Current Status of Low-Density Lipoprotein Cholesterol Target Achievement in Patients with Type 2 Diabetes Mellitus in Korea Compared with Recent Guidelines
Article information
Abstract
Background
We evaluated the achievement of low-density lipoprotein cholesterol (LDL-C) targets in patients with type 2 diabetes mellitus (T2DM) according to up-to-date Korean Diabetes Association (KDA), European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS), and American Diabetes Association (ADA) guidelines.
Methods
This retrospective cohort study collected electronic medical record data from patients with T2DM (≥20 years) managed by endocrinologists from 15 hospitals in Korea (January to December 2019). Patients were categorized according to guidelines to assess LDL-C target achievement. KDA (2019): Very High-I (atherosclerotic cardiovascular disease [ASCVD]) <70 mg/dL; Very High-II (target organ damage [TOD], or cardiovascular risk factors [CVRFs]) <70 mg/dL; high (others) <100 mg/dL. ESC/EAS (2019): Very High-I (ASCVD): <55 mg/dL; Very High-II (TOD or ≥3-CVRF) <55 mg/dL; high (diabetes ≥10 years without TOD plus any CVRF) <70 mg/dL; moderate (diabetes <10 years without CVRF) <100 mg/dL. ADA (2019): Very High-I (ASCVD); Very High-II (age ≥40+ TOD, or any CVRF), for high intensity statin or statin combined with ezetimibe.
Results
Among 2,000 T2DM patients (mean age 62.6 years; male 55.9%; mean glycosylated hemoglobin 7.2%) ASCVD prevalence was 24.7%. Of 1,455 (72.8%) patients treated with statins, 73.9% received monotherapy. According to KDA guidelines, LDL-C target achievement rates were 55.2% in Very High-I and 34.9% in Very High-II patients. With ESC/EAS guidelines, target attainment rates were 26.6% in Very High-I, 15.7% in Very High-II, and 25.9% in high risk patients. Based on ADA guidelines, most patients (78.9%) were very-high risk; however, only 15.5% received high-intensity statin or combination therapy.
Conclusion
According to current dyslipidemia management guidelines, LDL-C goal achievement remains suboptimal in Korean patients with T2DM.
INTRODUCTION
The number of people with diabetes is increasing globally, with an estimated 463 million people having diabetes in 2019 [1]. The number of patients with diabetes in Korea has also increased continuously; based on an analysis of Korea National Health and Nutritional Examination Survey (KNHANES) data from 2013 to 2016, approximately 14.4% of Korean adults aged 30 years and older had diabetes mellitus [2]. Shaw et al. [3] estimated the number of people worldwide with diabetes for 2010 and 2030, predicting an increase to approximately 4.32 million (11.4%) individuals in Korea by 2030. However, the estimated prevalence in 2016 already surpassed their prediction. The prevalence of Korean individuals with type 2 diabetes mellitus (T2DM) increased constantly from 5.6% (1.6 million) in 2006 to 13.8% (4.9 million) in 2018 [4].
According to a report from Statistics Korea, diabetes was the 6th and 7th leading cause of death in females and males, respectively, in 2018 [5]. The growing burden of diabetes also increases the risk of developing cardiovascular disease (CVD). Diabetes itself is a strong risk factor for CVD, which is a common cause of death in patients with diabetes [6-8]. Although the annual mortality rate from CVD in diabetes has been tending to decrease, it was still two times higher than that in the non-diabetes population in 2013 [9]. Low-density lipoprotein cholesterol (LDL-C) is also known as a modifiable risk factor for CVD and thus should be controlled.
Established clinical guidelines, including those from the Korean Diabetes Association (KDA), European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS), and American Diabetes Association (ADA), highlight the importance of primary and secondary prevention of CVD in patients with diabetes [6,10]. KDA guidelines suggest specific LDL-C target goals based on the risk level of patients with diabetes. Target LDL-C level is <100 mg/dL for patients without CVD and <70 mg/dL for patients with diabetes and CVD, target organ damage (TOD; albuminuria or glomerular filtration rate [GFR] <60 mL/min/1.73 m2) or CVD risk factors (hypertension, smoking, family history of premature atherosclerotic cardiovascular disease [ASCVD]). Statin therapy is recommended for all patients when the therapeutic target is not achieved. Recently updated 2019 ESC/EAS guidelines recommend a more aggressive treatment strategy on LDL-C according to cardiovascular (CV) risk categories in patients with diabetes (very-high CV risk <55 mg/dL, high CV risk <70 mg/dL, and moderate CV risk <100 mg/dL) [11].
However, LDL-C target levels in patients with diabetes are often not reached. The rate for an adequately controlled level, defined as LDL-C <100 mg/dL, was only 44.2% according to the Korean Diabetes Fact Sheet 2018 [2]. In the past, it was recommended to lower LDL-C to <70 mg/dL for patients with diabetes and ASCVD and <100 mg/dL for patients with diabetes without ASCVD; however, recent guidelines suggest more intensive treatment to achieve lower than <70 or even <55 mg/dL according to specific conditions such as ASCVD, TOD, CV risk factors, or long duration of diabetes [6,10,11]. However, there is still a lack of evidence on the current status of dyslipidemia management in patients with T2DM in the real-world setting.
Therefore, this study aims to evaluate the status of LDL-C management in patients with T2DM and investigate whether current real-world treatment practices at referral hospitals comply with updated clinical guidelines for strict control of LDL-C. This could lead to a better understanding of dyslipidemia management, which may consequently yield more favorable CV outcomes in T2DM.
METHODS
Study design
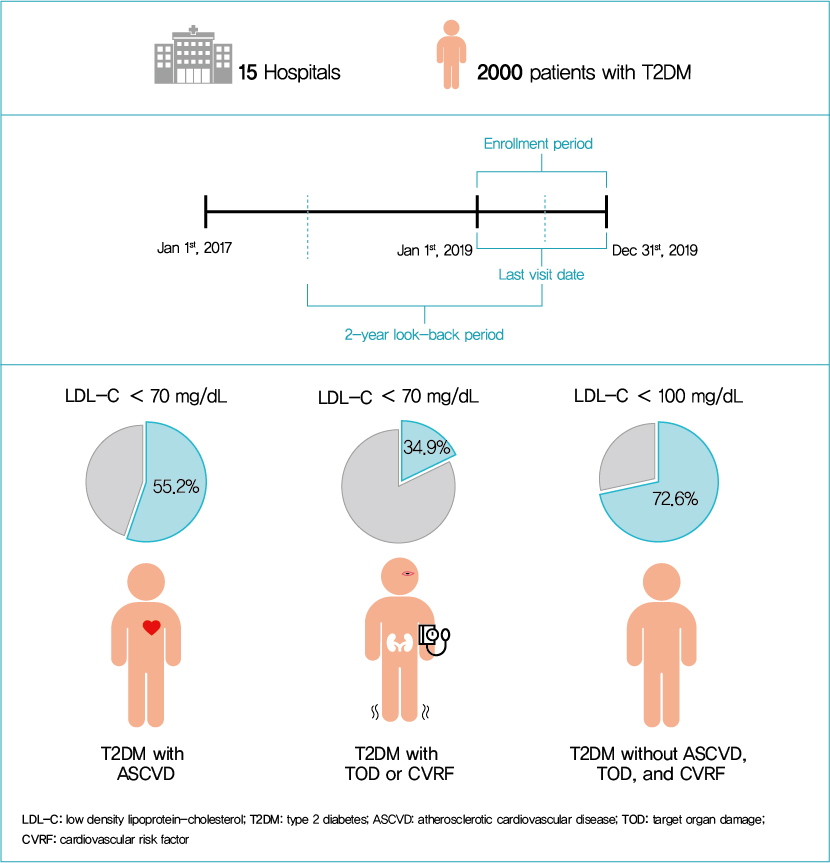
This was a retrospective, observational study using electronic medical record (EMR) data. Data were derived from a large hospital cohort of patients with T2DM followed by endocrinologists at 15 major hospitals in Korea. Research data were collected with the approval of each Institutional Review Board from all participating centers (IRB# KHNMC 2020-02-033 et al.) (Supplementary Table 1).
All adult patients with T2DM who had a recorded cholesterol level from January 1, 2019 to December 31, 2019 were eligible for data collection and inclusion in the study. Exclusion criteria were patients: (1) who were participating in other interventional study using medication or (2) who were in a critical or unstable medical condition. Patients who met inclusion criteria were selected from each hospital using a random sampling method.
The randomization protocol was as follows: the site investigator in each hospital assigned consecutive screening numbers (e.g., a series of numbers such as 1, 2, 3) for all adult T2DM patients who visited and had results of serum lipid levels at the participating hospitals between January 1, 2019 and December 31, 2019. Subsequently, only screening numbers were transferred to the study biostatistician. A total of 52,248 screening numbers were collected from 15 hospitals, after which the study biostatistician performed random sampling for each hospital. At this stage, sufficient random numbers (e.g., more than 1.5 to 2 times the number of samples assigned to each hospital) were sampled. The sampled random numbers were then transferred back to each site investigator. Patients who matched with the sampled random numbers and satisfied the inclusion criteria were enrolled to obtain data from their medical records (Fig. 1). EMR data (including demographics, clinical features, and lipid-lowering treatment patterns) were extracted from 15 hospitals from May to June 2020. We collected data over a 2-year look-back period from patients who visited between January 2019 and December 2019.
The site investigators carefully examined each patient's medical records and identified the patient's medical history and accompanying illness. Investigators entered the patient’s data into the electronic case report form (eCRF, Procuratio, Seoul, Korea). Patients who were diagnosed with, or treated for, unstable angina, myocardial infarction, stroke, or peripheral arterial disease were defined as being established ASCVD patients. TOD was defined as albuminuria, GFR <60 mL/min/1.73 m2, or retinopathy. Albuminuria was defined as a urine albumin creatinine ratio higher than 30 mg/g. Left ventricular hypertrophy (LVH) was confirmed by electrocardiogram, and the presence of retinopathy was confirmed by medical and examination records. The most recent results were used for laboratory test data. The average interval between the test day and medical appointment day was 12 days.
LDL-C treatment criteria according to dyslipidemia guidelines for patients with diabetes
Study populations were risk-stratified according to recent guidelines to assess LDL-C target goal achievement as shown in Table 1. TOD and CV risk factors were also applied by the definition which is specifically described in each guideline. We divided the very-high risk group into Very High-I (with established ASCVD) and Very High-II (with TOD or CV risk factors).
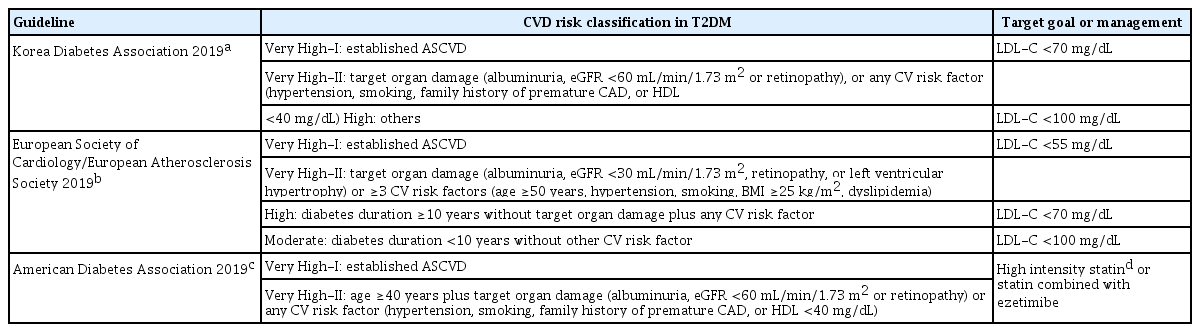
Summary of several guidelines for the management of dyslipidemia in patients with type 2 diabetes mellitus
KDA guidelines (2019) suggest the goals for treating dyslipidemia for three categories. If patients with diabetes have no CVD history or risk factor, the target LDL-C level is <100 mg/dL. For patients with diabetes and established ASCVD (Very High-I group), the target LDL-C level is <70 mg/dL. For patients with diabetes and TOD (albuminuria, GFR <60 mL/min/1.73 m2, or retinopathy) or any CV risk factor (hypertension, smoking, family history of premature ASCVD) (Very High-II group), the target LDL-C level is also <70 mg/dL. For others, the LDL-C target is <100 mg/dL [6].
According to ESC guidelines (2019), treating dyslipidemia in patients with diabetes was further specified and intensified. More stringent treatment is recommended for patients with diabetes and established ASCVD to lower the LDL-C level to <55 mg/dL (Very High-I group). Patients with diabetes who have TOD (microalbuminuria, estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2, retinopathy, or LVH) or with ≥3 CV risk factors (age ≥50 years, hypertension, smoking, body mass index [BMI] ≥25 kg/m2, dyslipidemia) are also recommended to lower the LDL-C level to <55 mg/dL (Very High-II group). Also, patients with diabetes whose disease duration is over 10 years without TOD plus any CV risk factor should adjust their LDL-C target to <70 mg/dL. Others with disease duration no longer than 10 years without any CV risk factor are recommended to keep their LDL-C target <100 mg/dL [11].
The 2019 ADA guideline recommends that patients with diabetes who have a history of ASCVD or aged >40 years with TOD or an additional CV risk factor, are managed with high-intensity statins or ezetimibe add-on to statin combination therapy [10]. Daily atorvastatin doses of 40 to 80 mg and rosuvastatin doses of 20 to 40 mg are defined as high-intensity statin. Moderate-intensity statins are defined as daily atorvastatin 10 to 20 mg, rosuvastatin 5 to 10 mg, pitavastatin 40 to 80 mg, and simvastatin 20 to 40 mg, according to the American College of Cardiology/American Heart Association (ACC/AHA) guidelines [12]. We investigated the treatment groups described in Table 1.
Statistical analysis
Descriptive statistical analyses were conducted to evaluate the distribution of patient characteristics. Categorical variables were described using number of observation (N), number and percent (%) within each category and number of missing observations. Numeric variables were described with number of observations, mean±standard deviation (SD). LDL-C target goal achievement was compared by statin dose using Mantel-Haenszel chi-square test within the same statin therapy groups, and Cochran-Mantel-Haenszel chi-square test between monotherapy and combination therapy groups. All tabulations of summary statistics and all statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) or higher. When statistical tests are performed, a two-tailed P<0.05 is considered as statistically significant.
RESULTS
Baseline characteristics of study subjects
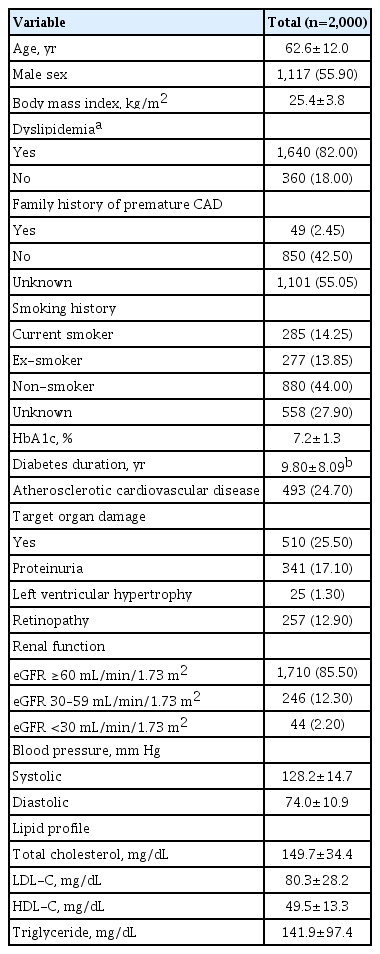
A total of 2,000 patients with DM were enrolled in the study (mean age 62.6 years, 55.9% men, mean BMI 25.4 kg/m2). Over 80% of study subjects were diagnosed with, or receiving medication for, dyslipidemia. The mean glycosylated hemoglobin level was 7.2% ±1.3%, and mean diabetes duration was 9.80±8.09 years. Overall, 24.7% of patients had established ASCVD, and 25.5% of patients had TODs such as proteinuria, LVH, or diabetic retinopathy. Mean systolic and diastolic blood pressure were 128±14.7 and 74±10.9 mm Hg. Mean±SD levels of total cholesterol, direct or calculated LDL-C, high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) were 149.7±34.4, 80.3±28.2, 49.5±13.3, and 141.9±97.4 mg/dL, respectively (Table 2). According to the LDL-C distribution of subjects, 78.9% of subjects had LDL-C <100 mg/dL and only 38.7% of subjects had LDL-C <70 mg/dL (Supplementary Fig. 1).
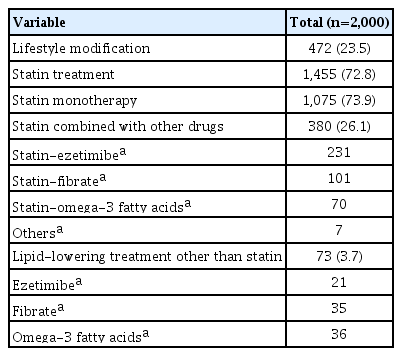
A total of 82% (n=1,640) of subjects were diagnosed with dyslipidemia and undergoing lifestyle modification or medical treatment, 72.8% (n=1,455) were taking lipid-lowering agents including statins, and 3.7% (n=73) were taking lipid-lowering agents other than statins. Among those taking lipid-lowering agents including statins, 73.9% took statin monotherapy, and 26.1% received combination therapy. The most common drugs in combination therapy were ezetimibe, followed by fibrate and omega-3 fatty acids. For the 73 (3.7%) patients not using statins, treatment included omega-3 fatty acids, fibrate, and ezetimibe (Table 3).
LDL-C target achievement rates according to recent guidelines
Based on KDA guideline risk categories, the mean LDL-C level in the established ASCVD group (Very High-I group) was 70.4±23.9 mg/dL, and 55.2% reached the LDL-C target of <70 mg/dL. The average LDL-C level was 82.4±28.8 mg/dL among those with TOD or CV risk factors (Very High-II group), and 34.9% of patients reached <70 mg/dL. The average LDL-C of the high risk group was 87.0±28.2 mg/dL and the target achievement rate was 72.6% (Fig. 2A).
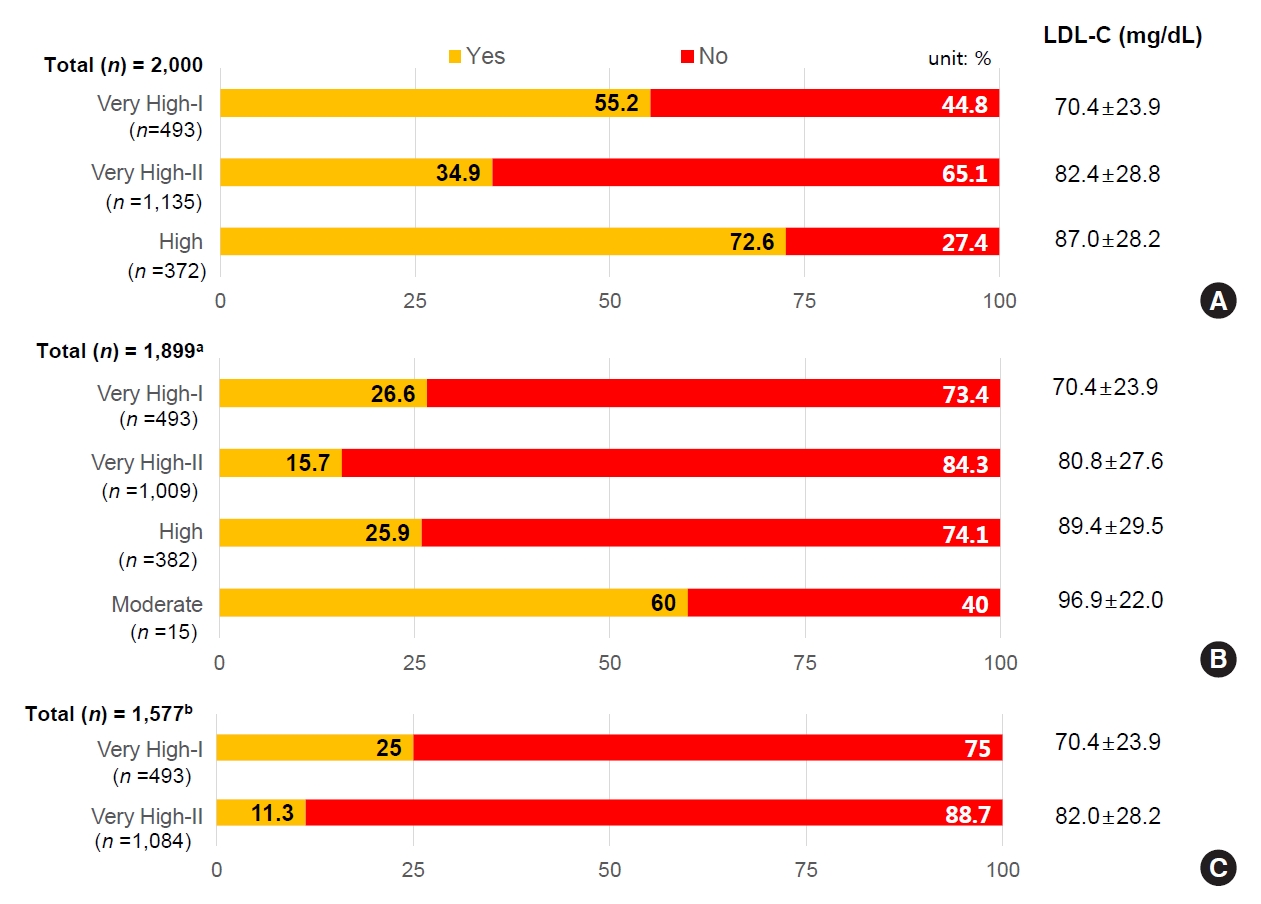
Low-density lipoprotein cholesterol (LDL-C) target achievement rates according to recent guidelines. (A) LDL-C target achievement rates according to Korean Diabetes Association (KDA) 2019 guidelines. (B) LDL-C target achievement according to European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) 2019 guidelines. (C) Lipid-lowering treatment pattern according to American Diabetes Association (ADA) 2019 guidelines. (A, B) ‘Yes’ indicates the portion of patients who meet the target goal and (C) ‘Yes’ denotes the portion of patients who received recommended lipid-lowering treatment, high-intensity statin or statin combined with ezetimibe. a101 patients out of the total 1,899 subjects were excluded since their cardiovascular (CV) risk was not clearly determined due to missing body mass index data, bThe treatment pattern for patient groups with very-high CV risk was reported selectively. Others are heterogeneous and are reported in Supplementary Table 1.
According to 2019 ESC/EAS guidelines, target LDL for patients with established ASCVD history (Very High-I), or TOD or ≥3 CV risk factors (Very High-II), is recommended to be <55 mg/dL. For the Very High-I group with ASCVD, 26.6% reached the LDL-C target, while only 15.7% of the Very High-II group reached the target. The LDL target for the high risk group with diabetes duration of more than 10 years and without TOD or CV risk factors is <70 mg/dL, with 25.9% of patients reaching this target. For patients with diabetes duration less than 10 years, the LDL target goal is <100 mg/dL for the Moderate risk group without other CV risk factors; 60.0% of patients reached this target (Fig. 2B).
Based on ADA 2019 guidelines, high-intensity statin or statin plus ezetimibe combination therapy is recommended for use in patients with established ASCVD (Very High-I), or patients over 40 years with TOD or any CV risk factor (Very High-II). The mean LDL-C for 493 patients in the Very High-I group was 70.4 mg/dL; mean LDL-C for 1,084 patients in the Very High-II group was 82.0 mg/dL. Only 25.0% of Very High-I group and 11.3% of Very High-II group patients were treated with high-intensity statins or statins plus ezetimibe therapy (Fig. 2C).
For patients aged over 40 years without ASCVD, TOD, or CV risk factors who did not meet Very High-I or Very High-II group criteria according to the ADA guideline, 48.0% were prescribed moderate-intensity statin, 11.8% received high-intensity statin or statin plus ezetimibe add-on therapy, and 34.8% received no treatment. For patients aged under 40 years with TOD or CV risk factors, but without ASCVD patients, 47.1% were prescribed moderate-intensity statin; high-intensity or statin plus ezetimibe therapy was used by 15.7% of patients, and 29.4% of patients were prescribed no statin. For patients aged under 40 years without ASCVD, TOD, or CV risk factors, only 56.3% were treated with moderate- to high-intensity statins, and no statin was prescribed for 43.8% of patients (Supplementary Table 2).
Prescribing pattern of lipid-lowering agents according to KDA guideline risk category and LDL-C target achievement
In patients with T2DM and established ASCVD (Very High-I), the prescribing pattern of lipid-lowering agents was: 47.3% statin monotherapy, 20.0% combination treatments including statin, and 10.1% no medication. The target LDL-C level according to the KDA 2019 guidelines is <70 mg/dL, and the achievement rate of this goal was 57.4% for statin monotherapy and 56.4% for statin combination therapy (Fig. 3A). Since this study was retrospective, it was not clear exactly whether a patient in the Very High-I group was previously medicated and stopped, or was treated at another medical institution. This group consisted of 64 individuals, with a mean age of 67.3 years, 68.8% males, all having ASCVD. LDL-C was 76±28.5 mg/dL, with a median of 71 mg/dL (interquartile range [IQR], 54 to 97), all below LDL-C 100 mg/dL.

The prescribing pattern of lipid-lowering agents and low-density lipoprotein cholesterol (LDL-C) target achievement rates of each treatment according to the risk categories of recent guidelines. (A) LDL-C target goal achievement according to lipid-lowering treatment methods by cardiovascular (CV) risk groups defined by Korean Diabetes Association (KDA) 2019 guidelines. (B) LDL-C target goal achievement according to lipid lowering treatment methods by CV risk groups defined by European Society of Cardiology (ESC)/European Foundation for the Study of Diabetes (EASD) 2019 guidelines. (C) Lipid lowering treatment by CV risk groups defined by American Diabetes Association (ADA) 2019 guidelines. TX, treatment; w/o, without. aRecommended treatment includes high-intensity statin or statin combined with ezetimibe treatment.
In patients with T2DM who had TOD such as albuminuria, CKD, or diabetic retinopathy, or any CV risk factor (Very High-II), the prescribing pattern was: 45% statin monotherapy, 15% combination therapy including stain, and 20% no medication. The target LDL-C level according to KDA 2019 guidelines is <70 mg/dL; the achievement rate of this goal was 39.8% for statin monotherapy and 44.6% for statin combination therapy (Fig. 3A). In the Very high-II group, it was not known exactly whether a patient was previously medicated and stopped, or was prescribed by another medical institution. This group consisted of 277 individuals, mean age 62.9 years, 66.4% male. There was no ASCVD, but 26.4% of individuals had TOD and 14.3% of patients had eGFR <60 mL/min/1.73 m2, all of whom had accompanying hypertension. LDL-C was 92.5±30.4 mg/dL, and median was 91 mg/dL (IQR, 74 to 109), so most of them were <100 mg/dL, and thought to have not been administered medication.
In the high risk group of patients with T2DM, the prescribing pattern was 40.8% for statin monotherapy, 12.2% for combination therapy including stain, and 30.7% for no medication. The target LDL-C level in the high risk group of patients with T2DM was less than 100 mg/dL. The achievement rate of this goal was 83.3% for statin monotherapy and 73.1% for statin combination therapy (Fig. 3A).
Prescribing pattern of lipid-lowering agents according to ESC/EAS guideline risk category and LDL-C target achievement
In the Very High-I group, monotherapy was used by 47.3% of patients, combination therapy was used by 20.0%, and 10.1% of patients received no treatment; respective target LDL achievement was 25.7%, 30.4%, and 25.4%. In the Very High-II group with TOD or ≥3 CV risk factors, 49.8% of patients received monotherapy, 15.7% received combination therapy, and 13.8% were not treated, with respective LDL target attainment of 17%, 15.2%, and 12.6%.
In the high risk group, 32.5% of patients were prescribed monotherapy, 8.6% combination therapy, and 45.1% were prescribed no treatment; respective target LDL achievement was 39.4%, 33.3%, and 14.7% (Fig. 3B).
Prescribing pattern of lipid-lowering agents according to ADA guideline risk category
In the Very High-I group, 60.1% of patients were prescribed monotherapy, 25.4% combination therapy, and 13% were untreated. Overall, 10.1% of monotherapy and 74.4% of combination therapy recipients were prescribed high-intensity statin or statin plus ezetimibe, which was the recommended treatment regimen.
In the Very High-II group, 54.2% of patients were prescribed monotherapy, 17.8% combination therapy, and 24.2% were untreated. High-intensity statin or statin combined with ezetimibe was prescribed in 2.9% of monotherapy and 56.5% of combination therapy recipients (Fig. 3C).
Change of prescription during the observation period
This study also identified whether any lipid-lowering agent prescription changes occurred during the 2-year observation period. Only 6.5% of statin prescriptions were changed. A total of 1.4% involved changing the type of statins at the same intensity, 0.9% were for increasing the statin dosage, 1.4% were for reducing the statin dosage, and 0.6% were for discontinuing and restarting statin. The addition of other lipid-lowering agents to statins accounted for 2.2% of prescription changes, of which ezetimibe was the most common, followed by fibrate and omega-3 fatty acids.
DISCUSSION
In this study, the LDL-C target achievement rate was investigated using real-world data from patients with diabetes currently under treatment following the recent publication of three major guidelines for treating dyslipidemia in patients with diabetes. First, we compared the LDL-C target attainment rate by assigning patients into a Very High-I risk group with established ASCVD history, a Very High-II risk group with CVD risk factors or TOD without ASCVD history, and the remaining patients into a high risk group.
Patients with ASCVD history accounted for 24.7% of all study subjects with diabetes. This ratio actually reflects the real-world results of patients with diabetes we encounter in the outpatient clinic [13]. Since previous guidelines recommended patients with diabetes and ASCVD history to lower target LDL-C to <70 mg/dL [14-16], this study showed a 55% achievement rate for a level of <70 mg/dL in the Very High-I group and an average LDL-C of 70.4±23.9 mg/dL (Fig. 2A).
Previous studies showed that the LDL-C target goal attainment rate for Korean patients with T2DM was less than 50% [17-20]. Compared to previous studies with low LDL-C target goal achievement of 17.6% for very-high risk and 47.2% for high risk groups, the recent LDL-C target goal attainment rate has improved. This is likely the result of recent studies highlighting the importance of statin use [21,22].
According to the KDA 2019 guideline, 55.3% of Very High-I groups were under LDL-C of 70 mg/dL and 88.0% were under 100 mg/dL (Supplementary Fig. 2). For the Very High-II group, 34.9% had LDL-C below 70 mg/dL and 77.2% had LDL-C below 100 mg/dL (Supplementary Fig. 3). A recent change in the KDA guidelines is that a stricter treatment target for T2DM patients with TOD or ASCVD risk factors has been recommended, aiming at achieving LDL-C <70 mg/dL, unlike previous guidelines in which it was <100 mg/dL. Nevertheless, Fig. 2 shows that the average LDL-C in the Very High-II group is 82.4±28.8 mg/dL, indicating that most physicians still treat these groups using treatment targets of <100 mg/dL, rather than 70 mg/dL. Because this study was conducted shortly after the announcement of the 2019 KDA guideline, many doctors followed the previous guideline for lipid management in T2DM. When we applied the previous KDA guideline (LDL-C target goal less than 100 mg/dL, 77% of patients reached the target LDL-C of <100 mg/dL (Fig. 2).
It has already been reported that in T2DM patients with microalbuminuria, the risk of CVD is higher than in those without [23]. Also, in patients with diabetes, the higher the number of ASCVD risk factors such as hypertension, smoking, and family history of early CVD, the higher the risk of CVD than in those who do not have risk factors [24]. Therefore, recent guidelines should be widely known and it should be accepted that T2DM patients with TOD or ASCVD risk factors need more aggressive LDL-C treatment.
An analysis of drug prescription patterns and LDL-C target goal achievement rate according to KDA guidelines shows that 47% of patients were prescribed statin monotherapy in the Very High-I group and only 57.4% of them reached the target LDL-C level (Fig. 3A).
Patients who do not reach the target goal may first be checked for drug compliance and, if they do not reach the target goal despite taking their medication regularly, should be considered for additional administration of high-intensity statins or ezetimibe add-on therapy. In the high risk group in our analysis, 40% of patients were prescribed statin monotherapy, and this is a remarkable result because 83.3% of patients in this group reached their target LDL-C level.
LDL-C is an important modifiable CV risk factor [25], and the recent trend has been for lower LDL targets to prevent CVD. A characteristic of dyslipidemia in patients with diabetes is atherogenic dyslipidemia with features such as small dense LDL, high TG, and low HDL-C levels [26]. Thus, more aggressive LDL-C level control is recommended for patients with diabetes than in those without diabetes.
The 2019 ESC/EAS guidelines on diabetes and CVD are much stricter than the KDA guidelines. Unlike most previous guidelines which recommended <70 mg/dL for patients with diabetes and ASCVD history, ESC/EAS guidelines recommend 55 mg/dL. This is concordant with the American Association of Clinical Endocrinologists and American College of Endocrinology guidelines published in 2017 [27]. Based on the results of the IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) study, the addition of ezetimibe 10 mg to simvastatin 80 mg, compared to 80 mg of simvastatin alone, lowered LDL-C levels below 55 mg/dL and the risk of CVD was reduced significantly [28].
According to ESC/EAS guidelines, in the Very High-I group, the LDL-C treatment target level is <55 mg/dL and the achievement rate was 26.6%, which is far below our expectations (Fig. 2B). This is not just a problem in Korea. In a French study, 59% of patients did not reach LDL-C targets, with achievement rates being especially low in the very-high risk group with diabetes [29]. In addition, in a Chinese study, 39.7% of patients in the very-high risk group reached LDL-C treatment targets, which were considered to be related to knowledge about LDL-C targets, physician specialty and professional status such as resident or attending physician [30]. A meta-analysis of guideline target attainment for glucose, blood pressure, and lipid control in patients with diabetes showed an LDL-C treatment target goal achievement of 49.2% (95% confidence interval, 39.0% to 59.4%) of. The study did not analyze the LDL-C target attainment rate according to CVD risk. However, the meta-analysis reported results from various countries according to guidelines from 2006 to 2013. Approximately 50% of patients reached LDL-C targets [31].
Previous studies have reported the LDL-C target achievement rate of dyslipidemia patients in Korea [32,33], but our study is the first to investigate the treatment status for dyslipidemia in diabetic patients in line with recently published strict treatment guidelines for LDL-C.
Moreover, for patients with diabetes who have TOD or ≥3 CV risk factors (Very High-II group), the LDL-C target of 55 mg/dL was very low (15.7%), as shown in Fig. 2B. This shows that specific recommendations considering the duration of diabetes or CV risk factors have not yet been applied to clinical sites. In addition, our study showed that statin monotherapy is prescribed for about 45% of patients in the Very High-I and -II groups (Fig. 3B), but only 25% and 17% of patients reached an LDL-C level of 55 mg/dL. High-intensity statins or combination therapy should be considered for more patients to enable them to reach their target LDL-C level.
In Korea, research is still underway to evaluate whether it is better to lower the LDL-C treatment target for patients with diabetes and ASCVD from 70 to 55 mg/dL. The IMPROVE-IT study found that in patients with diabetes, the benefits on CVD were significantly higher than in those without diabetes. Since no racial differences have been observed in the study, it may still be accepted in Asians that lower LDL-C is better, and <55 mg/dL is likely to have a more significant effect, but more research is still needed on whether to change the lower LDL-C treatment target to <55 mg/dL.
Considering the ADA guideline, less than 30% of patients with diabetes and ASCVD were prescribed high-intensity statin therapy or statin-ezetimibe combination therapy (Fig. 3C). As Asian individuals have reported better LDL-C treatment with lower doses of statins than Western individuals, it is believed that this reflects more use of moderate-intensity statins than high-intensity statins [34,35].
Even if the treatment target was not reached, less than 5% of the treatments were changed to high-intensity statins or added ezetimibe. This shows that there is clinical inertia regarding doctors’ treatment habits.
One of the limitations of this study was the analysis of results for patients with diabetes who were treated by endocrinology specialists in university hospitals, which does not reflect the status of other patients who were treated in primary care clinics. In the future, analysis of data including primary medical institutions is needed. The second is that this was retrospective study that reviewed data from the previous 2 years that failed to assess drug compliance. Therefore, it is necessary to assess patient compliance or adherence through a prospective cohort study. Third, in 2019, many new guidelines were published; however, it is believed that clinical inertia remained between patients and physicians regarding changing previously administered medication. It is expected that LDL-C target achievement rates will be improved through more aggressive treatment with education and promotion of the new guidelines.
In conclusion, despite recent changes in active treatment guidelines for dyslipidemia in diabetes, the LDL-C target achievement rate remains low. In particular, for diabetic patients with TOD or CV risk factors, it is recommended that, to achieve LDL-C targets equivalent to those for ASCVD patients, prescriptions need to be strengthened more aggressively. Furthermore, drug prescription patterns suggest that ezetimibe add-on therapy or high-intensity statin therapy may be helpful, as statin monotherapy is still prescribed for many patients who do not meet their goals.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0088.
The approval of each Institutional Review Board from all participating centers
Treatment pattern among patients defined as `others’ according to ADA 2019 guidelines
Low-density lipoprotein cholesterol (LDL-C) distribution.
Low-density lipoprotein cholesterol (LDL-C) distribution of Very High-I according to Korean Diabetes Association (KDA) 2019 guidelines.
Low-density lipoprotein cholesterol (LDL-C) distribution of Very High-II according to Korean Diabetes Association (KDA) 2019 guidelines.
Notes
CONFLICTS OF INTEREST
This study was sponsored by Viatris Korea. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
In-Kyung Jeong was editor in chief of the Diabetes & Metabolism Journal from 2020 to 2021. Sung Hee Choi, Seung-Hyun Ko have been editorial board member of the Diabetes & Metabolism Journal since 2020. Junghyun Noh was associate editors of the Diabetes & Metabolism Journal from 2020 to 2021. They were not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: I.K.J., S.R.K.
Acquisition, analysis, or interpretation of data: S.J.Y., I.K.J., J.H.C., J.L., H.C.C., S.H.C., S.W.C., H.J.J., H.C.K., S.S.K., S. H.K., G.K., S.K.K., J.H.L., M.K.M., J.N., C.Y.P., S.R.K.
Drafting the work or revising: S.J.Y., I.K.J., S.R.K.
Final approval of the manuscript: I.K.J., S.R.K.
FUNDING
This study was funded by Viatris Korea.
Acknowledgements
Editorial assistance was provided by David P. Figgitt PhD, ISMPP CMPPTM, Content Ed Net.