Sodium-Glucose Cotransporter-2 Inhibitor for Renal Function Preservation in Patients with Type 2 Diabetes Mellitus: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement
Article information
Abstract
Diabetes is a leading cause of end-stage renal disease. Therefore, prevention of renal dysfunction is an important treatment goal in the management of diabetes. The data of landmark cardiovascular outcome trials of sodium-glucose cotransporter-2 (SGLT2) inhibitor showed profound reno-protective effects. The Korean Diabetes Association and the Korean Society of Nephrology reviewed clinical trials and performed meta-analysis to assess the effects of SGLT2 inhibitors on the preservation of estimated glomerular filtration rate (eGFR). We limited the data of SGLT2 inhibitors which can be prescribed in Korea. Both eGFR value and its change from the baseline were significantly more preserved in the SGLT2 inhibitor treatment group compared to the control group after 156 weeks. However, some known adverse events were increased in SGLT2 inhibitor treatment, such as genital infection, diabetic ketoacidosis, and volume depletion. We recommend the long-term use SGLT2 inhibitor in patients with type 2 diabetes mellitus (T2DM) for attenuation of renal function decline. However, we cannot generalize our recommendation due to lack of long-term clinical trials testing reno-protective effects of every SGLT2 inhibitor in a broad range of patients with T2DM. This recommendation can be revised and updated after publication of several large-scale renal outcome trials.
INTRODUCTION
Diabetic kidney disease (DKD) is a global problem, and the prevalence and incidence are increasing strikingly. Along with the accelerating incidence of DKD, the total number of patients with end-stage renal disease (ESRD) undergoing maintenance dialysis has grown by approximately 7% to 10% per year in Korea [123]. DKD is the most prevalent cause of ESRD (50.2% of new ESRD patients in 2016) in Korea from 1994 [4]. All current efforts for type 2 diabetes mellitus (T2DM) are devoted to the control of hyperglycemia to prevent the development of micro- and macrovascular complications. The cornerstone of therapy to prevent DKD is the strict control of blood pressure with the renin-angiotensin-aldosterone system (RAAS) blockade and blood glucose levels [5]. However, many patients with diabetes progress to chronic kidney disease (CKD) despite standard treatment. These kinds of patients usually have profound amounts of albuminuria despite the use of RAAS blocking agents, and renal function declines rapidly. Furthermore, reduced estimated glomerular filtration rate (eGFR) is independently associated with all-cause mortality and cardiovascular disease [6]. There is an unmet clinical need for diabetes treatment to prevent or delay DKD progression.
Sodium-glucose cotransporter-2 (SGLT2) inhibitor is an emerging antidiabetic medication, and its cardio-protective effect has been proven from the large-scale cardiovascular outcome trials of Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes (EMPA-REG) OUTCOME [7], Canagliflozin Cardiovascular Assessment Study (CANVAS) Program [8], and Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) [9]. In these studies, renal outcome was analyzed as a secondary outcome. Empagliflozin showed 49% reduction of incident or worsening nephropathy (progression to macroalbuminuria, doubling of the serum creatinine level, initiation of renal-replacement therapy, or death from renal disease) [10], and dapagliflozin reduced 47% of renal-specific outcomes (a sustained decline of at least 40% in eGFR to less than 60 mL/min/1.73 m2, ESRD, or death from renal or cardiovascular causes) [11]. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial [12] was the first trial to show its reno-protective effects as a primary outcome from a large-scale randomized clinical trial (among 4,401 subjects with T2DM). However, canagliflozin is not available in Korea. Therefore, it is necessary to investigate the renal benefits of SGLT2 inhibitors, which can be prescribed in Korea [1314]. In real clinical practice, we monitored patient's renal function using eGFR as described in other clinical trials assessed it. Therefore, among various renal outcomes, we aimed to analyze the eGFR after long-term treatment of SGLT2 inhibitors.
RECENT RECOMMENDATIONS REGARDING SGLT2 INHIBITOR USE IN PATIENTS WITH T2DM
In August 2019, the American Diabetes Association revised the online version of the Standards of Medical Care in Diabetes, adopting the results of the CREDENCE trial [15]. It recommended to consider SGLT2 inhibitor treatment in patients with T2DM and CKD (level of evidence C). After that, it updated the recommendation level to level of evidence A and specified the indication of SGLT2 inhibitor, for patients with T2DM and DKD with eGFR ≥30 mL/min/1.73 m2 and albuminuria in 2020 [16]. Diabetes Canada also recommended SGLT2 inhibitor in T2DM with clinical cardiovascular disease and with eGFR ≥30 mL/min/1.73 m2 to reduce the risk of progression of CKD (Grade B, level 2 for empagliflozin and Grade C, level 3 for canagliflozin) in 2018 [17]. The Committee of Clinical Practice Guideline of the Korean Diabetes Association updated the 6th Clinical Practice Guideline in 2019. In this guideline, SGLT2 inhibitor was recommended as a second-line drug, and the committee emphasized its cardioprotective effect [18]. However, it did not yet contain renal outcomes of SGLT2 inhibitor. In this statement, we firstly announced the reno-protective effects of SGLT2 inhibitor considering the situation of Korea.
EFFECTS OF SGLT2 INHIBITOR ON RENAL FUNCTION
Results of cardiovascular outcome trials using SGLT2 inhibitors
Neuen et al. [19] reported the meta-analysis data of EMPA-REG Outcome, CANVAS Program, CREDENCE, and DECLARE-TIMI 58. This meta-analysis demonstrated that SGLT2 inhibitors reduced the risk of ESRD and death due to kidney disease (relative risk of 0.67). This beneficial effect has been shown consistently across baseline eGFR levels in those studies. However, a meta-analysis result excluding CREDENCE trial showed that the reno-protective effects were attenuated in patients with more advanced renal dysfunction at baseline eGFR below 60 mL/min/1.73 m2 [20]. The inclusion and exclusion criteria of the original randomized clinical trials were not the same. For example, the EMPA-REG trial [10] included subjects with their eGFR of at least 30 mL/min/1.73 m2, in contrast DECLARE-TIMI 58 study [11] included subjects whose eGFR were equal to or higher than 60 mL/min/1.73 m2. In this regard, 25.5% of subjects in the EMPA-REG study and only 7.4% of participants in DECALRE-TIMI 58 had eGFR <60 mL/min/1.73 m2. Furthermore, a large number of participants were not followed up, and Asian patients made up the minority of participants in each study, 21.6% in the EMPA-REG study and 13.4% in the DECLARE-TIMI 58 study. For this reason, uncertainty remains about the reno-protective effect of SGLT2 inhibitor in Asian populations with a broad range of renal function, especially in subjects with reduced renal function. In this study, we reviewed current evidence considering patients' baseline renal function and performed meta-analysis to determine whether SGLT2 inhibitor can be recommended to Korean subjects with T2DM.
Meta-analysis of large clinical trials using SGLT2 inhibitors
Search strategy and selection criteria
We searched PubMed, Embase, and Cochrane Central Register of Controlled Library up to March 2020, using search terms including dapagliflozin, empagliflozin, ipragliflozin, and ertugliflozin, which are drugs available in Korea. We included long-term large-scale randomized placebo controlled trials whose treatment duration was at least 52 weeks in T2DM with more than 100 participants in total. No studies on ipragliflozin and ertugliflozin met these criteria. Finally, we included 12 articles for 11 clinical studies (Supplementary Table 1) [91021222324252627282930]. In this meta-analysis, final eGFR level or delta eGFR from baseline was compared between SGLT2 inhibitor treatment group and placebo-control according to the method presented in the original studies, and we analyzed the data of low and high doses of empagliflozin separately.
Effect of SGLT2 inhibitor on eGFR
First, we compared the eGFR levels at each time point between SGLT2 inhibitor and control. As shown in Fig. 1A, SGLT2 inhibitor treatment showed a lower eGFR level than the control at 12 weeks, −1.81 mL/min/1.73 m2 (95% confidence interval [CI], −3.03 to −0.58) and at 24 weeks, −1.33 mL/min/1.73 m2 (95% CI, −2.52 to −0.15). However, a favorable effect on eGFR was observed after at least 156 weeks of treatment. At 208 weeks of treatment, the mean difference of eGFR was 3.96 mL/min/1.73 m2 (95% CI, 3.13 to 4.80) between groups. In general, the improving trend of eGFR was observed in the SGLT2 inhibitor group as the treatment duration was prolonged. In terms of eGFR change from baseline, mean difference was 1.42 mL/min/1.73 m2 (95% CI, 0.42 to 2.41) at 156 weeks (Fig. 1B). Therefore, SGLT2 inhibitor treatment showed reno-protective effects after long-term treatment.
Effect of SGLT2 inhibitor on glycosylated hemoglobin, body weight, and blood pressure
The glucose lowering effect of SGLT2 inhibitors was consistently observed: the mean difference in decline of glycosylated hemoglobin (HbA1c) was −0.54 (95% CI, −0.67 to −0.41) at 52 weeks and −0.62 (95% CI, −0.75 to −0.48) at 104 weeks (Fig. 2A). The magnitude of body weight reduction was also greater in SGLT2 inhibitor treatment than control treatment (Fig. 2B). Both systolic and diastolic blood pressure were more decreased in SGLT2 inhibitor treatment (Fig. 2C and D).
Adverse effects of SGLT2 inhibitor
There was no difference between SGLT2 inhibitor and control groups in hypoglycemia events and urinary tract infection (Fig. 3A and B). However, more subjects were diagnosed with genital infection (risk ratio of 3.34) (Fig. 3C) and diabetic ketoacidosis (risk ratio 2.22) (Fig. 3D). Acute kidney injury was less in SGLT2 inhibitor compared to control (risk ratio 0.71) (Fig. 3E), but volume depletion was slightly more common in the SGLT2 inhibitor group (risk ratio 1.16) (Fig. 3F).
Effects of SGLT2 inhibitor on renal function among patients with eGFR below 60 mL/min/1.73 m2
The clinical studies we included for this systemic review and meta-analysis did not enroll a sufficient number of patients with an eGFR below 60 mL/min/1.73 m2. The patients with eGFR below 60 mL/min/1.73 m2 included 7.4% of participants in the dapagliflozin study (DECLARE-TIMI 58) and 25.5% of participants in the empagliflozin study (EMPA-REG). Dapagliflozin did not demonstrate the prevention of eGFR decline in patients with an eGFR below 60 mL/min/1.73 m2 compared to placebo group during 4-year follow-up (P=0.053) [11]. This negative result for preventing the eGFR decline with dapagliflozin could be due to an insufficient number of enrolled patients with an eGFR below 60 mL/min/1.73 m2 since dapagliflozin reduced the rate of eGFR decline in patients with an eGFR above 60 mL/min/1.73 m2. In the empagliflozin study, patients with eGFR below 60 mL/min/1.73 m2 treated with the empagliflozin showed higher eGFR compared to the placebo group from 156 weeks, while the analysis with total enrolled patients showed higher eGFR at 104 weeks (Fig. 4A vs. Fig. 1A). In terms of eGFR change from baseline, SGLT2 inhibitors showed beneficial effect at 208-week treatment compared to control (Fig. 4B). Both SGLT2 inhibitor studies revealed the initial decrease of the eGFR after SGLT2 inhibitors treatment since the mechanism of SGLT2 inhibitors may be due to relieving vasodilation of the afferent arteriole and following decrease of glomerular hyperfiltration in diabetes [31]. This initial decrease of the eGFR after SGLT2 inhibitor treatment was more significant in patients with an eGFR below 60 mL/min/1.73 m2.
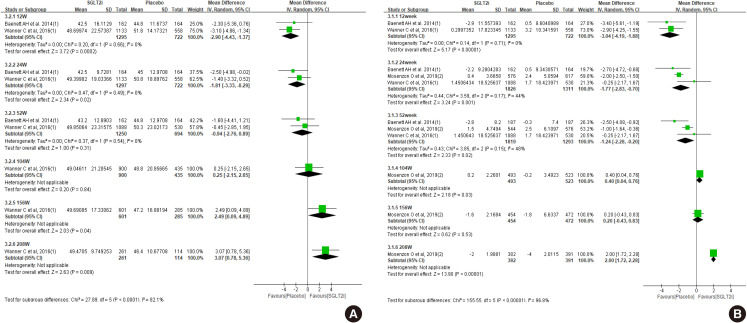
Results of patients with estimated glomerular filtration rate (eGFR) below 60 mL/min/1.73 m2. Effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on (A) eGFR, and (B) change of eGFR from baseline. SD, standard deviation; IV, inverse variance; CI, confidence interval.
The risk of a sustained decrease in eGFR by at least 40% to less than 60 mL/min/1.73 m2, ESRD, or renal death was lower in the dapagliflozin group than those in the placebo group, but there was no statistical significance in patients with eGFR below 60 mL/min/1.73 m2 (hazard ratio, 0.60; 95% CI, 0.35 to 1.02; P=0.059) [11]. In the EMPA-REG study, incident or worsening nephropathy was significantly lower in the empagliflozin group with eGFR below 60 mL/min per 1.73 m2 than in the placebo group (hazard ratio, 0.58; 95% CI, 0.47 to 0.71; P<0.001) [10]. The adverse events in the EMPA-REG study were similar between the empagliflozin group and the placebo group in patients with an eGFR of 60 mL/min/1.73 m2 or more and in patients with an eGFR of 59 mL/min/1.73 m2 or less. In our meta-analysis, patients with eGFR <60 mL/min/1.73 m2 showed less hypoglycemia and more genital infection in SGLT2 inhibitor treatment compared to control (Supplementary Fig. 1).
Results of Asian dominant study
We arbitrarily defined Asian-dominant study when Asians made up more than 40% of total subjects. Kaku et al. [21] analyzed the subgroup data of the Asian population from the EMPA-REG OUTCOME trial, which was the longest and largest trial in our meta-analysis. Asian-dominant studies showed no significant difference in eGFR change between groups (Fig. 5A). Only one study was analyzed after 156-week treatment in this meta-analysis; therefore, it is not conclusive whether Asians may experience similar benefits of renal preservation in long-term treatment of SGLT2 inhibitor compared to the non-Asian population. Other parameters, such as HbA1c, body weight, blood pressure, and adverse events were similar with meta-analysis of total population (Fig. 5B, Supplementary Fig. 2).
KOREAN DIABETES ASSOCIATION AND KOREAN SOCIETY OF NEPHROLOGY JOINT CONSENSUS STATEMENTS ON THE USE OF SGLT2 INHIBITOR IN T2DM FOR RENAL FUNCTION PRESERVATION
Long-term treatment of SGLT2 inhibitor has a preventive effect on decline of renal function in some patients with T2DM; therefore, long-term treatment of SGLT2 inhibitor is recommended under continuous monitoring of renal function (eGFR) (weak recommendation, low quality of evidence).
Strength of the recommendation
Study participants were mainly from Western countries. Their baseline body mass index was near 30 kg/m2, which is relatively higher than Korean subjects with T2DM. Furthermore, Asian dominant studies are lacking, and meta-analysis including only Asian dominant studies did not show statistically significant effects on renal preservation. In addition, only two studies included subjects whose eGFR was less than 60 mL/min/1.73 m2 in this analysis. The immediate decline of renal function was frequently observed in subjects with eGFR <60 mL/min/1.73 m2; therefore, more attention should be focused on this population. In this regards, we cannot recommend the use of SGLT2 inhibitors to all of the patients with T2DM. We need further studies aiming to see renal effects as a primary outcome and including a meaningful number of Asian patients and subjects with a broad range of renal function. The Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) trial (NCT03036150) and EMPA-KIDNEY study (NCT03594110) will provide evidence in this field. This recommendation can be revised and updated after publication of these two landmark studies.
Quality of evidence
Authors independently assessed risk of bias at the study level using the revised Cochrane risk of bias tool for randomized trials (risk of bias 2.0), and any disagreements were resolved by consensus among authors. Risk of bias was generally low except two studies that had high risk of bias due to missing outcome data (Supplementary Fig. 3). A large number of patients were not followed up at final assessment. More than 50% of participants were not followed up in three studies. We do not know distinct clinical characteristics between subjects who were followed up successfully and those who were not. Therefore, there is uncertainty due to high dropout rate.
Other consideration
The mainstay of prevention and treatment for CKD in T2DM included optimal treatment of hyperglycemia, dyslipidemia, obesity, and blood pressure using RAAS blockade. These general recommendations should be followed, and also consider patient's preference regarding the benefits of SGLT2 inhibitors and the possible disadvantages, such as urinary frequency, unwanted body weight loss, genital infection, and cost. We should educate patients for preventing volume depletion and genital infection. From the physicians' perspectives, immediate decline of renal function is concerned, and a follow-up plan for monitoring eGFR must be developed.
ACKNOWLEDGMENTS
This work was performed through the cooperation of the Korean Diabetes Association and the Korean Society of Nephrology.
Notes
This manuscript is simultaneously published in The Korean Journal of Medicine, Diabetes & Metabolism Journal, Kidney Research and Clinical Practice, and The Journal of Korean Diabetes.
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2020.0172.
Supplementary Table 1
Characteristics of randomized clinical trials included in meta-analysis
Supplementary Fig. 1
Results of patients with estimated glomerular filtration rate below 60 mL/min/1.73 m2. Effect of sodium-glucose cotransporter-2 (SGLT2) inhibitors on (A) change of glycosylated hemoglobin, (B) hypoglycemia, and (C) genital infection. SD, standard deviation; IV, inverse variance; CI, confidence interval.
Supplementary Fig. 2
Result of Asian dominant studies. Effects of sodium-glucose cotransporter-2 (SGLT2) inhibitors on (A) body weight, (B) systolic blood pressure, (C) diastolic blood pressure, (D) hypoglycemia, (E) genital infection, (F) urinary tract infection, and (G) volume depletion. SD, standard deviation; IV, inverse variance; M-H, Mantel-Haenszel; CI, confidence interval.
Supplementary Fig. 3
Risk of bias assessment.




