Study on Risk Factors of Peripheral Neuropathy in Type 2 Diabetes Mellitus and Establishment of Prediction Model
Article information
Abstract
Background
Diabetic peripheral neuropathy (DPN) is one of the most serious complications of type 2 diabetes mellitus (T2DM). DPN increases the risk of ulcers, foot infections, and noninvasive amputations, ultimately leading to long-term disability.
Methods
Seven hundred patients with T2DM were investigated from 2013 to 2017 in the Sanlin community by obtaining basic data from the electronic medical record system (EMRS). From September 2018 to July 2019, 681 patients (19 missing) were investigated using a questionnaire, physical examination, biochemical index test, and follow-up Toronto clinical scoring system (TCSS) test. Patients with a TCSS score ≥6 points were diagnosed with DPN. After removing missing values, 612 patients were divided into groups in a 3:1 ratio for external validation. Using different Lasso analyses (misclassification error, mean squared error, –2log-likelihood, and area under curve) and a logistic regression analysis of the training set, models A, B, C, and D were established. The receiver operating characteristic (ROC) curve, calibration plot, dynamic component analysis (DCA) measurements, net classification improvement (NRI) and integrated discrimination improvement (IDI) were used to validate discrimination and clinical practicality of the model.
Results
Through data analysis, model A (containing four factors), model B (containing five factors), model C (containing seven factors), and model D (containing seven factors) were built. After calibration, ROC curve, DCA, NRI and IDI, models C and D exhibited better accuracy and greater predictive power.
Conclusion
Four prediction models were established to assist with the early screening of DPN in patients with T2DM. The influencing factors in model C and D are more important factors for patients with T2DM diagnosed with DPN.
INTRODUCTION
In recent years, with change in diet and lifestyle, type 2 diabetes mellitus (T2DM) has gradually become a major public health problem worldwide and one of the main causes of blindness, amputation, heart disease, renal failure, and premature death. According to the International Diabetes Federation report published in 2019, 463 million patients are diagnosed with diabetes worldwide, and China ranks first in the number of patients with diabetes at approximately 116.4 million.
Diabetic peripheral neuropathy (DPN) is one of the most common, complex, and serious complications of diabetes experienced by patients. DPN is defined as damage to the nervous system caused by chronic hyperglycemia and various pathophysiological changes. DPN increases the risk of ulceration, noninvasive amputation, and foot infection, which will eventually lead to long-term disability [1], and it imposes substantial economic and psychological burdens on patients with T2DM. According to the study, patients with DPN are two to three times more likely to fall than patients without DPN [2]. Currently, the pathogenesis of DPN is unclear, leading to the lack of a specific clinical treatment. Therefore, the early diagnosis and timely reduction of multiple risk factors are the keys to reducing DPN.
Based on the T2DM population s in the Chinese communities this study explored the risk factors for DPN and established four prediction models. The predictive nomogram may help hospitals and communities to determine early predictions of the occurrence of DPN in patients with T2DMand facilitate early control and intervention in the clinic.
METHODS
The previous study and data collection were approved by the Ethics Committee of Shanghai Oriental Hospital affiliated with Tongji University (Batch number: [2017] Research Review No. 20) and was performed according to the principles of the Declaration of Helsinki.
Acquisition of indicators
With the authorization of Sanlin Community Health Center Hospital affiliated with Shanghai University of Traditional Chinese Medicine, our team reviewed the electronic medical record systems (EMRS) of 700 patients with T2DM who were diagnosed from 2013 to 2017 to obtain a history of DPN. From September 2018 to July 2019, 700 patients were followed up, with 19 missed visits. The 681 T2DM patients were investigated using a questionnaire, physical examination biochemical index test, and examination based on the Toronto clinical scoring system (TCSS) scale. The questionnaire mainly collected basic information about age, sex, disease course, medical history, and other basic information of the patients. Finally, 69 of the 681 patients who were followed were excluded from the study due to the partial absence of data, and 612 patients (219 patients with DPN) were included in the subsequent statistical analysis.
During the physical examination, systolic blood pressure (SBP), diastolic blood pressure (DBP), height, weight, waist circumference were measured. All patients fasted to measure fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), blood urea nitrogen, uric acid (UA), and serum creatinine. Two hours after the meal, blood samples were collected again to determine postprandial blood glucose (PBG). According to Chinese guideline for T2DM published in 2017, the ankle reflex, acupuncture pain, vibration, pressure, and temperature are used to screen for DPN in the clinic. Specific test items and corresponding scores are shown in Supplementary Table 1. Informed consent was obtained from all participants (Fig. 1).
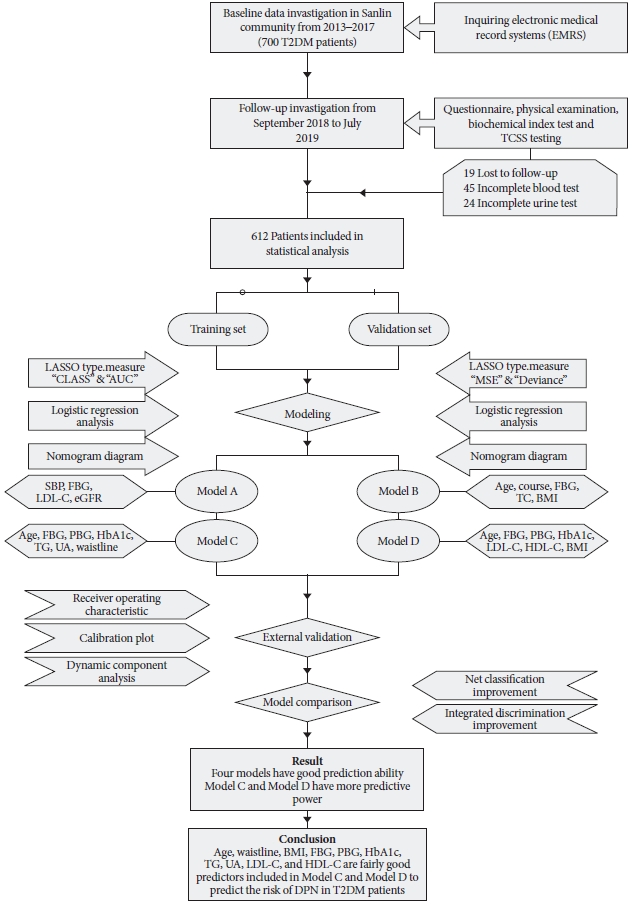
Flowchart of the procedure used in this study. The flowchart shows the entire process of the study from the acquisition of indicators, diagnosis of patients, handing of missing and abnormal values, statistical analysis, and conclusions. TCSS, Toronto clinical scoring system; LASSO, Lead Absolute Shrinkage and Selection Operator; CLASS, misclassification error; AUC, area under curve; MSE, mean squared error; SBP, systolic blood pressure; FBG, fasting blood glucose; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate; TC, total cholesterol; BMI, body mass index; PBG, postprandial blood glucose; HbA1c, glycosylated hemoglobin; TG, triglyceride; UA, uric acid; HDL-C, high-density lipoprotein cholesterol; DPN, diabetic peripheral neuropathy; T2DM, type 2 diabetes mellitus.
Diagnostic criteria for patients
T2DM patients diagnosed with DPN in the Sanlin community from 2013 to 2017 underwent nerve conduction velocity or cutaneous sympathetic nerve response tests in specialist clinics. From September 2018 to July 2019, follow-up of the population was performed to diagnose DPN using the TCSS. Many studies have confirmed that the consistency between the screening of DPN with TCSS score ≥6 points is good [3,4], and the sensitivity and specificity of the TCSS are greater than 70% when the scores are ≥6 [5]. Therefore, patients with a TCSS score ≥6 points were diagnosed with DPN in the follow-up survey.
Statistical analysis
All statistical analyses included in this study were conducted using R software version 3.6.3 (https://www.R-project.org). First, the reliability and validity of the questionnaire data were tested. The scale of Cronbach’s α was 0.77. The test value of the Kaiser-Meyer-Olkin (KMO) Measure was 0.680, with P<0.01 calculated using Bartlett’s test. The reliability and validity of the questionnaire were good. The training set and validation set of this study were divided at a ratio of 3:1. A descriptive statistical analysis, t-test, and chi-square tests were performed. Lead Absolute Shrinkage and Selection Operator (LASSO) regression analysis was used to screen the 17 independent variables of the training set to identify the factors. Four different types of measures of the LASSO, including the area under curve (AUC), misclassification error (CLASS), mean squared error (MSE), and –2log-likelihood (deviance), were used to filter the variables. A type measure is used to specify the target covariates that were minimized when cross-validating the selected model. After features with nonzero coefficients in the LASSO regression model were selected, a multivariate logistic regression analysis was performed on the training set to identify all significant risk factors.
The features were assessed by calculating odds ratios with 95% confidence intervals, and the corresponding P values were then obtained. Features with P≤0.05 were used to build the nomogram. The receiver operating characteristic (ROC) curve, calibration plot, dynamic component analysis (DCA) were performed to assess the accuracy of nomogram. We compared the models by determining the net classification improvement (NRI) and integrated discrimination improvement (IDI) to select the best model.
RESULTS
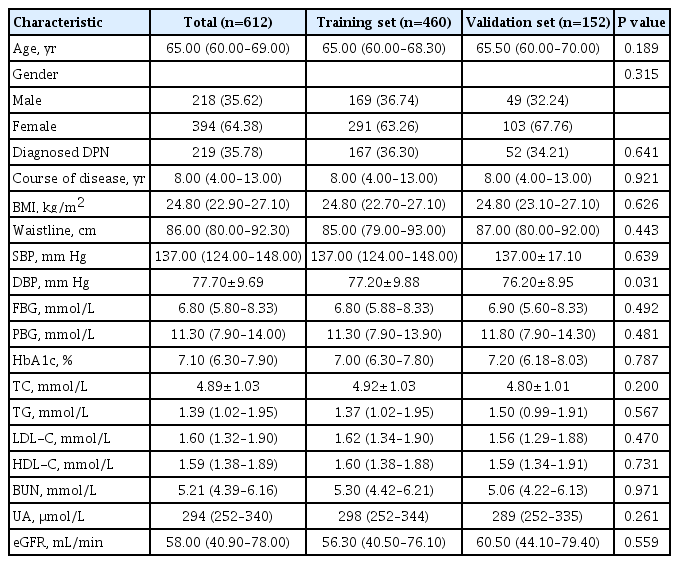
Six hundred and twelve patients with valid data were included in this study, 218 male patients and 394 female patients. The average age of the patients was 65.00 years old (range, 60.00 to 69.00). Two hundred and ninety patients (35.78%) had DPN. Patients was divided into training set (460 people) and validation set (152 people). Specific demographic and clinical characteristics are shown in Table 1.
The data from the training set were analyzed using a LASSO regression analysis. AUC was used to screen four factors, including FBG, estimated glomerular filtration rate (eGFR), SBP, and LDL-C (Fig. 2A and E). CLASS was used to screen five factors, namely, age, body mass index (BMI), disease course, FBG, and TC (Fig. 2B and E). MSE was used to screed seven factors, namely, age, HbA1c, FBG, PBG, TG, waist circumference, and UA (Fig. 2C and E); deviance was used to screen seven factors, including age, BMI, and HbA1c, FBG, PBG, LDL-C, and HDL-C (Fig. 2D and E). The specific coefficients corresponding to the variables and the lambda.1se obtained for different types of measures are shown in Supplementary Table 2. The risk factors selected were included in the logistic regression analysis. The P values of all the included variables was less than 0.05 (Table 2). Then, four nomogram diagrams corresponding to model A, B, C, D were established (Fig. 3).
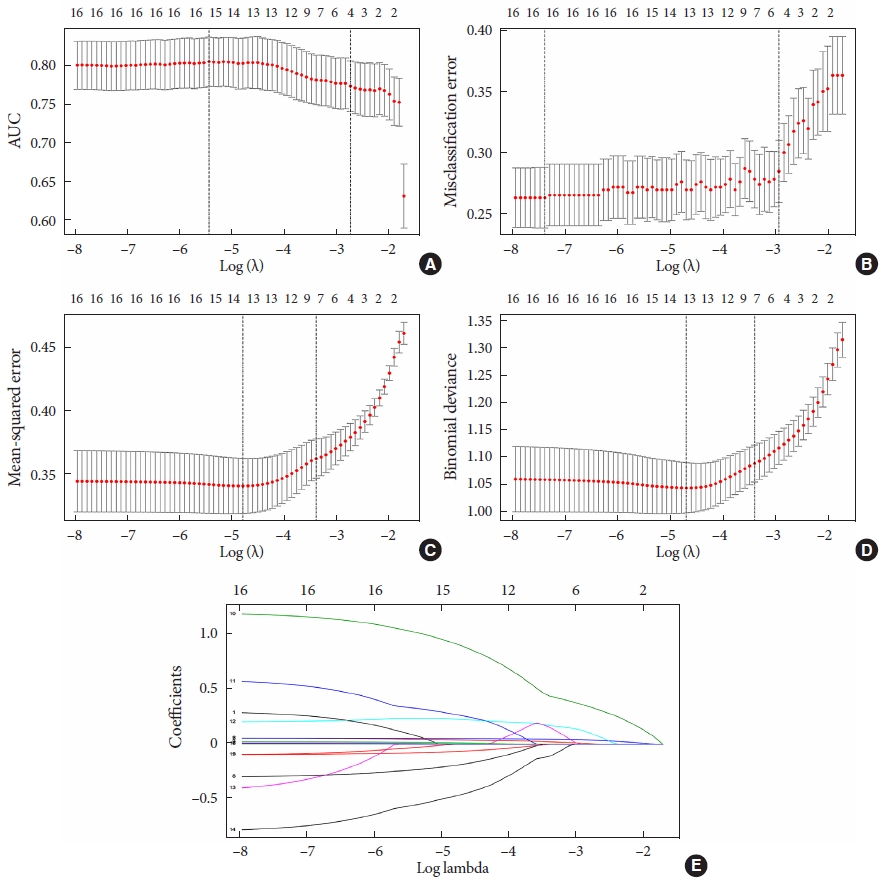
Demographic and clinical features selected using the Lead Absolute Shrinkage and Selection Operator (LASSO) analysis. (A) Area under curve (AUC): LASSO coefficient profiles of the four features. A coefficient profile plot was produced with the log(lambda) sequence. A vertical line was drawn at the value selected using fivefold cross-validation, where the optimal lambda value resulted in four features with nonzero coefficients. (B) Misclassification error (CLASS): LASSO coefficient profiles of the five features. A coefficient profile plot was produced with the log(lambda) sequence. A vertical line was drawn at the value selected using five-fold cross-validation, where the optimal lambda value resulted in five features with nonzero coefficients. (C) Mean squared error (MSE): LASSO coefficient profiles of the seven features. A coefficient profile plot was produced with the log(lambda) sequence. A vertical line was drawn at the value selected using five-fold cross-validation, where the optimal lambda value resulted in seven features with nonzero coefficients. (D) Deviance: LASSO coefficient profiles of the seven features. A coefficient profile plot was produced with the log(lambda) sequence. A vertical line was drawn at the value selected using five-fold cross-validation, where the optimal lambda value resulted in seven features with nonzero coefficients. (E) CLASS & MSE & deviance & AUC: optimal parameters (lambda) selected in the LASSO model using five-fold cross-validation based on the minimum criteria. The partial likelihood deviance (binomial deviance) curve was plotted versus log(lambda). Dotted vertical lines were drawn at the optimal values using the minimum criteria and the 1-standard error (SE) of the minimum criteria.
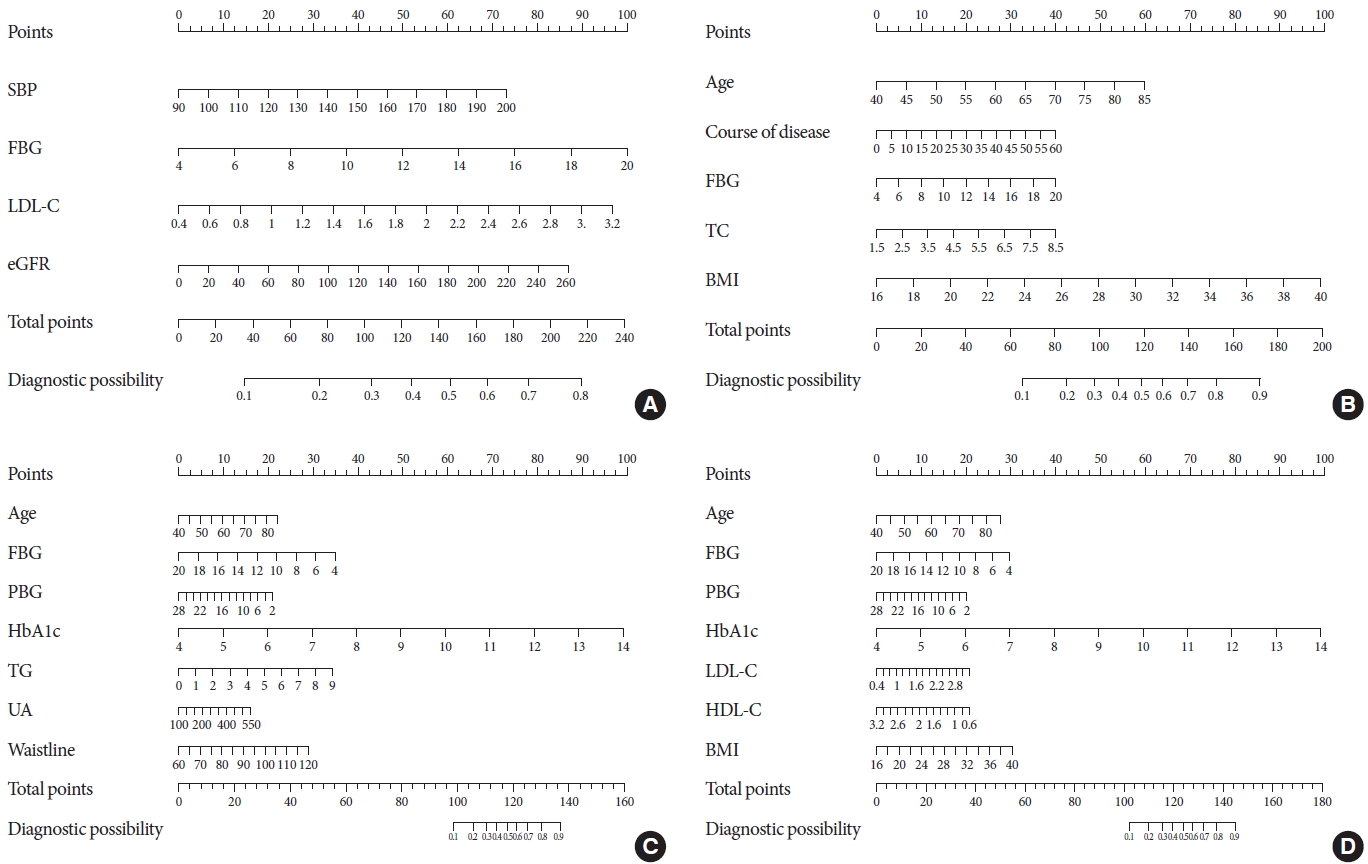
The nomograms for diabetic peripheral neuropathy (DPN) based on models A, B, C, and D. (A) Model A: The nomogram for DPN in patients with type 2 diabetes mellitus (T2DM) was developed in the cohort by integrating systolic blood pressure (SBP), fasting blood glucose (FBG) levels, low-density lipoprotein cholesterol (LDL-C) levels, and estimated glomerular filtration rate (eGFR). (B) Model B: The nomogram for DPN in patients with T2DM was developed in the cohort by integrating age, disease course, FBG levels, total cholesterol (TC) levels, and body mass index (BMI). (C) Model C: The nomogram for DPN in patients with T2DM was developed in the cohort by integrating age, postprandial blood glucose (PBG) levels, FBG levels, glycosylated hemoglobin (HbA1c) levels, TC levels, uric acid (UA) levels, and waist circumference. (D) Model D: The nomogram for DPN in patients with T2DM was developed in the cohort by integrating age, PBG levels, FBG levels, HbA1c levels, LDL-C levels, high-density lipoprotein cholesterol (HDL-C) levels, and BMI.
The ROCs of model A, B, C, D were reported in Supplementary Table 3 and Supplementary Fig. 1. The ROC value of model A, B, C, D is 0.345 (0.594 to 0.683), 0.324 (0.590 to 0.760), 0.327 (0.727 to 0.796), and 0.313 (0.689 to 0.808) in training set, and the ROC value of model A, B, C, D is 0.354 (0.650 to 0.635), 0.395 (0.720 to 0.654), 0.430 (0.800 to 0.712), and 0.556 (0.910 to 0.654) in validation set. The calibration test produced S:P values for models A, B, C, and D in the training set and validation set of 0.951, 0.983, 0.915, 0.990, and 0.987, 0.713, 0.906, 0.520, which showed in Supplementary Fig. 2, respectively. Hosmer-Lemeshow tests were performed using the four models for the training set and validation set. In the training set, the corresponding P values of the four models were 0.333, 0.917, 0.379, and 0.915, respectively. In the validation set, the corresponding P values of the four models were 0.611, 0.179, 0.353, and 0.353, respectively. The P values of the four models were greater than 0.05, indicating that these models had good fits and were valid. The DCA revealed threshold probabilities of models A, B, C, and D in the training set of 36% to 60%, 36% to 66%, 36% to 80%, and 36% to 78%, respectively (Supplementary Fig. 2). The DCA decision curve indicated threshold probabilities of models A, B, C, and D in the validation set of 34% to 43%, 34% to 60%, 34% to 77%, and 34% to 71%, respectively (Supplementary Fig. 2).
By calculating the NRI of the continuous variables in the training set, the cutoff was 0.327 (0.727 to 0.796), model C was better than model D, model D was better than model B, model B was better than model A (Supplementary Fig. 3). In the validation set, the cutoff was 0.556 (0.910 to 0.654), model D was better than model C, model C was better than model B, model B was better than model A (Supplementary Fig. 3). After calculating the IDI of the continuous variables based on the training set, model C was better than model D, model D was better than model B, model B was better than model A. Based on the validation set, model C was better than model D, model D was better than model B, model B was better than model A. Therefore, models C and D were improved compared with models A and B, indicating that the characteristic risk factors included in models C and D met the clinical prediction modeling standard (Table 3).
DISCUSSION
Four DPN models were established in this study. According to the results, models C and D were more excellent models. Since the selected variables included in models A and B were all significant in the logistic regression analysis, these factors in model A and model B significantly correlated with DPN. Models C and D were validated by constructing an ROC curve and calibration curve, and were compared with NRI and IDI models, indicating that the accuracy of these two models was significantly better than model A and model B. Therefore, the influencing factors included in model C and model D are the risk factors that patients with T2DM presenting with DPN must closely monitor. According to the four models, seven factors, FBG, PBG, LDL-C, age, TC, BMI, and HbA1c, appear in two or more models and significantly modulate DPN.
Although most of the factors ultimately obtained in this study have a certain coincidence and similarity with the results of previous studies, this study differs from the perspective of statistical research methods and the previous studies using a logistic regression analysis alone. We tried to use the new statistical methods and models to study the risk factors for DPN based on previous studies and explained the problem from different perspectives. Previous researches have used a single factor analysis to validate a multivariate analysis or performed stepwise regression and recycling logistic regression analyses of the process to obtain the results. For example, Pai et al. [6] used a multivariate logistic regression analysis to explore the risk factors for DPN in patients with T2DM by investigating the prevalence of and biochemical risk factors for DPN in patients with or without neuropathy. After adjusting for all other potential confounding factors, Khawaja et al. [1] performed a binary logistic regression analysis to determine independent predictors of peripheral neuropathy. However, in this process, various confounding factors must be considered as variables along with the problem of multicollinearity. In the present study, the LASSO regression analysis provided a better solution to this problem with more accurate results. The greatest difference between this method and previous studies using a logistic regression analysis was that the population was randomly divided into groups at a 3:1 ratio for external verification. Variables were screened using the LASSO regression analysis, and a traditional logistic regression analysis was also performed. ROC, calibration and DCA curves were constructed for the training and validation sets to verify the accuracy and stability of the two models. NRI and IDI were introduced to compare the models and assess their stability.
In fact, the present study lacks a review of other factors contributing to DPN in patients with T2DM, including smoking, alcohol consumption, diet, other lifestyle factors, some biochemical parameters, and some pharmacological parameters. For example, a cross-sectional survey showed a strong relationship between a family history of diabetes and the development of DPN [7]. Another survey of 2837 patients showed that insulin therapy, microalbuminuria and apparent albuminuria were independently related to DPN [6]. The leukocyte count is also related to DPN, while oral hypoglycemia will reduce the incidence of DPN [8]. The aforementioned factors, including smoking, alcohol consumption, and diet, were not included in the initial investigation of this study. Therefore, the team was unable to determine whether these factors would cause DPN in patients with T2DM. The research team will conduct a more detailed investigation by collecting samples and analyzing indicators in the population of patients with T2DM in the future and will further analyze the factors influencing peripheral neuropathy in Chinese patients with T2DM by including more people with T2DM and a more comprehensive list of factors.
As shown in the present study, SBP was one of the risk factors for DPN among patients withT2DM, consistent with previous studies. A systematic review showed a 2.6-fold higher SBP in T2DM patients with DPN than in T2DM patients without DPN [9]. Regardless of whether T2DM is accompanied by hypertension, an elevated SBP always increased the risk of DPN [10]. According to the study by Yokoyama et al. [11], the occurrence of diabetic neuropathy is significantly correlated with SBP.
In the data analysis, FBG was one of the important factors influencing the risk of comorbid DPN in T2DM patients. A study of 110 healthy individuals, 83 T2DM patients, and 65 patients with DPN concluded that the FBG was a risk factor for DPN [12]. Higher FBG are associated with a higher probability of developing DPN [8].
The higher LDL-C, the greater the risk of DPN in T2DM patients [13]. The blood viscosity of patients with diabetes increases because of the abnormal blood lipid levels, which impedes blood flow, results in the formation of a micro thrombus, and substantially affecting blood circulation [5]. An insufficient blood supply in the nervous system leads to an energy metabolism disorder, which substantially impairs the transmission of signals in the nervous system [14]. A study of T2DM patients in Taiwan identified elevated LDL-C as an independent risk factor for DPN [15]. The amount of filtrate produced by both kidneys per unit time is called the eGFR, which is an indicator of renal function. This study revealed a close relationship between the eGFR and DPN. Zhang et al. [16] analyzed 1,059 T2DM patients and observed a higher eGFR in the DPN group than in the non-DPN group. The eGFR is an important risk factor for concurrent DPN [16]. DPN is also related to FBG, the diabetes duration and a decreased eGFR [17].
With aging, the resistance of the human body will decrease, the level of organ function will decrease, and the incidence of many diseases will increase. A cross-sectional study identified an older age as a risk factor for DPN [6]. A survey in Myanmar also reported an increased risk of diabetic peripheral disease with aging [18]. Using multivariate logistic regression analysis, a survey of 248 patients with diabetes indicated that DPN was independently related to aging [19]. Sendi et al. [7] identified a significant correlation between DPN and increasing age. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study identified age as the most significant risk factor for DPN other than HbA1c levels [20].
A longer disease course increased the probability of T2DM patients diagnosed with DPN. A cross-sectional study showed a positive correlation between disease course and DPN [21]. The duration of diabetes and smoking were significant risk factors for DPN [18]. By performing a logistic regression analysis, Khawaja et al. [1] showed that long-standing diabetes (≥5 years of diabetes) was significantly associated with DPN.
TC is the sum of the cholesterol contained in all lipoproteins in the blood. The analysis performed in this study concluded that TC were associated with DPN. A study of 200 patients with T2DM showed a significant correlation between a TC >5.2 mmol/L and DPN [22].
T2DM patients with DPN are at risk of developing foot ulcers, which substantially affect the quality of life of the patients. The plantar pressure is abnormal in T2DM patients presenting with DPN [23], and the plantar pressure is related to ulcers [24]. In other words, plantar pressure is related to the risk and severity of peripheral neuropathy. Some studies conducted in western countries have shown that obesity (BMI >30 kg/m2) causes plantar hypertension [25,26]. Therefore, obese patients with T2DM are more likely to suffer from DPN. In the study by Zhen et al. [27], the prevalence of DPN was 62.62% in obese patients with T2DM and 46.99% in patients with T2DM presenting a normal weight. The prevalence of DPN was significantly different between the two groups [27]. The possible pathogenic mechanism is that the obese patients are too heavy, which increases the pressure on the sole of the foot. This increased pressure causes the direct mechanical destruction of the soft tissue of the foot, ischemia and necrosis due to longterm local tissue compression and repeated forces that induce inflammation [28].
According to the results of the LASSO and logistic regression analyses, PBG are strongly correlated with the risk of DPN in T2DM patients. A related study using a multivariate logistic regression analysis of risk factors for DPN concluded that the 2-hour PBG was positively correlated with DPN [12].
Based on the results of the present study, HbA1c were significantly related to DPN, consistent with other studies. Pai et al. [6] noted significant differences in age, the disease course, and HbA1c between patients with and without DPN. A survey of 37,375 people concluded that T2DM patients and HbA1c greater than 7.0% had an increased risk of DPN. Both T2DM and HbA1c have a linear relationship with DPN [21]. Another group survey also described an association of HbA1c with DPN in patients with diabetes [26]. A study of 388 T2DM patients identified a positive correlation between HbA1c and DPN by performing a multivariate logistic regression analysis [27]. Under normal conditions, the human body can maintain a certain blood sugar level through hormonal and nerve regulation, but under the joint actions of genetic factors and environmental factors (such as an unreasonable diet, obesity, etc.), the regulatory function will be disrupted and the blood sugar level will increase. Short-term and single hyperglycemic event do not cause serious damage to the human body. However, long-term hyperglycemia will cause pathological changes in various tissues and organs of the body, leading to acute and chronic complications, such as decreased resistance, impaired renal function, neuropathy, fundus diseases, cardiovascular and cerebrovascular diseases, and diabetic foot, among others. Therefore, effective control of HbA1c is helpful to protect against DPN in T2DM patients.
The higher the UA, the higher the risk of cooccurring DPN in T2DM patients, consistent with the results of the study by Papanas et al. [29]. A study by Lin et al. [22] showed a significant correlation between elevated blood UA and DPN, indicating that this parameter is a predictor of DPN. Serum UA were significantly elevated in a meta-analysis of patients with diabetes. Hyperuricemia is significantly associated with an increased risk of DPN, and hyperuricemia is associated with an increased risk of peripheral blood disorders [30]. A positive correlation has been observed between TCSS scores and UA levels [5]. However, further studies are needed to determine whether UA is involved in the pathogenesis of peripheral neuropathy in patients with T2DM.
This paper has suggested an association between the waist circumference and DPN. A study of diabetes in a young follow-up cohort showed that an increase in waist circumference was significantly associated with DPN [31]. A Danish study also observed associations between a greater weight, waist circumference, and baseline BMI with DPN [32]. An analysis of potential confounding factors for neuropathy by Aubert et al. [33] also showed that the waist circumference was independently associated with peripheral neuropathy. Another Chinese study divided 100 middle-aged subjects into a group of healthy subjects, a group of subjects with T2DM but without DPN within the last 5 years, and a group of subjects with T2DM who were diagnosed with DPN within the last 5 years. DPN was significantly correlated with serum levels of biochemical indicators (TG and HbA1c) and anthropometric indicators (weight and waist circumference) [34]. Oh et al. [35] concluded that subjects with DPN had a higher BMI and greater waist circumference than subjects without DPN, suggesting an association between abdominal obesity and DPN.
Hypertriglyceridemia is a disorder in the synthesis or degradation of heterologous TG. It is an important risk factor for the occurrence of diseases related to metabolic syndrome, such as coronary heart disease, hypertension and diabetes. We concluded that the TG is one of the risk factors for DPN. A higher TG correlates with a greater risk [15]. According to another study, hypertriglyceridemia is an independent risk factor for DPN in obese T2DM patients [27]. Three different groups of subjects were analyzed in a study conducted in Taiwan using the percussion impact entropy index (PEIppi). A significant correlation was observed between TG and DPN [34]. A high TG tends to cause “consistence,” namely, a change in the blood viscosity caused by a high lipid content in the blood, deposits on the blood vessel wall, and the gradual formation of small plaques known as atherosclerosis. However, the area and thickness of these massive deposits on the wall of the blood vessel will gradually increase, resulting in a decrease in the internal diameter of the blood vessel, a slower blood flow, and an acceleration of the process of blocking the blood vessel that may even interrupt the blood flow in serious cases. In addition to the interruption of blood flow, the obstruction might also cause a thrombus. If a thrombus occurs in the lower extremities, the blood flow of the extremities is not impaired, leading to necrosis. A 7-year follow-up survey of 8,379 people suggested that reducing cardiovascular risk factors may help prevent DPN. Cardiovascular risk factors, including hypertension and high TG, were positively correlated with DPN.
HDL-C correlated with DPN in the present study. Lower HDL-C correlated with a greater risk of DPN in patients with T2DM. In a comparative study by Sun et al. [36], HDL-C in the diabetic group was lower than in the healthy group. In fact, HDL-C exerts an anti-inflammatory effect when present at normal levels. In T2DM patients, a decrease in HDL-C will reduce its anti-inflammatory effect or even promote inflammation [37]. The specific mechanism is that the low HDL-C in T2DM patients activates monocytes and increases the secretion of tumor necrosis factor α (TNF-α). TNF-α is a key factor contributing to the development of atherosclerosis [38]. In other words, HDL-C is transformed into atherogenic granules in patients with T2DM [39] to modulate the cardiovascular function, subsequently causing pathological changes in the nervous system due to the lack of an energy supply.
The study still had some limitations. Regarding the samples, the T2DM patients analyzed were recruited from only one community in Shanghai, which does not represent the conditions of all T2DM patients in Shanghai. T2DM patients who were treated at the hospital or at home were unable to participate in the study. This study also lacked information on other potential risk factors for DPN, including lifestyle factors and drug indicators. The patients who were newly diagnosed with DPN based on the TCSS score may have been false positives. Because DPN is related to other complications of T2DM, the team will incorporate relevant factors associated with these diseases and strive to establish a more perfect DPN prediction model in future studies.
In the present study, models A, B, C, and D were established. Based on the NRI and IDI, model C and D are better predictive models. Thus, the influencing factors included in model C and D are more important risk factors for T2DM patients. FBG, PBG, LDL-C, age, TC, BMI, and HbA1cwere appeared in two or more models and significantly contributed to the risk of DPN.
Supplementary Materials
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0100.
TCSS score of diabetic peripheral neuropathy
Coefficients and lambda.1se value of different LASSO in training set
Comparison of ROC among four models
The pooled area under curves (AUCs) of the receiver operating characteristic curves for models A, B, C, and D. (A) Training set analyzed using model A, (B) model B, (C) model C, and (D) model D. The y-axis indicates the true positive rate of the risk prediction. The x-axis indicates the false positive rate of the risk prediction. The blue line represents the performance of the nomogram. (E) Validation set analyzed using model A, (F) model B, (G) model C, and (H) model D. The y-axis indicates the true positive rate of the risk prediction. The x-axis indicates the false positive rate of the risk prediction. The blue line represents the performance of the nomogram. (I) Training set and (J) validation set analyzed using models A, B, C, and D. The yaxis indicates the true positive rate of the risk prediction. The x-axis indicates the false positive rate of the risk prediction. The black line represents the performance of the nomogram in model A, and the red line represents the performance of the nomogram in model B. The green line represents the performance of the nomogram in model C, and the blue line represents the performance of the nomogram in model D.
Calibration curves of the diabetic peripheral neuropathy (DPN) incidence risk prediction nomogram in the array and decision curve analysis of the incidence risk nomogram of DPN. (A) Training set analyzed using model A, (B) model B, (C) model C, and (D) model D. The x-axis represents the predicted incidence risk. The y-axis represents the actual diagnosis of DPN. The diagonal dotted line represents a perfect prediction by an ideal model. The solid line represents the performance of the nomogram; a closer fit to the diagonal dotted line represents a better prediction. (E) Validation set analyzed using model A, (F) model B, (G) model C, and (H) model D. The x-axis represents the predicted incidence risk. The y-axis represents the actual diagnosis of DPN. The diagonal dotted line represents a perfect prediction by an ideal model. The solid line represents the performance of the nomogram; a closer fit to the diagonal dotted line represents a better prediction. (I) Training set and (J) validation set. The x-axis represents the threshold probability. The y-axis indicates the net benefit. The thin solid line represents the assumption that all patients are diagnosed with DPN. The thick solid line represents the assumption that no patients are diagnosed with DPN. The black dotted line represents the incidence risk nomogram of DPN in model A, and the red dotted line represents the incidence risk nomogram of DPN in model B. The green dotted line represents the incidence risk nomogram of DPN in model C, and the blue dotted line represents the incidence risk nomogram of DPN in model D.
Comparison of the models using net classification improvement. (A) Model B is 0.161 better than model A. (B) Model C is 0.330 better than model A. (C) Model D is 0.302 better than model A. (D) Model C is 0.169 better than model B. (E) Model D is 0.161 better than model B. (F) Model C is 0.013 better than model D. (G) Model B is 0.223 better than model A. (H) Model C is 0.348 better than model A. (I) Model D is 0.431 better than model A. (J) Model C is 0.193 better than model B. (K) Model D is 0.269 better than model B. (L) Model C is 0.039 better than model D.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: F.H.
Acquisition, analysis, or interpretation of data: F.H.
Drafting the work or revising: B.W., Z.N.
Final approval of the manuscript: B.W., Z.N., F.H.
FUNDING
None
Acknowledgements
The authors thank the Sanlin Community Health Center, which cooperated with us in this study, for helping to recruit participants, as well as all participants who volunteered to be studied. And thank the financial supportion of the fourth round of Shanghai Public Health Three-Year Action Plan Key Discipline Construction–Health Education and Health Promotion (Grant No. 15GWZK1002).