months, and were in a well-controlled diabetic state (glycosylated hemoglobin [HbA1c] less than 7.5%). A full list of the inclusion and exclusion criteria is provided in the Supplementary Material. Among 10 participants, one dropped out within 3 days due to personal circumstances. The nine remaining patients were included in final analysis. The subjects voluntarily participated in the study and provided written informed consent. The study protocol was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by the Institutional Review Boards of SMC (no. 2019-11-220-011) and AMC (no. S2019-2494-0001).
End points
The co-primary end points were patch pump usage time for one attachment and number of serious adverse events. Serious adverse events were defined as severe hyperglycemia (glucose >400 mg/dL for at least 20 minutes), severe hypoglycemia characterized by altered mental and/or physical functioning that requires assistance from another person for recovery, or DKA [9,10]. The secondary end points were total amount of insulin injected per patch and changes in HbA1c, glycoalbumin, and continuous glucose monitoring (CGM) data.
Statistical analysis
Data are presented as mean±standard deviation or median (interquartile range [IQR]) for continuous variables or as number with percentage for categorical variables. Comparisons of glycemic outcomes and CGM data before and after using the investigational device were performed using the paired t-test or Wilcoxon’s signed rank test. All P values were two-tailed, and P values <0.05 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
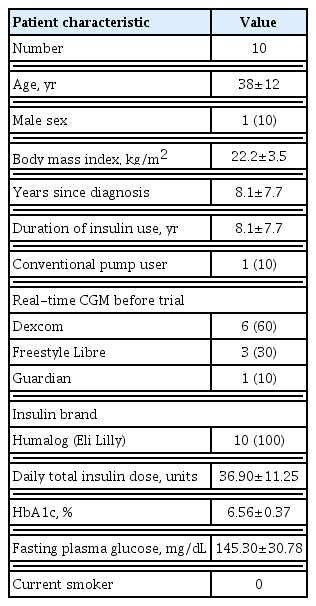
Baseline characteristics of the study sample are shown in Table 1. The mean age was 38 years, most participants were female (90%), and the mean duration of DM was 8.1 years. The mean of total patch usage time is 683.2 hours and the patch was attached for 99.3% of 687.7 hours of study period except for replacement time. The median patch usage time was 84.00 hours (IQR, 64.50 to 92.50). Nine of 84 patches (10.7%) were used for less than 24 hours, six patches (7.1%) were used for less than 48 hours, nine patches (10.7%) were used for less than 72 hours, 11 patches were used for 84 hours, and 49 patches were used over than 84 hours. The reasons for less than 84 hours were due to inlet blockage, pump malfunction, or ADM smart controller malfunction, unexplained hyperglycemia that occurs without apparent medical, dietary insulin dosage, or pump explanation, and last visit. The median insulin amount per patch was 122.90 units (IQR, 86.05 to149.86), with a minimum of 2.60 units and maximum of 186.60 units. Serious adverse events related to investigational devices did not occur in any participants. There were no significant differences in HbA1c, glucose management indicator (GMI), and glycoalbumin (Table 2). The time in range (TIR, 70 to 180 mg/dL) significantly increased (70.71%±17.14% vs. 82.96%±9.14%, P=0.01) (Table 2). The times spent below range (TBR, <54 mg/dL) and above range (TAR >180 mg/dL) also improved (Table 2). The total daily dose of insulin (TDD) was not different in the nine participants before and after using a patch pump, 38.33±10.92 and 37.55±8.99 units, respectively (P=0.639). There were significant differences in simple satisfaction scores between conventional pump or MDI and investigational device (5.78±1.99 vs. 8.78±1.09, P=0.002). In the EOPatch satisfaction questionnaire, most participants reported they were satisfied with the device and its usefulness (Supplementary Table 1).
DISCUSSION
We observed a median patch usage time of 84.00 hours (IQR, 64.50 to 92.50), and the median insulin amount per patch was 122.90 units (IQR, 86.05 to 149.86). Serious adverse events did not occur during the trial. After the trial, TIR 70 to 180 mg/dL, TBR <54 mg/dL, and TAR >180 mg/dL were significantly improved. In addition, patients were more satisfied with tubeless patch pump than with MDI or other conventional pumps.
The Omnipod, manufactured by the Insulet Corporation, is the most widely used patch pump, can be used for 3 days (72 hours). Treatment outcomes using the Omnipod system include improved glycemic control and reduction of hypoglycemic events compared to MDI usage and were not inferior to those of conventional CSII [7,11]. The EOPatch uses Teflon cannulas like the Omnipod, minimizing the inconvenience caused by needles [12,13]. The motor mechanism is different from that of actuation of the Omnipod, which is a shape memory alloy [8]. The EOPatch uses an electroosmotic pump that allows precise automatic insulin delivery using electrochemistry, which allows a more compact size and enables correct insulin delivery capacity with less noise and heat than existing pumps [14]. EOPatch usage time is 3.5 days compared to 3 days for the Omnipod. In this study, 71.4% of patch pumps were used for more than 72 hours, and median patch usage time was 84 hours. Based on that, the cost burden should be decrease and allow patients to replace the pump only twice per week with regular usage.
This was a short 4-week study that did not allow a sufficient length of time to evaluate HbA1c. Therefore, we observed no significant statistical differences in HbA1c, GMI, or mean plasma glucose level. However, results of CGM data were improved. In particular, mean TIR significantly improved from 70% to 83% without change in TDD, which is a considerable advantage considering that 70% and 80% of each TIR corresponds to HbA1c 7.0% and 6.5% [15]. Considering that all the participants in our study already had used CGM before study entry and HbA1c was less than 7.5%, effective carbohydrate counting and precise insulin dose calculation using bolus calculator might allow glycemic improvements. However, these advantages are not limited to tubeless patch pump and can be experienced with all insulin pumps.
According to T1DM exchange clinical registry data, compared with the fair/poor control group (HbA1c same or over 8.5%), the excellent control group (HbA1c less than 6.5%) was characterized by fewer missing insulin doses and more frequent meal or correction bolus [16]. In other words, with more frequent bolus insulin injections, patients achieve better control of diabetes, highlighting the importance of pump usage. However, CSII is used in only about 5% in T1DM in Korea compared to 63% in United States [17], which can be explained by lack of reimbursement for prescription, education and training of insulin pump by National Health Insurance, and the low prevalence of T1DM [18].
This study has several limitations. First, this is a single-arm study, with a small number of participants and a short observation period. Second, the effects of selection bias could not be excluded because of the nonrandomized study design. Third, there might be volunteer bias because we enrolled patients with well-controlled T1DM (HbA1c less than 7.5%) and provided structured education. Therefore, it might be difficult to generalize our glycemic outcomes to real clinical settings.
In conclusion, the median usage time of the tubeless patch pump was 3.5 days, and 4-week usage was safe and led to clinically meaningful improvement in glycemic control, including severe hypoglycemia and TIR in patients with well-controlled T1DM.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0299.
Inclusion and exclusion criteria
EOPatch satisfaction questionnaire items
Study flow. CGM, continuous glucose monitoring.
EOPatch.
Advanced diabetes manager.
EOBridge. CGM, continuous glucose monitoring; BG, blood glucose; SG, sensor glucose.
ACKNOWLEDGMENTS
We appreciated the trial participants for taking part in the study.
Notes
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0299.
CONFLICTS OF INTEREST
Nammi Park and Sangjin Han are employees of EOFLOW. No other potential conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
Conception or design: N.P., W.J.L., J.H.K.
Acquisition, analysis, or interpretation of data: J.P., N.P., S.H., Y.B.L., G.K., S.M.J.
Drafting the work or revising: J.P., N.P., J.H.K.
Final approval of the manuscript: J.P., N.P., J.H.K.
FUNDING
Funding and material supports for this study were provided by EOFLOW.