A Real-World Study of Long-Term Safety and Efficacy of Lobeglitazone in Korean Patients with Type 2 Diabetes Mellitus
Article information
Abstract
Background
Thiazolidinediones (TZDs) have been associated with various safety concerns including weight gain, bladder cancer, and congestive heart failure (CHF). This study evaluated the efficacy and safety of lobeglitazone, a novel TZD in patients with type 2 diabetes mellitus (T2DM) in real practice.
Methods
In this non-interventional, multi-center, retrospective, and observational study conducted at 15 tertiary or secondary referral hospitals in Korea, a total of 2,228 patients with T2DM who received lobeglitazone 0.5 mg for more than 1 year were enrolled.
Results
Overall adverse events (AEs) occurred in 381 patients (17.10%) including edema in 1.97% (n=44). Cerebrovascular and cardiovascular diseases were identified in 0.81% (n=18) and 0.81% (n=18), respectively. One case of CHF was reported as an AE. Edema occurred in 1.97% (n=44) of patients. Hypoglycemia occurred in 2.47% (n=55) of patients. Fracture occurred in 1.17% (n=26) of all patients. Lobeglitazone significantly decreased HbA1c level, resulting in a mean treatment difference of −1.05%± 1.35% (P<0.001), and decreased total cholesterol, triglyceride, and low-density lipoprotein cholesterol. However, it increased high-density lipoprotein cholesterol, regardless of statin administration. The patients who received lobeglitazone 0.5 mg showed an apparent reduction in glycosylated hemoglobin (HbA1c) from baseline during the first 6 months of treatment. The HbA1c levels remained stable between months 6 and 42.
Conclusion
Lobeglitazone has long-term safety profile, good glycemic-lowering effect and long-term durability of glycemic control in real-world clinical settings.
INTRODUCTION
Lobeglitazone (trade name, Duvie, Chong Kun Dang Pharmaceutical Corporation, Seoul, Korea) is a novel thiazolidinedione (TZD). TZD-based drugs improve insulin resistance by regulating the activity of genes involved in glucose and lipid metabolism by stimulating peroxisome proliferator-activated receptor-γ (PPARγ) receptors [1]. Lobeglitazone increases cell response to insulin without increasing insulin secretion in the pancreas. Therefore, the burden on the pancreas is less than that in other anti-diabetic treatments that increase insulin secretion. In addition, it is reported to be effective in protecting pancreatic β-cells [2,3]. Furthermore, TZD exhibit cardioprotective effects by increasing the secretion of adiponectin [4], expanding blood vessels [5], and alleviating inflammation [5,6]. It is also involved in lipid metabolism, which is known to lower small-dense low-density lipoprotein cholesterol (LDL-C) [7].
Lobeglitazone clinical trials were conducted in Korean type 2 diabetes mellitus (T2DM) atients for 6 months to up to 12 months [8,9], and the efficacy and safety results of lobeglitazone are relatively limited. In pre-marketing clinical trials, a controlled group of patients was enrolled but elderly or patients taking concomitant drugs were excluded to minimize other possible effects in determining the efficacy and safety of the drug. Consequently, in real clinical practice, unforeseen events not detected in the pre-marketing stage may occur because of the extensive and longer duration of treatment involving a variety of patients with various underlying conditions and diseases. Therefore, an ongoing safety assessment under real-world settings is needed. TZD use has been associated with the risk of congestive heart failure (CHF), fractures, bladder cancer (long-term use), edema, and weight gain [1,10–12]. Treatment with lobeglitazone has been shown to be safer than other TZDs in patients with bladder cancer and bone fractures [8]. However, currently, there is a lack of large-scale, long-term safety and efficacy data of lobeglitazone in Korea. This is a non-interventional, multi-centered, retrospective and observational study designed to evaluate the efficacy and safety of lobeglitazone in patients with T2DM in real world.
METHODS
Subjects and study design
This non-interventional multi-center observational study (Retrospective study to Evaluate the Safety of DuvieR in Korean Patients with Type 2 Diabetes Mellitus [DISCOVERY] study) was conducted at 15 tertiary or secondary referral hospitals in Korea. A total of 2,228 patients with T2DM who received lobeglitazone 0.5 mg for more than one year between February 1, 2014 and December 20, 2018 were enrolled. The subjects’ data was collected from anonymized medical records in a clinical setting during the study period, and were recorded in the electronic case report form (eCRF) at the discretion of the researcher. The study protocol was registered at ClinicalTrials.gov (registration number NCT05043467).
Study assessments
In brief, the survey items covered baseline demographic characteristics such as age, sex, height, body weight, body mass index (BMI), duration of diabetes, diabetic complications, medical history, lobeglitazone administration information (total treatment period, start and end date of administration, reason for discontinuation of lobeglitazone), and concomitant medication (type and dose).
Safety assessment items included the incidence of major adverse events (AEs) and any AEs that occurred during the lobeglitazone therapy. Major AEs were: edema, weight gain, fractures, bladder cancer, anemia, hypoglycemia, macular edema, cardiac death, myocardial infarction, stroke, transient ischemic attack, coronary arterial occlusion, and CHF. AEs including blood pressure change, increased liver enzymes (>3×), and dizziness were also identified.
To assess the efficacy of lobeglitazone 0.5 mg, changes in glycosylated hemoglobin (HbA1c) and glucose levels, and lipid parameters (total cholesterol, triglyceride, LDL-C, and high-density lipoprotein cholesterol [HDL-C]) at 3, 6, 12, 18, 24, 36, 42, and 48 months after administration were identified.
Ethics statement
This study proposal was approved by the Institutional Review Boards (IRBs) of each study center listed below: Soonchunhyang University Bucheon Hospital IRB (2020-02-025), Yeouido St. Mary’s Hospital IRB, Konkuk University Hospital IRB, Inje University Ilsan Paik Hospital IRB, Kangbuk Samsung Hospital IRB, Soonchunhyang University Seoul Hospital IRB, Inje University Sanggye Paik Hospital IRB, Hallym University Kangnam Sacred Heart Hospital IRB, Wonju Severance Christian Hospital IRB, Myongji Hospital IRB, Seoul National University Bundang Hospital IRB, Soonchunhyang University Cheonan Hospital IRB, Kyung Hee University Hospital IRB, Ajou University Hospital IRB, and Bucheon St Mary’s Hospital IRB. Written informed consent by the patients was waived due to a retrospective nature of our study.
Statistical analysis
Statistical methods
Safety analysis involved all patients who received at least one dose of lobeglitazone and at least one post-safety follow-up. Efficacy analysis involved patients who were naïve to lobeglitazone or lobeglitazone add-on therapy.
Data management and statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). The patient characteristics are summarized and tabulated. Data are presented as mean±standard deviation (SD) for continuous variables, whereas the categorical variables are expressed as percentages of patients and number of events. Since the data were not normally distributed, a Wilcoxon signed-rank sum test was performed to evaluate changes in laboratory variables from baseline to follow-up. All tests were two-sided and performed at 5% level of significance.
Sample size
According to the retrospective study of Rajagopalan et al. [10], the frequencies of CHF and related hospitalization were 2.0% and 0.7%, respectively. Based on the rates of hospitalization due to CHF, the sample size needed was at least 2,180 to detect a rare serious AE assuming a rate of 0.7% at a fraction of 50% and a two-sided significance level of 0.05.
RESULTS
Patient characteristics
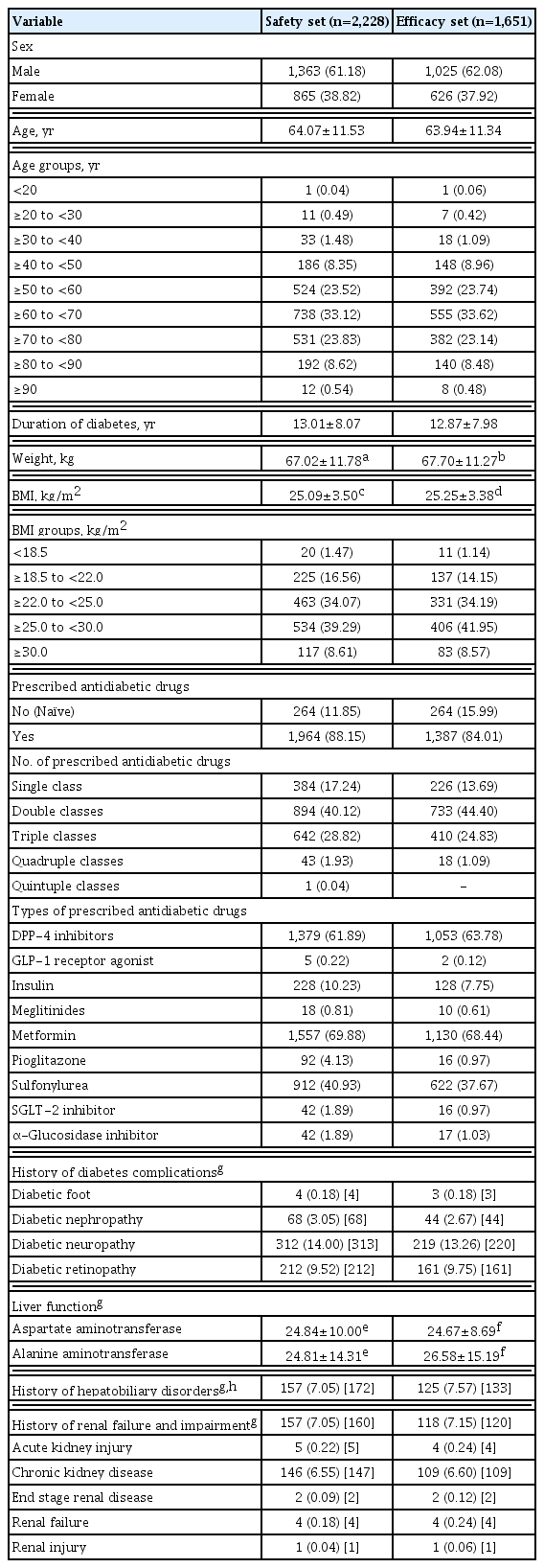
The baseline characteristics of study participants in the safety set are presented in Table 1. The safety analysis set included total patients (n=2,228), and the efficacy analysis set involved drug-naïve patients at the baseline and following the addition of only lobeglitazone without changing other anti-diabetic agents (n=1,651). Among patients in the safety analysis set, the mean age was 64.07±11.53 years, and males constituted 61.18%. The mean duration of diabetes was 13.01±8.07 years. The mean BMI was 25.09±3.50 kg/m2. In terms of diabetic treatment status, 264 patients (11.85%) in the safety analysis set were drug-naïve and 1,964 patients (88.15%) were exposed to concomitant anti-diabetic medications, which mainly included metformin (n=1,557, 69.88%), dipeptidyl peptidase-4 inhibitor (n=1,379, 61.89%), sulfonylurea (SU; n=912, 40.93%), sodium glucose cotransporter-2 inhibitor (n=42, 1.89%), and insulin (n=228, 10.23%). Mean duration of lobeglitazone treatment was 28.59±9.84 months in the safety analysis set.
Safety
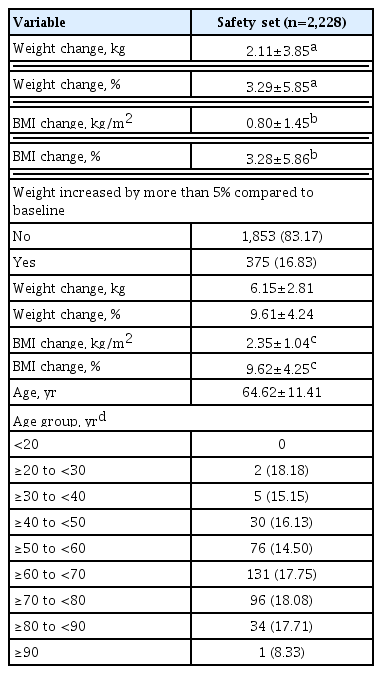
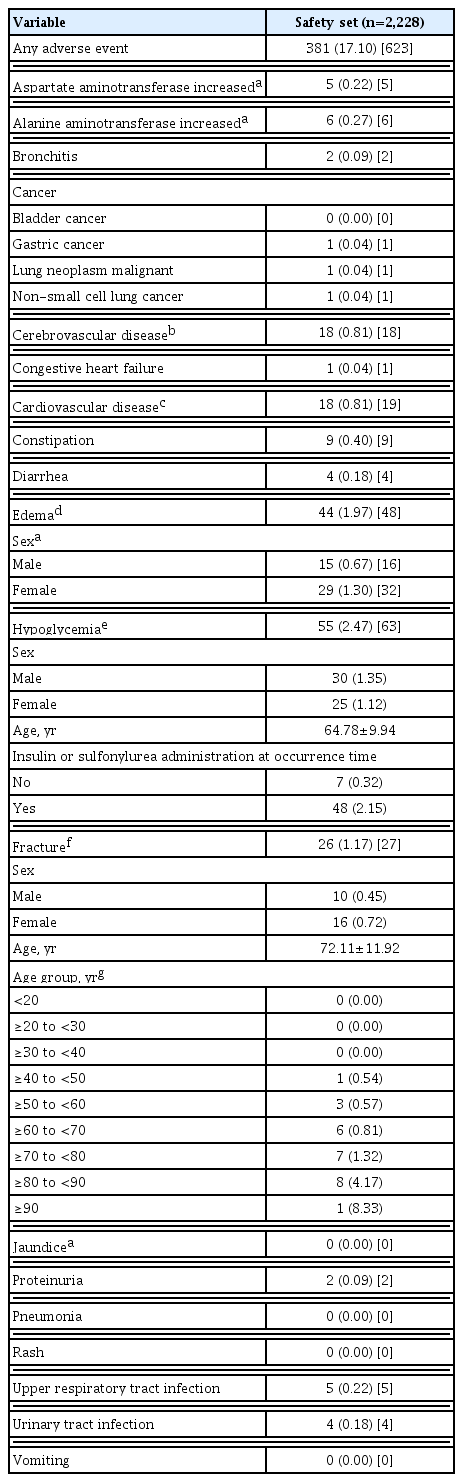
The safety analysis set included 2,228 patients. The results of weight gain after taking lobeglitazone 0.5 mg are presented in Table 2. The mean weight change was 2.11±3.85 kg (3.29%± 5.85%). The percentage of the study participants with weight gain exceeding 5% compared to baseline was 16.83% in the safety analysis set. The percentage of patients who gained weight did not vary with age. Overall, AEs occurred 381 patients (17.10%) (Table 3). AEs of special interest are listed in Table 3. Of these AEs, edema was detected in 1.97% (n=44). In this study, edema was observed more frequently in females than in males (n=29 [1.30%] vs. n=15 [0.67%], respectively). Cerebrovascular and cardiovascular diseases occurred in 0.81% (n=18) and 0.81% (n=18), respectively. One case of CHF was reported as an AE. Hypoglycemia occurred in 2.47% (n=55) patients. Insulin or SU was used to treat hypoglycemia in 87.27% of patients. Fractures occurred in 1.17% (n=26) of all patients. Of these, 61.54% were female and the number of fractures increased with age. Increases in aspartate aminotransferase and alanine aminotransferase occurred in 0.22% (n=5) and 0.27% (n=6) of patients, respectively. No cases of jaundice were reported as an AE.
Efficacy
Effects of lobeglitazone on glucose and lipid parameters
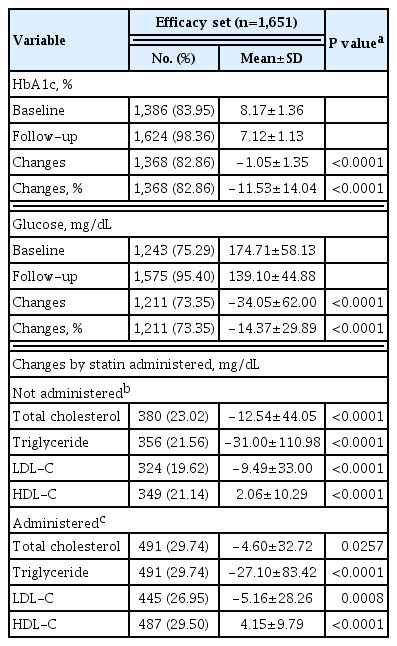
The effects of lobeglitazone were analyzed in the efficacy analysis set (n=1,651) and expressed as mean±SD changes in HbA1c, glucose, and lipid parameters from baseline until the end of treatment (Table 4). Lobeglitazone 0.5 mg significantly decreased HbA1c from the baseline level of 8.17%±1.36% to the final level at the end of study, 7.12%±1.13%, resulting in a mean treatment difference of −1.05%±1.35% (P<0.001). Glucose was also improved at the end of treatment (mean treatment difference −34.05±62.00 mg/dL, P<0.001). Treatment with lobeglitazone 0.5 mg significantly decreased the levels of total cholesterol, triglyceride, and LDL-C and increased HDL-C, regardless of statin therapy (not administrated statin, all P<0.001) (Table 4).
Durability of glycemic control following lobeglitazone therapy
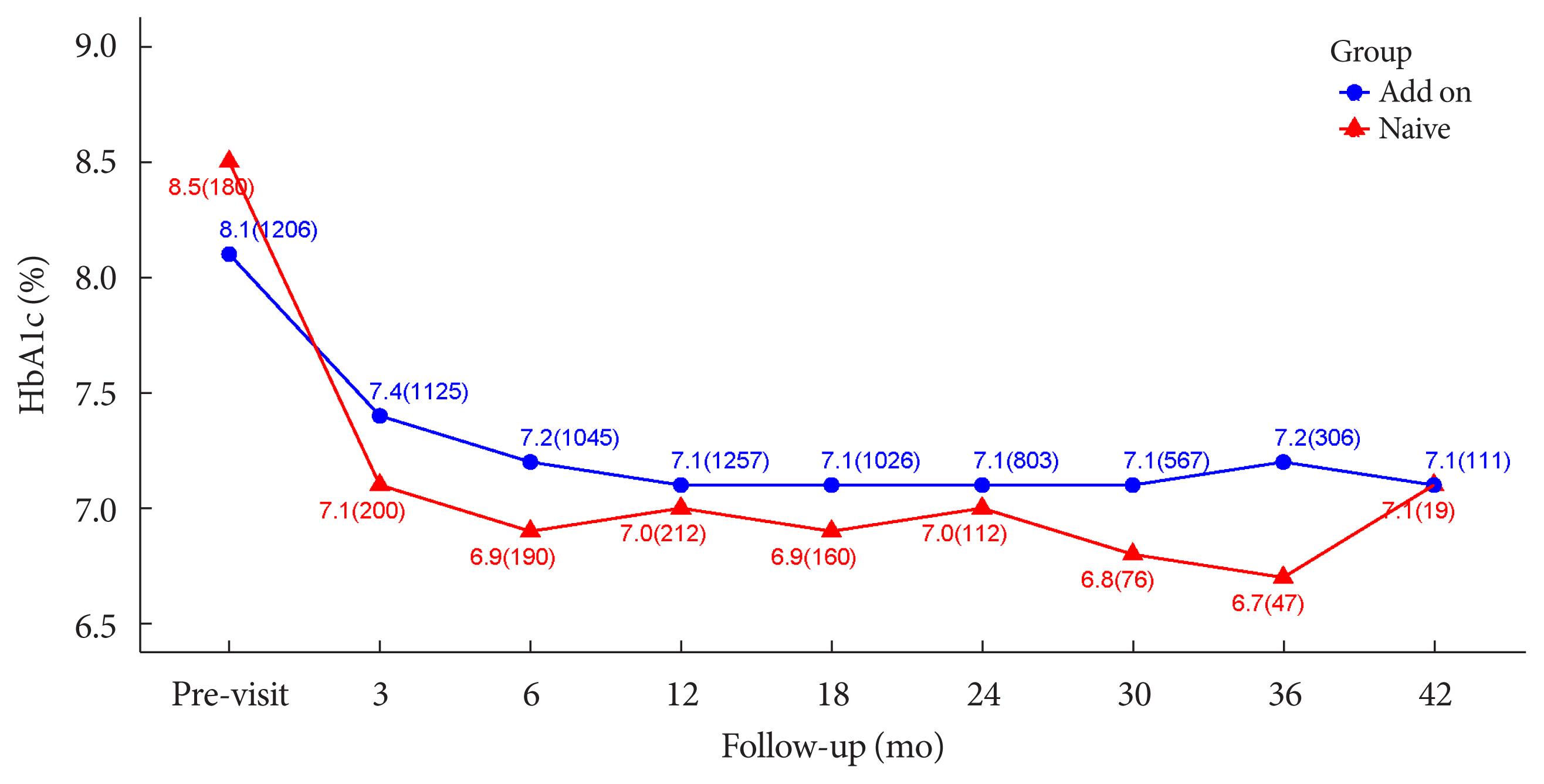
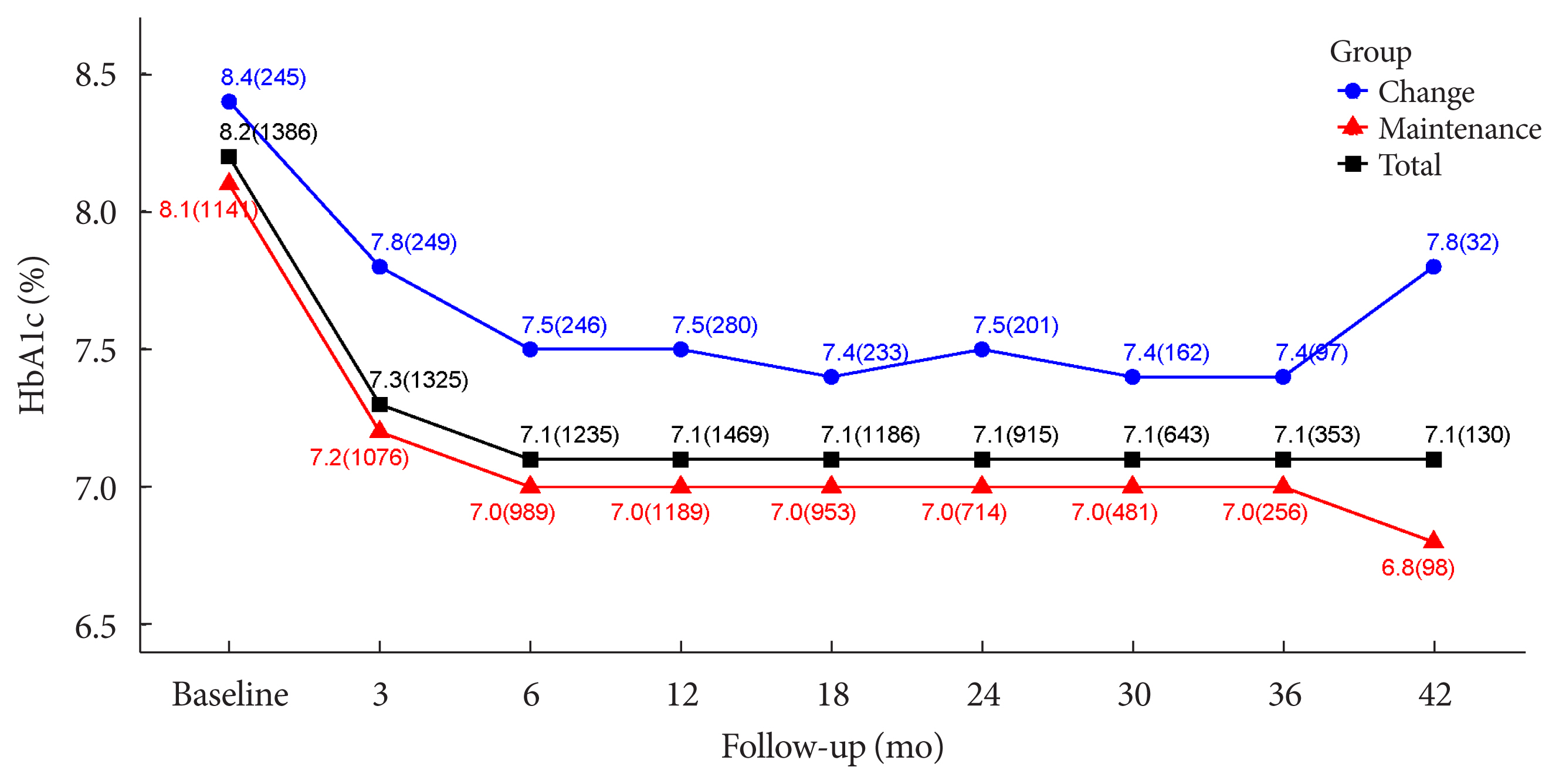
The durability of glycemic control was analyzed in 1,651 patients. Changes in HbA1c levels for 42 months in different groups are shown in Figs. 1 and 2. Patients who received lobeglitazone 0.5 mg (add-on group vs. drug naïve group) showed an apparent reduction in HbA1c from baseline during the first 6 months of treatment (add-on, 8.1%±1.3% and 7.2%±1.2%, respectively; drug naïve, 8.5%±1.9% and 6.9%±1.2%, respectively), and the HbA1c levels remained stable in both groups of patients between months 6 and 42 (Fig. 1). Fig. 2 showed change in HbA1c levels for 42 months in the three groups (the efficacy analysis set vs. maintenance of anti-diabetic medications vs. change of anti-diabetic medications). HbA1c levels at baseline, 6 and 42 months in the efficacy analysis set were 8.2%±1.4%, 7.1%±1.2%, and 7.1%±1.2%, respectively and the HbA1c levels remained stable between months 6 and 42 (Fig. 2). Treatment in anti-diabetic medications starting from the initial lobeglitazone remained unchanged until the final HbA1c test (maintenance group) in 81.0% (n=1,337) patients. In this group, HbA1c levels at baseline, 6 and 42 months in the efficacy analysis set were 8.1%±1.4%, 7.0%±1.1%, and 6.8%±0.9%, respectively (Fig. 2). Glycemic control and long-term durability were higher in the maintenance group following treatment with anti-diabetic medications than in those who switched to other anti-diabetic medications (Fig. 2).
Changes in glycosylated hemoglobin (HbA1c, %) level during 42 months in the add-on and drug-naïve groups are shown. Values are presented as HbA1c mean (number of patients).
DISCUSSION
In the present study, treatment with lobeglitazone 0.5 mg showed a good long-term safety profile. However, lobeglitazone treatment increased body weight by 2.11 kg (3.29%) and led to edema in 1.97% patients during the treatment. Lobeglitazone treatment improved glycemic control in patients with T2DM, and also significantly improved lipid parameters. In particular, lobeglitazone was associated with excellent long-term glycemic control.
Previous TZDs were associated with various safety concerns including weight gain, bladder cancer, CHF, and so on [1,11,13–18]. Both efficacy and safety are important in determining the clinical benefit of anti-diabetic agents. Weight gain and edema are well known AEs of TZDs. As previously stated, lobeglitazone treatment increased body weight by 2.11 kg (3.29%) and resulted in edema in 1.97% patients during the study period. Lobeglitazone treatment increased body weight by 0.89 and 1.65 kg at 24 and 52 weeks, respectively, in previous efficacy and safety trials of lobeglitazone monotherapy [8,9]. In a meta-analysis, pioglitazone therapy led to a weight gain of 1.76 kg [19]. Compared with previous studies of lobeglitazone and pioglitazone, the present real-world study revealed a higher body weight gain, plausibly due to the effects of other concomitant therapies such as SU and insulin, and also the real-world, retrospective design and long-term treatment duration.
Overall, AEs occurred in 17.10% of patients in this study. Cerebrovascular and cardiovascular diseases were found in 0.81% and 0.81%, respectively. Lobeglitazone is associated with a very low risk of cerebrovascular and cardiovascular disease in this study. Only a single case of CHF was reported as an AE despite long-term observation of a large number of patients. The findings suggest that clinicians do not need to desist from prescribing lobeglitazone due to the risk of CHF in patients with T2DM. Edema was detected in 1.97% of patients. In this study, edema was observed more frequently in females than in males (1.30% vs. 0.67%, respectively). Edema is already known to be related to TZDs, and it may result from a reduction in the renal excretion of sodium along with an increase in sodium and free water retention [20]. Other possible mechanisms for edema include increased sympathetic nervous system activity, altered interstitial ion transport, and alterations in endothelial permeability [21–23]. In the results of a previous study, more female patients and more insulin users developed TZD-related fluid retention [20]. Hypoglycemia occurred in 2.47% of all patients, but it occurred in 87.27% of those exposed to add-on therapy comprising insulin or SU, suggesting the hypoglycemic effects of concomitant anti-diabetic therapies.
TZD, a PPARγ agonist, promotes adipogenesis, and inhibits osteoblastogenesis, and its long-term use is associated with impaired bone quality and an increased risk of bone fracture [24–26]. In this study, fractures were reported in 1.17% patients. Although 61.18% of total patients were male, 61.54% of fractures occurred in females and the incidence of fracture increased with age. Compared with clinical studies involving other TZDs [12,27], lobeglitazone showed lower risk of bone fracture (1.17%) than those of other TZDs. Lobeglitazone monotherapy did not significantly alter femur neck and total hip bone mineral density (BMD) compared with placebo [8,9]. Lim et al. [28] evaluated the effects of a 52-week treatment with lobeglitazone 0.5 mg on BMD as a primary end point. The study showed that treatment with lobeglitazone 0.5 mg over 52 weeks was not detrimental to BMD compared with placebo.
Compared with rosiglitazone or pioglitazone, a novel TZD such as lobeglitazone requires lower doses for glycemic control [29]. Lobeglitazone also affected adipocyte biology in a previous study [30]. Further, in a previous animal study, lobeglitazone had no detrimental effects on osteoblast biology [31]. Therefore, the lower dose and the distinct effect of lobeglitazone on adipocyte biology may contribute to a reduced risk of bone fracture.
A few observational studies have reported that TZD increases the risk of bladder cancer [11,15,18]. No cases of bladder cancer have been reported in our study. Based on previous preclinical studies [32,33], lobeglitazone does not increase the risk of bladder cancer. Because lobeglitazone shows a lower effective dose—due to its higher affinity to PPARγ—and as it is mainly metabolized by the liver with negligible renal excretion, lobeglitazone may have a lower risk of bladder cancer than other TZDs [34,35]. However, this study cannot confirm the safety of lobeglitazone on bladder cancer, underscoring the need for a further large prospective study to investigate the risk.
In this study, lobeglitazone showed an apparent reduction in HbA1c from baseline during the first 6 months of treatment (add-on, 8.1%±1.3% and 7.2%±1.2%, respectively; drug naïve, 8.5%±1.9% and 6.9%±1.2%, respectively). Further, the HbA1c levels remained stable in patients in both groups by month 42. These results suggest glycemic efficacy and long-term durability of lobeglitazone. No change in anti-diabetic medications was required in 81.0% of all patients who were treated with upfront lobeglitazone until the final HbA1c test. Glycemic control and long-term durability were better in the maintenance group exposed to anti-diabetic medications. Maintenance therapy without changing anti-diabetic agents implies strong long-term durability in glycemic control. In a previous study investigating the role of different anti-diabetic agents as add-on treatments to metformin in patients with T2DM, the addition of a TZD to metformin yielded the most durable glycemic response [36]. Lobeglitazone, a novel PPARγ agonist, was based on a modification of the rosiglitazone structure to introduce a p-methoxyphenoxy group at the 4-position of the pyrimidine moiety [34,37]. This contributes to the enhanced binding affinity of lobeglitazone for PPARγ; docking analysis suggests that the binding affinity of lobeglitazone is 12 times higher than those of rosiglitazone and pioglitazone [34,38]. TZDs represent peripheral insulin sensitizers [1], but lobeglitazone showed beneficial effects on pancreatic β-cell survival and function in an animal study [2]. These factors may affect the long-term durability of glycemic control with lobeglitazone. Treatment with lobeglitazone also improved lipid parameters in this study consistent with other clinical studies of lobeglitazone [8,9,39].
This study has several limitations. First, the mean age of participants in this study was 64.07±11.53 years, and the proportion of young patients was extremely low. Further, the mean diabetic duration of participants was relatively long. These limitations may affect the study results. In a previous Korean study, young adults with diabetes were more likely to manifest higher insulin resistance [40]. Second, as this study enrolled patients who had received lobeglitazone 0.5 mg for more than 1 year between February 1, 2014 and December 20, 2018, there were no data on patients who were excluded due to AE or lack of effectiveness within one year. However, in general, there is limited convincing data with a paucity of long-term research in this area, so this study is nonetheless expected to represent a meaningful contribution. Third, due to its retrospective design, the results might have been affected by selection bias. Nonetheless, as our study involved multiple centers including 15 hospitals and various patient groups, the results of the study can be generalized.
In conclusion, our results reinforce the long-term safety and durability of the glycemic lowering effect of lobeglitazone 0.5 mg in real-world clinical practice.
ACKNOWLEDGMENTS
We thank all members of DISCOVERY study group involved in conducting and managing this study.
Notes
AUTHOR CONTRIBUTIONS
Conception or design: B.Y.K., J.H.N., C.Y.P., K.H.S., S.K.
Data acquisition, analysis, or interpretation: S.K.K., H.S.K., J.Y.M., S.K.
Drafting the work or revision: B.Y.K., H.S.K., J.H.L., S.L., S.W.C., I.K.J., C.H.C., S.J.H.
Final approval of the manuscript: B.Y.K., H.S.K., S.K.K., J.H.N., C.Y.P., H.K.P., K.H.S., J.C.W., J.M.Y., M.Y.L., J.H.L., S.L., S.W.C., I.K.J., C.H.C., S.J.H., H.S.K., J.Y.M., S.K.
CONFLICTS OF INTEREST
In-Kyung Jeong was editor in chief of the Diabetes & Metabolism Journal from 2020 to 2021. Jung Hyun Noh was associate editor of the Diabetes & Metabolism Journal from 2020 to 2021. They were not involved in the review process of this article. Otherwise, there was no conflict of interest. Statistical analysis was supported by Chong Kun Dang Pharmaceutical Corporation. H.S.K and J.Y.M are employees of Chong Kun Dang Pharmaceutical Corporation.
FUNDING
This work was funded by Chong Kun Dang Pharmaceutical Corporation and partly supported by the Soonchunhyang University Research Fund.