Long-Term Glycaemic Durability of Early Combination Therapy Strategy versus Metformin Monotherapy in Korean Patients with Newly Diagnosed Type 2 Diabetes Mellitus
Article information
Abstract
We assessed the glycaemic durability with early combination (EC; vildagliptin+metformin [MET], n=22) versus MET monotherapy (n=17), among newly-diagnosed type 2 diabetes mellitus (T2DM) enrolled (between 2012 and 2014) in the VERIFY study from Korea (n=39). Primary endpoint was time to initial treatment failure (TF) (glycosylated hemoglobin [HbA1c] ≥7.0% at two consecutive scheduled visits after randomization [end of period 1]). Time to second TF was assessed when both groups were receiving and failing on the combination (end of period 2). With EC the risk of initial TF significantly reduced by 78% compared to MET (n=3 [15%] vs. n=10 [58.7%], P=0.0228). No secondary TF occurred in EC group versus five patients (29.4%) in MET. Patients receiving EC treatment achieved consistently lower HbA1c levels. Both treatment approaches were well tolerated with no hypoglycaemic events. In Korean patients with newly diagnosed T2DM, EC treatment significantly and consistently improved the long-term glycaemic durability as compared with MET.
INTRODUCTION
Diabetes is a global epidemic with 60% of the affected population living in Asian countries [1]. In 2016, approximately 5.02 million adults were diagnosed with type 2 diabetes mellitus (T2DM) in Korea [2]. The prevalence is expected to increase, as approximately 8.7 million people have been diagnosed with impaired fasting glucose [2] and a quarter of these individuals have a high likelihood of developing T2DM over a period of 3 to 5 years [3].
As per the Korean Diabetes Association (KDA), 43.1% of all diagnosed patients with T2DM were reported to be untreated [2]. Among those undergoing treatment, 75% patients fail to achieve the KDA recommended glycosylated hemoglobin (HbA1c) target of <6.5% with current standard-of-care [2].
Although international guidelines recommend initial monotherapy for treatment of T2DM [4], studies have showed that treatment with initial combination of dipeptidyl peptidase 4 inhibitors (DPP4i) with metformin resulted in significant HbA1c reductions compared with a traditional stepwise approach [5].
The recent Vildagliptin Efficacy in combination with metfoRmIn For earlY treatment of type 2 diabetes (VERIFY) trial demonstrated that early intervention strategy with a combination therapy of vildagliptin (DPP4i) plus metformin in treatment-naïve patients with T2DM provides greater and more durable long-term clinical benefits compared with the current standard-of-care metformin monotherapy [6].
The aim of this regional analysis from the VERIFY study is to elucidate the clinical benefits of an early intervention approach in patients with newly diagnosed T2DM from Korea.
METHODS
Study design and patients
VERIFY was a Phase IV, randomized, double-blind, parallelgroup study involving patients (18 to 70 years) with T2DM (HbA1c, 6.5% to 7.5%) across 34 countries. Early combination (EC) treatment included metformin (stable daily dose of 1,000, 1,500, or 2,000 mg) and vildagliptin 50 mg twice daily, or standard-of-care initial metformin monotherapy and placebo twice daily (Supplementary Fig. 1). The detailed study design has been published previously [7].
The VERIFY study enrollment period was from March 30, 2012 to April 10, 2014. The last trial visit was on April, 2019. The study was performed in accordance with the ethical principles laid down in the Declaration of Helsinki and was registered with ClinicalTrials.gov (NCT01528254). The results for the VERIFY study have been posted on ClinicalTrials.gov and EU Clinical Trials Register (www.clinicaltrialsregister.eu). This study was approved by the Institutional Review Board of Catholic Medical Center, College of Medicine, The Catholic University of Korea (IRB no. F1-06-2). Written informed consent was obtained from all patients.
Assessments
The primary efficacy endpoint was the time to confirmed initial treatment failure, defined as HbA1c ≥7.0% at two consecutive planned post-randomization visits, 13 weeks apart. Secondary endpoints were time to second treatment failure (two consecutive values of HbA1c ≥7.0% when all patients were receiving combination therapy); change in HbA1c (%) from baseline; and safety and tolerability. Adverse events (AEs) and serious AEs were recorded, with their severity and relationship to study drug over the entire study duration. The detailed statistical analysis of the VERIFY study was published earlier [8]. A P value of 0.05 (2-sided) was considered significant. The statistics program used was SAS versions 9.2 and 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
A total of 39 eligible patients were randomized to receive either EC (n=22) or initial metformin monotherapy (n=17) and the majority (n=28, 71.8%) completed the 5-year study. The overall median (interquartile range [IQR]) age for Korean patients was 53.5 years (IQR, 46.0 to 59.0) with 48.7% being females with a mean±standard deviation body mass index (BMI) of 26.3±2.6 kg/m2. Median duration of T2DM was 3.2 months (IQR, 0.8 to 8.0) in the EC group and 1.7 months (IQR, 0.1 to 9.0) in the monotherapy group. The other baseline demographics and clinical characteristics were similar between the two treatment groups (Supplementary Table 1).
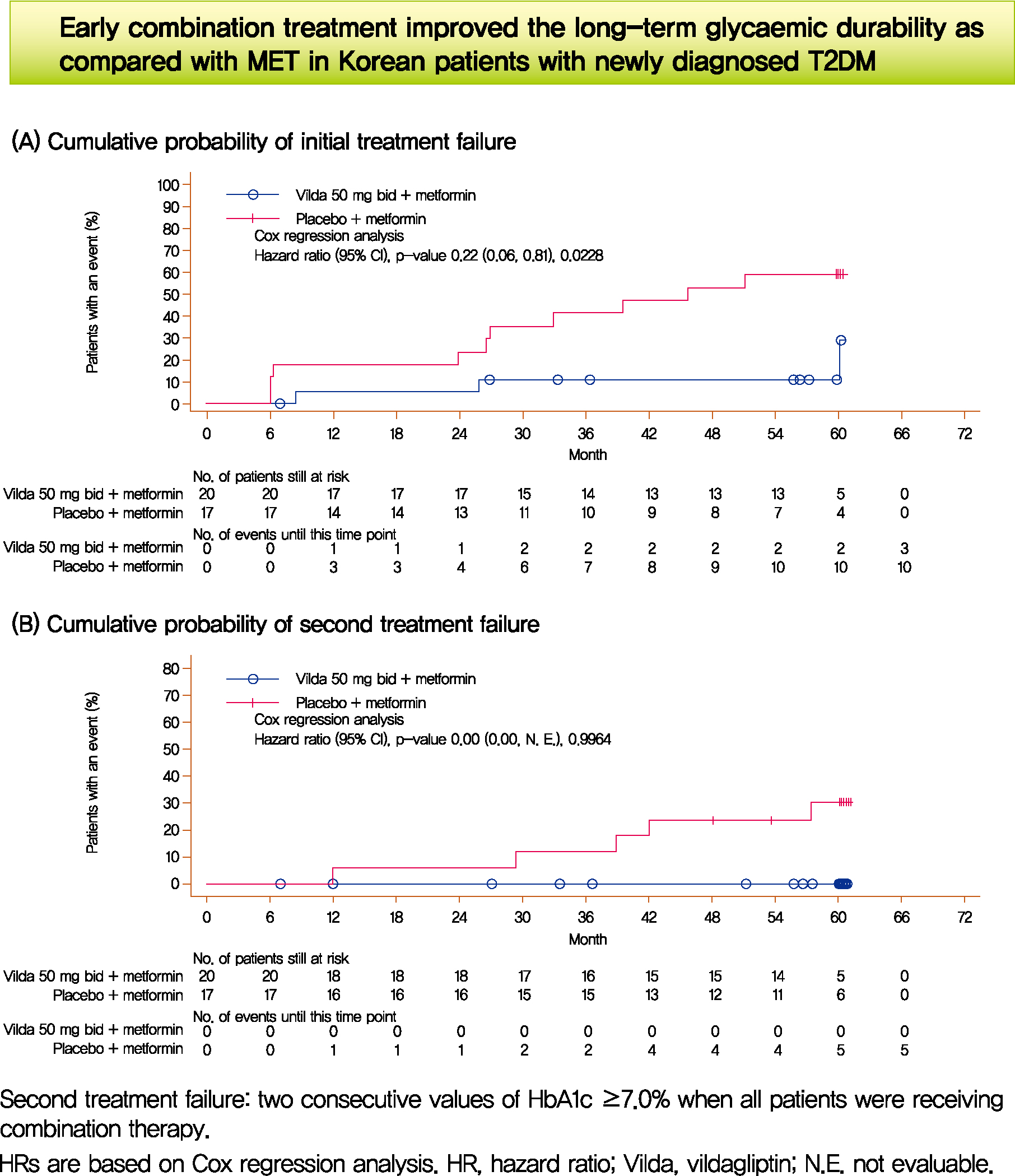
At the end of the study, 15% (n=3) of the patients in EC group versus 58.8% (n=10) in the monotherapy group attained the primary end-point (P=0.0228). The median observed time to treatment failure in the monotherapy group was 45.7 months (IQR, 26.6 to 59.9) compared with 58.5 months (IQR, 30.1 to 60.0) for those receiving EC. These three patients in the EC group with initial treatment failure later attained glycaemic control (HbA1c <7%) without insulin treatment. In the EC group, no patient experienced secondary treatment failure compared to five patients (29.4%) in the metformin monotherapy group (Fig. 1). Similar benefits with EC was shown across baseline subgroups (Supplementary Fig. 2).
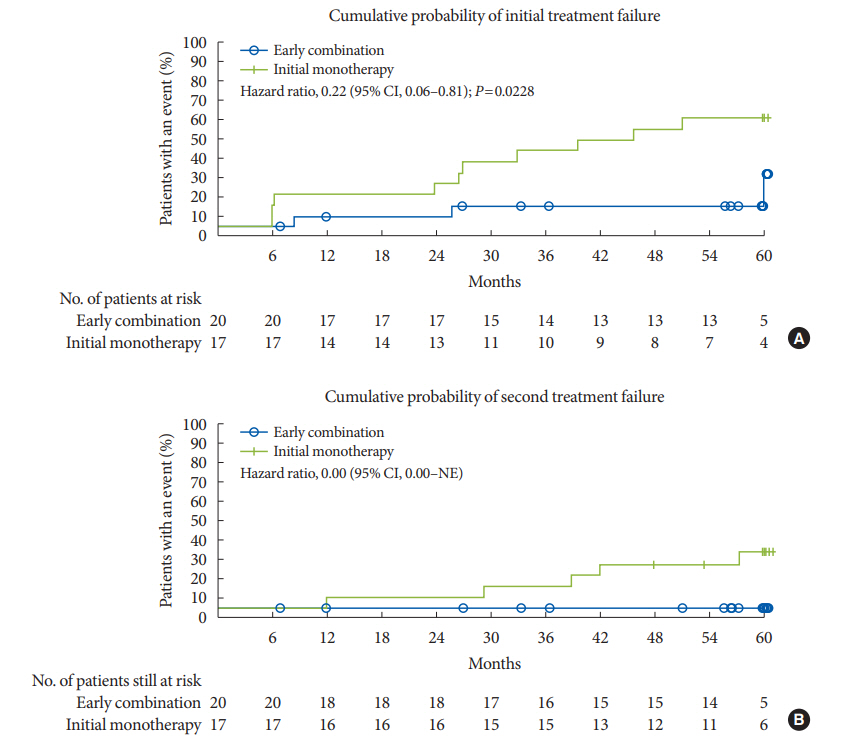
Time to treatment failure. (A) Cumulative probability of initial treatment failure. (B) Cumulative probability of second treatment failure. The Kaplan-Meier (KM) estimates were performed for patients who had received at least one randomized medication and one post-randomization efficacy parameter assessed. Thus, not all patients included in the KM analysis had data at month 0. Hazard ratios are based on Cox regression analysis. CI, confidence interval; NE, not evaluable.
Compared to metformin monotherapy a greater proportion of patients with EC presented with HbA1c cut-off values <7.0%, <6.5%, and <6.0% over 5 years (Supplementary Fig. 3). Overall the pattern of HbA1c reduction in the EC group had a characteristic rapid and sustained decline immediately after randomization (Fig. 2). Glycaemic control and long-term durability was better with EC than the initial monotherapy (Fig. 2) even if the analysis of coefficient of failure does not apply to the study design with protocol-defined rescue therapy implementation (vs. intention-to-treat).

A) Glycosylated hemoglobin (HbA1c) levels over 12 months by treatment approaches and (B) long-term glycaemic durability in patients without treatment failure in both treatment groups (those remaining in Period 1). (A) Early combination: This group includes all patients who started treatment with vildagliptin plus metformin. Initial monotherapy: This group includes all patients who started treatment with metformin plus placebo. The analysis was performed for patients who had received at least one randomized medication and one post-randomization efficacy parameter assessed. (B) Patients failing on initial monotherapy: These patients received combination therapy after initial treatment failure with metformin monotherapy. Patients not failing on initial monotherapy: These patients continued with metformin monotherapy till the end of the study. Patients failing on early combination: These patients continued to receive combination therapy until secondary treatment failure. Patients not failing on early combination: These patients continued with the combination therapy till the end of the study.
No hypoglycaemic events were reported in Korean patients and no AE led to treatment discontinuation. Both treatment approaches were well tolerated with no new safety findings (Supplementary Table 2).
DISCUSSION
This regional analysis of the VERIFY study has shown that EC with vildagliptin plus metformin improved glycaemic durability in Korean patients with newly diagnosed T2DM compared with standard-of-care initial metformin monotherapy followed by sequential combination with vildagliptin. Implementation of EC treatment strategy significantly reduced the risk of time to initial treatment failure among Korean participants by 78% compared to the metformin monotherapy throughout the 5-year study duration.
A recent Korean study reported that only 25% of patients with T2DM receiving standard-of-care achieved the treatment goal of HbA1c <6.5% in clinical practice [2], whereas 85% of Korean patients achieved long-term glycaemic control with EC in this regional analysis. Furthermore, the lack of secondary oss of glycaemic control among those few failing on EC supports the overall conceptual success and claims of long-term durability with this rather novel combination therapy approach. A rapid reduction of HbA1c with EC within the first 3 months was observed along with well-maintained long-term glycaemic durability. This suggests that lowering HbA1c around 6.0% with EC might be recommended for management of newly diagnosed patients with T2DM. These clinical observations led to the expected, rather recent updates in the position statement from the American Diabetic Association and European Association for the Study of Diabetes mentioning that healthcare providers should engage in shared decision making around initial combination therapy in new-onset cases of T2DM [9].
This regional analysis also demonstrated long-term glycaemic control with EC without any hypoglycaemic events despite sustained attainment of low HbA1c levels. This can be attributed to vildagliptin which leads to improved, glucose-dependent response by both α- and β-pancreatic cells, which in turn contributes to its low hypoglycaemic potential [10].
Diabetes pathophysiology and management requirements in the Asian population differ from that of Western population. Early insulin secretion failure, rather than insulin resistance plays a primary role in development of T2DM in the Asian population [11-14]. Also, diabetes in the Asian population is characterised by a younger age and lower BMI [15-17], which is reflected in this analysis of Korean patients with T2DM when compared to the global VERIFY population (Supplementary Table 3) [18].
The results of this regional analysis should be extrapolated with caution due to the small sample size, narrow HbA1c cutoff (6.5% to 7.5%) at baseline and the assessment of only one treatment combination. Other studies addressing individualised EC for all the possible permutations of the recommended oral therapeutic alternatives must follow for solving the questions regarding the generalisability of the VERIFY study results.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0173.
Patient characteristics and disposition
Adverse events and serious adverse events in the study population
Comparison between the global VERIFY study and the Korean data in terms of results, patient baseline characteristics and adverse events
Study design. The duration of period 1 can differ between the two treatments. HbA1c, glycosylated hemoglobin. Adapted from Del Prato et al. [7].
Subgroup analysis of time to initial treatment failure. Hazard ratios (HRs) and the associated confidence intervals (CIs) and P values were obtained from a Cox proportional hazards model containing terms for treatment approach, geographical region, and baseline glycteraction P values are provided for tests of homogeneity of between-group differences among subgroups, with no adjustment for multiple testiosylated hemoglobin (HbA1c). Significance was established on the basis of a two-sided 0.05 significance level. The treatment-by-subgroup inng. BMI, body mass index; GFR, glomerular filtration rate; NE, not evaluable. aThe analysis was performed for patients who had received at least one randomized medication and one post-randomization efficacy parameter assessed, bTwo patients were of Korean mixed race, so were not included in the predominant race category and were denoted as ‘others’, cGFR was estimated using the Modification of Diet in Renal Disease (MDRD) formula. Baseline GFR is calculated using the serum creatinine and body weight value at the Day 1 measurement, or the value obtained at an earlier visit (scheduled or unscheduled) which was closest to Day 1, if the Day 1 measurement is missing. Age is the value at screening. The P value for treatment comparison in the overall population is also provided.
Success rate of early combination and initial monotherapy approaches for (A) glycosylated hemoglobin (HbA1c) <7.0%, (B) HbA1c <6.5%, and (C) HbA1c <6.0% over the entire study duration.
Notes
CONFLICTS OF INTEREST
Soon-Jib Yoo, Sang-Ah Chang, Tae Seo Sohn, Hyuk-Sang Kwon, Jong Min Lee, and Sungdae Moon have nothing to disclose. Pieter Proot is employed by and own stocks in Novartis. Päivi M. Paldánius was the medical lead of the VERIFY study and employed by Novartis Pharma AG at and up to the time of study completion. Kun Ho Yoon has served as a consultant for Novo Nordisk and MSD; received honorarium as a speaker from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceutical, MSD, Novo Nordisk, Sanofi, and Takeda; and received research support from AstraZeneca and Takeda.
AUTHOR CONTRIBUTIONS
Conception or design: K.H.Y
Acquisition, analysis, or interpretation of data: S.J.Y., S.A.C., T.S.S., H.S.K., J.M.L., S.M., P.P., P.M.P., K.H.Y.
Drafting the work or revising: S.J.Y., S.A.C., T.S.S., H.S.K., J.M.L., S.M., P.P., P.M.P., K.H.Y.
Final approval of the manuscript: K.H.Y.
FUNDING
None
Acknowledgements
The authors thank Ritika Paul and Shaswati Khan, Novartis Healthcare Pvt. Ltd., India for medical writing support and Hana Kim, Novartis Korea, for medical advice during the manuscript preparation.
Novartis is committed to sharing with qualified external researchers, access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel based on scientific merit. All data provided are anonymised to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The criteria and process for trial data availability are described online.