Changes in Patterns of Physical Activity and Risk of Heart Failure in Newly Diagnosed Diabetes Mellitus Patients
Article information
Abstract
Background
Exercise is recommended for type 2 diabetes mellitus (T2DM) patients to prevent cardiovascular disease. However, the effects of physical activity (PA) for reducing the risk of heart failure (HF) has yet to be elucidated. We aimed to assess the effect of changes in patterns of PA on incident HF, especially in newly diagnosed diabetic patients.
Methods
We examined health examination data and claims records of 294,528 participants from the Korean National Health Insurance Service who underwent health examinations between 2009 and 2012 and were newly diagnosed with T2DM. Participants were classified into the four groups according to changes in PA between before and after the diagnosis of T2DM: continuously inactive, inactive to active, active to inactive, and continuously active. The development of HF was analyzed until 2017.
Results
As compared with those who were continuously inactive, those who became physically active after diagnosis showed a reduced risk for HF (adjusted hazard ratio [aHR], 0.79; 95% confidence interval [CI], 0.66 to 0.93). Those who were continuously active had the lowest risk for HF (aHR, 0.77; 95% CI, 0.62 to 0.96). As compared with those who were inactive, those who exercised regularly, either performing vigorous or moderate PA, had a lower HF risk (aHR, 0.79; 95% CI, 0.69 to 0.91).
Conclusion
Among individuals with newly diagnosed T2DM, the risk of HF was reduced in those with higher levels of PA after diagnosis was made. Our results suggest either increasing or maintaining the frequency of PA after the diagnosis of T2DM may lower the risk of HF.
INTRODUCTION
The number of patients with heart failure (HF) has been increasing globally and its prevalence is expected to rise continuously as the population ages. By 2030, the prevalence of HF will increase to 46% and total medical expenditures for HF patients are expected to increase from $20.9 billion to $53.1 billion [1]. As the aged population rapidly increases in South Korea, it is predicted that the prevalence of HF will increase. Recent study estimated that over 1.7 million (3.35%) Koreans will have HF by 2040 [2].
This increase in HF may be the result of an increased prevalence of risk factors such as diabetes and ischemic heart disease [3]. To date, several studies have reported that individuals with diabetes have a much higher risk for HF compared to those without [4]. As diabetes and HF have become major health problems in many countries, there is a greater focus on preventing the onset or delaying the progression of HF in patients with diabetes.
Increasing the amount of daily physical activity (PA) is one recommendation given to prevent cardiovascular disease (CVD). Recent studies have suggested that augmenting PA is associated with lower HF risk in general population and high-risk groups including diabetics [5,6]. However, epidemiological data linking changes in the patterns of PA to effects on HF prevention are rare, especially in patients with newly diagnosed type 2 diabetes mellitus (T2DM).
Therefore, using data from the National Health Insurance Service (NHIS) in the Republic of Korea, we examined the association between changes in patterns of PA after the diagnosis of T2DM and incident HF.
METHODS
Source of data
This study analyzed data from the Korean NHIS and claims database. The NHIS system is a mandatory health insurance program that covers 97.1% of the Korean population. In Korea, the NHIS is the single health care insurer and is managed by the government. The NHIS includes an eligibility database (i.e., including data such as age, sex, socioeconomic variables, type of eligibility, household income level); a medical treatment claims database (compiled based on medical bills that were claimed by medical service providers for medical expenses); a health examination database (including results of general health examinations and questionnaires on lifestyle and behavior); a medical care institution database (including types of medical care institutions, location, equipment, and number of physicians); and a death register. For this study, we used the general health examination data and NHIS claims data including diagnoses, procedures, prescription records, and mortality.
This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital of Korea (KBSMC 2020-02-003). Participants who underwent national health check-up examinations provided written informed consent for the use of their data for research purposes. All personal information was deleted and only nonidentifiable data were included for analysis.
Study population and design
This study investigated adults without HF older than 40 years of age who received general health check-ups at least twice. We selected 17,314,795 participants who had undergone a health examination between 2009 and 2012, then identified participants with fasting blood glucose levels above 126 mg/dL at the baseline examination (n=1,915,024). Among these individuals, we selected patients whose diagnoses of T2DM were newly made within 2 years from the date of their baseline health examination using the claims data. A diagnosis of T2DM was defined according to the following criteria: (1) by the presence of International Classification of Diseases, 10th revision, clinical modification (ICD-10-CM) codes E11, E12, E13, or E14 and claims for at least one oral antidiabetic agent or insulin at the baseline or (2) a fasting glucose level of 126 mg/dL or higher (obtained from the health examination database). Participants who did not undergo a follow-up health examination within 18 to 30 months after their baseline examination were subsequently excluded (n=154,795). We tracked their data while observing whether HF occurred or not from 2010 to 2017. We also excluded 2,263 participants with a history of HF (ICD-10 code I50 and a history of hospitalization) to ensure that all diagnoses of HF were newly made. Further, subjects with any missing data were excluded (n=5,052). Finally, 132,418 participants were included in the analyses. The incidence of HF was analyzed using the claims data from January 1, 2010 to December 31, 2017 or until the date of death, whichever came first (Supplementary Fig. 1).
Anthropometric and laboratory measurements
Data on medical history, medication use, and health-related behaviors were collected through the administration of a self-reported questionnaire, whereas physical measurements and serum biochemical parameters were obtained by trained staff. Body mass index (BMI) was defined as the patient’s weight (kg) divided by the square of their height (m). Fasting blood glucose, aspartate aminotransferase, alanine aminotransferase, and total cholesterol levels were measured after 12 hours of fasting.
Classification of change in physical activity
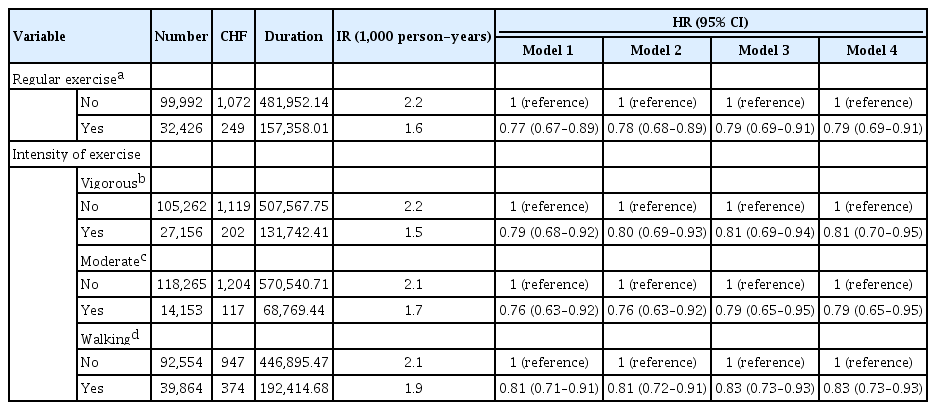
Each participant was asked to report their weekly PA levels according to three categories: vigorous (≥20 min/day; e.g., running, aerobic, or fast cycling at least three times per week), moderate (≥30 min/day; e.g., brisk walking, bicycling at a usual speed, or gardening at least five times per week), and walking (≥30 min/day). Walking was defined as usual-pace walking for at least 10 minutes at a time [7]. Regular exercise was defined as performing at least 30 minutes of moderate PA at least five times per week or at least 20 minutes of strenuous PA at least three times per week.
The study participants were classified into four groups based on changes in their PA levels apparent at the time of the second examination as follows: (1) continuously physically inactive, (2) inactive to active, (3) active to inactive, and (4) continuously physically active. The validity and reliability of the self-administered questionnaire on PA deployed in the NHIS cohort are described in a previous study [8].
Definition of HF and comorbidities
A diagnosis of HF was defined by the presence of ICD-10-CM code I50 and hospitalization [9]. Hypertension was defined according to the presence of at least one claim per year for the prescription of antihypertensive agents, under ICD-10-CM codes I10 through I15, or a systolic/diastolic blood pressure of 140/90 mm Hg or higher [10,11]. The presence of dyslipidemia was defined according to the presence of at least one claim per year for the prescription of antihyperlipidemic agents under ICD-10 code E78 or a total cholesterol level of 240 mg/dL or higher [10,12]. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate of less than 60 mL/min [13]. CVD included myocardial infarction (MI) and stroke. MI was defined by the occurrence of hospitalization with the diagnostic codes of I21 and I22, while stroke was defined by the presence of ICD-10-CM codes I63 and I64 as well as a history of hospitalization with claims for brain magnetic resonance imaging or brain computed tomography [11,12].
Statistical analysis
Anthropometric and laboratory data from the baseline examination was used in analyses. Continuous variables were presented as mean±standard deviation and categorical variables were expressed as percentages. Clinical characteristics between the participants were compared using one-way analysis of variance for continuous variables and the chi-square test for categorical variables, respectively. The incidence rate of HF is presented per 1,000 person-years. Cox proportional-hazards regression analysis was adopted and we calculated the hazard ratio (HR) and 95% confidence interval (CI) for incident HF according to changes in the pattern of PA. The adjusted hazard ratio (aHR) was calculated after adjusting the following variables: age, sex, smoking, alcohol consumption, household income, BMI, hypertension, dyslipidemia, CKD, stroke, and MI.
For subgroup analysis, we stratified the participants by age (65 years and older), sex (male and female), hypertension, dyslipidemia, CKD, CVD, malignancy (yes and no), and weight change. Weight changes were calculated for each subject as the difference from follow-up health examination to baseline examination; stable, gain or loss <5% of body weight at baseline; gain, weight gain of ≥5% and loss, weight loss of ≥5% [14]. All reported P values were two-tailed and <0.05 were considered to be statistically significant. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and R version 3.2.3 (http://www.Rproject.org; The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline characteristics
The mean age of the participants was 57.7±9.58 years and 84,504 (63.8%) of the participants were male. During 639,310.15 person-years of follow-up, 1,321 total HF events occurred. Table 1 presents the baseline characteristics of the participants. As compared with subjects who did not increase their PA after diagnosis of T2DM, those who were continuously physically active or became physically active following the diagnosis of T2DM had smaller waist circumferences and were less obese (P<0.001) (Table 1). Additionally, those who were physically active at the second health check-up examination had lower fasting blood glucose levels and total cholesterol levels than those of participants in the continuously inactive group.
Risk for incident HF according to the intensity of physical activity during follow-up
We investigated the relationship between the level of PA and the development of HF during follow-up. As compared with those who did not exercise regularly, those who were exercising regularly, either performing vigorous or moderate activity, had a lower HF risk (aHR, 0.79; 95% CI, 0.69 to 0.91). The crude incidence rates of HF were 1.5, 1.7, and 1.9 per 1,000 person-years in the vigorous-intensity group, moderate-intensity group, and walking group, respectively. Both the vigorous- and moderate-exercise groups showed a reduced risk for HF (HR, 0.81; 95% CI, 0.69 to 0.94 in the vigorous-activity group vs. HR, 0.79; 95% CI, 0.65 to 0.95 in the moderate-activity group; P<0.001) relative to the control group. After adjustment for confounding variables, the HRs were attenuated but showed consistently reduced risks for HF (Table 2).
Risk for incident HF according to changes in the pattern of physical activity
When the HR for incident HF was analyzed according to changes in the pattern of PA at follow-up, taking the continuously physically inactive group as the reference, patients in the continuously physically active group showed the lowest risk for HF after adjusting for confounding variables (aHR, 0.78; 95% CI, 0.69 to 0.97) (Fig. 1). Also, participants who became physically active during follow-up showed a reduced risk for HF in comparison with those who remained continuously physically inactive (decrease of 0.6/1,000 person-years in the incidence rate: aHR, 0.79; 95% CI, 0.67 to 0.93). Meanwhile, those who were physically active but became inactive did not show a significantly reduced risk for HF (aHR, 0.92; 95% CI, 0.78 to 1.10).
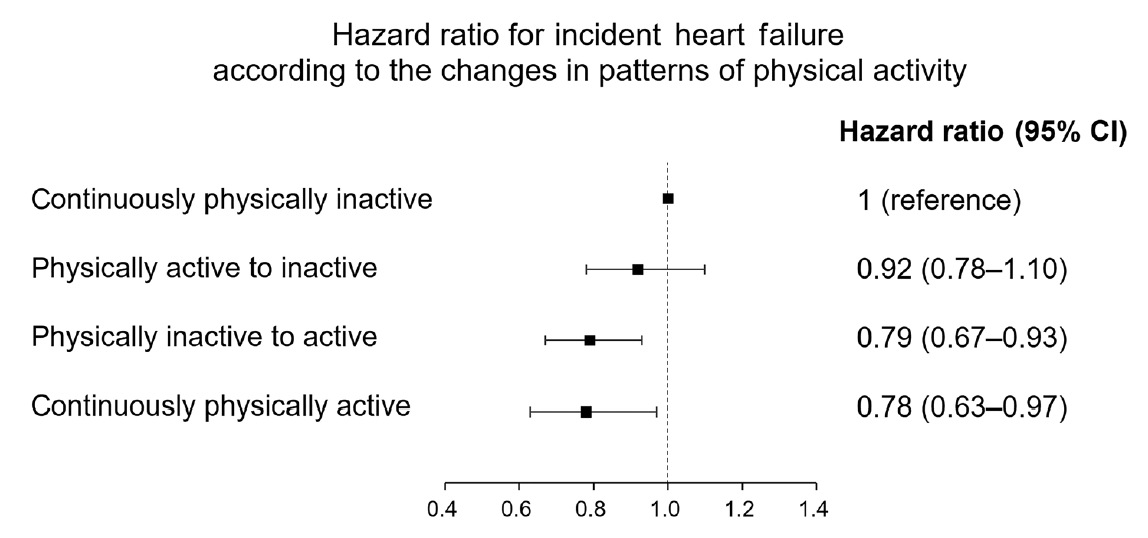
Hazard ratio for incident heart failure (HF) according to changes in the pattern of physical activity. Cox proportional-hazards regression analysis was adopted and we calculated the hazard ratio and 95% confidence interval for incident HF according to changes in the pattern of physical activity. Adjusted for age, sex, current smoking, alcohol, income, waist circumference, hypertension, dyslipidemia, chronic kidney disease, stroke, myocardial infarction, and fasting blood glucose. CI, confidence interval.
Subgroup analyses
To analyze the effects of changes in PA on the development of HF, subgroup analyses were conducted by stratifying study participants according to age, sex, comorbid chronic disease (i.e., hypertension, dyslipidemia, CKD, and CVD), presence of malignancy, and weight change (Table 3, Supplementary Table 1). When we divided the study subjects according to sex, there was a significant interaction between sex and the risk of HF according to changes in the pattern of PA; specifically, men who were continuously active showed the lowest risk for HF. However, no significant interactions between changes in PA and other subgroup variables with respect to the risk for HF were apparent (all P for interaction >0.05). The overall trends and effects of PA on risk estimates for HF were similar in most of the subgroups including those established according to age and the presence of hypertension, dyslipidemia, and CVD, respectively.
DISCUSSION
In our study, we observed the appearance of beneficial effects of increasing PA on reducing the risk of HF after 4.8 years of follow-up. Our findings indicate that increasing or maintaining high levels of PA following the diagnosis of T2DM is associated with a lower risk of HF as compared with in individuals who are continuously inactive. Those who altered their PA from active to inactive during follow-up also had a lower risk of HF as compared with continuously inactive controls. This result could be partially explained by the sustained legacy effect of exercise [15].
In addition to these results, we observed the existence of beneficial effects of PA regardless of the amount or intensity: both vigorous exercise and walking were able to lower the risk of HF. In a meta-analysis conducted by Swain and Franklin [16], exercise performed at a vigorous intensity appeared to have greater cardioprotective benefits than exercise of a moderate intensity. Regarding the frequency and intensity of PA, our moderate-intensity group showed the greatest reduction in risk relative to the vigorous-intensity group. We found concordant results supporting that moderate-intensity exercise may have greater metabolic benefits than vigorous-intensity exercise [15]. Slentz et al. [17] reported that moderate-intensity exercise may improve the disposition index (a marker of pancreatic beta-cell function) more than the high-amount/vigorous-intensity exercise group. The data from the Studies of a Targeted Risk Reduction Intervention through Defined Exercise (STRRIDE) suggest that the same amount of exercise at lower intensity increases the percentage of energy coming from fat oxidation, which may improve metabolic parameters [18]. While the exact mechanism for this result is unclear, this finding may be partially mediated by hemodynamic effects or other biological effects such as lipid metabolism and insulin sensitivity.
There are several potential mechanisms by which PA may interact with the mechanisms of diabetes and pathophysiology of HF. There is growing evidence that insulin resistance has considerable effects on myocardium, which may be responsible for raising the risk of HF in individuals with T2DM [19]. In the hearts of diabetics, glucose utilization may be decreased and free fatty acid use is increased, resulting in insulin resistance [20]. Augmenting PA may improve insulin sensitivity, therefore, associated with a reduced rate of coronary heart disease, as well as with a lower risk of HF [19,21]. Review articles discussed other possible mechanisms by which PA might have beneficial effects in diabetic patients. PA can have beneficial effects on neurohormonal, inflammatory, metabolic adaptations, as well as on endothelial dysfunction. Exercise counterbalances the long-term detrimental effects of neurohormonal activation [22], promotes nitric oxide release, resulting in endothelium-dependent vasodilation [23]. Also, regular exercise training can have anti-inflammatory effect by increasing plasma levels of interleukin 10 (IL-10), reducing inflammatory cytokines (e.g., IL-6, tumor necrosis factor-α), platelet-related inflammatory mediators, and peripheral markers of endothelial dysfunction [24,25]. All these exercise-induced changes can be associated with halting the progression of HF in patients with diabetes.
Several studies have documented the benefits of regular exercise and PA in reducing the coronary heart disease risk in both primary and secondary prevention [26]. However, clinical studies have reported a higher incidence of HF in diabetics even without ischemic heart disease. In this study, we observed an inverse association between PA and risk of HF, regardless of the patient’s previous history of MI or stroke. Previous studies have suggested that the introduction of purposeful weight loss programs and exercise training may improve symptoms and survival rates among patients with HF [27]. Interactions between weight changes and the risk of HF were further examined in our study. We expected the benefits of increased PA might be greater in participants who lose weight via exercise than those who gained weight. However, there was no significant interaction between weight loss and incident HF.
There are expected to be other mechanisms viable in the prevention of HF besides weight loss. One possible explanation is that higher PA improves myocardial perfusion by alleviating endothelial dysfunction and therefore dilating the coronary vessels [28].
When we conducted subgroup analyses according to the presence of malignancies, we could not observe the benefits of greater PA in reducing the risk of HF in subjects with malignancies. These results may be related to their exposure to cardiotoxic chemotherapy agents. There was no benefits of being continuously active among participants with CKD, which could be partially explained by the cardiorenal syndrome among participants in advanced stages of CKD.
Our study has potential limitations that should be considered during its interpretation. First, the definition of study outcome and comorbidities was based on claims data. Participants who have not yet been diagnosed with HF may have decreased PA. Also, information on the frequency and intensity of PA was based on a self-reported questionnaire, which is subject to recall response bias. Self-report questionnaires may provide a reliable approximation of PA at the population level [29]; however, we cannot completely rule out the possibility of bias. We also did not collect the information regarding the type of exercise (i.e., resistance exercise, aerobic exercise, or both). Large, randomized controlled trials are required to determine the most effective exercise training regimen. Second, detailed information for evaluating HF such as pro-brain natriuretic peptide level and left ventricular ejection fraction was not available in the NHIS database. HF in T2DM patients is not a homogenous complication rather a heterogenous condition; however, lack of echocardiographic data is a major limitation of our study. Third, we did not consider the effects of medications, which might have potential effects on the development of cardiovascular complications. We were unable to obtain study individuals’ prescribed drug data, especially which might have beneficial effects on the development of HF (i.e., sodium-glucose co-transporter-2 [SGLT-2] inhibitor or angiotensin receptor blockers); however, according to Ko et al. [30], metformin has been the most commonly used antidiabetic drug in Korea (80.4% in 2013), while sulfonylurea is the most commonly prescribed second-line agent following metformin. According to the literature regarding the prescription patterns in T2DM in Korea so far, SGLT-2 inhibitor was first introduced in Korea at the end of 2013, has not been commonly prescribed in newly diagnosed patients due to reimbursement restriction [31,32]. We also did not consider changes in blood pressure or antihypertensive medication, other health-related behaviors including medication adherence, smoking status, and alcohol consumption after the diagnosis of diabetes. We could not adjust for changes in blood pressure or health status during follow-up, because we could not obtain time-varying confounders in our dataset. These changes in study participants during follow-up period should be considered in future studies.
Participants who exercise regularly might have healthier lifestyles involving good diets and better compliance with their medical treatments. However, after adjustment for various factors including smoking status and alcohol habits, associations between increased PA and reduced risk of HF were attenuated, yet still suggested significant benefits. When we divided participants according to their weight changes between biennial health examinations, we could not obtain clinical information about their weight changes. Patients with HF may experience a range of weight changes as manifestations of HF decompensation, from peripheral edema to cachexia [33]. Lastly, the generalization of our results may be limited because of the single ethnicity among patients included in this study.
Despite these limitations, our study has several strengths. Until now, no study has analyzed the relationship between changes in the pattern of PA and HF, especially in patients with newly diagnosed T2DM via a longitudinal design. Because of the difficulty of conducting exercise detraining studies, only a few studies have examined the effects of exercise or PA on the development of HF. Though the causal relationship of PA and the risk of HF could not be fully addressed here, our results suggest that augmenting PA might have positive effects on mitigating the onset of HF in patients with newly diagnosed T2DM. Additional studies assessing other ethnic groups with longer follow-up periods are required to clarify the role of PA in the progression of HF in high-risk diabetic patients.
Among individuals with newly diagnosed T2DM, either increasing the level of PA or remaining continuously physically active after the diagnosis of T2DM may lower the risk of HF. Even in individuals with known risk factors of HF such as a history of MI, maintaining or increasing PA was associated with a reduced risk for HF. Patients with newly diagnosed T2DM should be encouraged to increase daily PA.
Supplementary Materials
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0046.
Subgroup analysis of study participants
Flow chart of study population.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: I.J.
Acquisition, analysis, or interpretation of data: H.K., S.E.P., K.D.H., Y.G.P.
Drafting the work or revising: I.J., H.K., S.E.P., E.J.R., W.Y.L.
Final approval of the manuscript: I.J., H.K., S.E.P., K.D.H., Y.G.P., E.J.R., W.Y.L.
FUNDING
None
Acknowledgements
The authors acknowledge the efforts of Department of R&D Management at Kangbuk Samsung Hospital, Korea for editing figures and tables. The authors would like to thank the National Health Insurance Service for cooperation.