Predictive Clinical Parameters for the Therapeutic Efficacy of Sitagliptin in Korean Type 2 Diabetes Mellitus
Article information
Abstract
Background
Sitagliptin is a highly selective dipeptidyl peptide-4 (DPP-4) inhibitor that increases blood levels of active glucagon-like peptide (GLP)-1 and glucose-dependent insulinotrophic polypeptide (GIP), resulting in increased insulin secretion. While studies conducted in other countries have indicated the efficacy and safety of using sitagliptin to treat type 2 diabetes mellitus (T2DM), its predictors of effects to sitagliptin are not well understood. Therefore, we evaluated the predictive clinical parameters for the therapeutic benefits of sitagliptin when added to an ongoing metformin or sulfonylurea therapy in Korean T2DM subjects.
Methods
We obtained data from 251 Korean T2DM subjects who had recently started taking sitagliptin as add-on therapy. Exclusion criteria included any insulin use. Changes in HbA1c (ΔHbA1c) and fasting plasma glucose (ΔFPG) were assessed by comparing baseline levels prior to sitagliptin administration to levels 12 and 24 weeks after treatment. Responders were defined as subjects who experienced decrease from baseline of >10% in ΔHbA1c or >20% in ΔFPG levels at 24 weeks.
Results
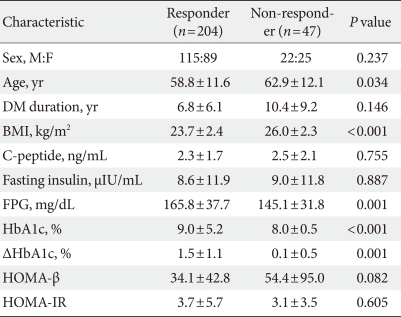
We classified 81% of the subjects (204 out of 251) as responders. The responder group had a lower mean body mass index (23.70±2.40 vs. 26.00±2.26, P≤0.01) and were younger (58.83±11.57 years vs. 62.87±12.09 years, P=0.03) than the non-responder group.
Conclusion
In Korean T2DM subjects, sitagliptin responders had lower body mass index and were younger compared to non-responders.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a complex metabolic disorder caused by insufficient insulin secretion and insulin resistance. Recently, the prevalences of T2DM and other metabolic abnormalities have increased in Korea due to their westernized lifestyle and nutritional status and together have become serious economic and social problems [1].
In the last 10 years, several studies have shown that strict glycemic control is a most important factor in the prevention of macrovascular and microvascular complications [2-6]. So T2DM treatments focus on lifestyle changes, reducing hyperglycemia, and improving insulin sensitivity. Management includes measures such as weight loss and oral medications that improve insulin sensitivity and lower blood glucose by decreasing insulin resistance. Since T2DM has progressively changing characteristics, the identification of a uniform treatment plan for all patients is difficult. Moreover recent reports on the pathogenesis of T2DM in Korea suggest that impaired insulin secretion, especially selective beta cell loss, is more prominent than insulin resistance, even in the stage of impaired glucose [7]. The reasons for the above are not entirely clear, but differences in body fat distribution, insulin resistance, lipid profile and prevalence of hypertension may contribute. Thus, these racial or ethnic differences rise from not only genetic predisposition but also environmental factors. Therefore individual treatment regimens need to be customized for each T2DM patients [8].
Recently developed dipeptidyl peptide-4 (DPP-4) inhibitors prevent the degradation of glucagon-like peptide (GLP)-1, an important incretin in the body. GLP-1 increases the secretion of insulin and decreases the secretion of glucagon, thereby reducing blood sugar. Since GLP-1 improves glucose tolerance [9,10], over 20 DPP-4 inhibitors are in development worldwide with sitagliptin and vildagliptin commonly used in Korea. DPP-4 inhibitor is clinically beneficial because its adverse effects are minimal by comparison other orally administered hypoglycemic agents. For example, it can safely be used by elderly individuals who are at increased risk of hypoglycemia, and weight gain and other gastrointestinal side effect are uncommon. Moreover in preclinical studies, DPP-4 inhibitor increased the number of pancreatic beta cells and improved the function of beta cells and improved function [11].
In the present study, we investigated the effectiveness of sitaglipin as add-on therapy for subjects with T2DM who had inadequetly controlled blood sugar level with conventional oral hypoglycemic agents such as metformin and sulfonylurea. Treatment responses were examined and the factors affecting the overall effectiveness of sitagliptin use were analyzed.
METHODS
Subjects
The medical records of subjects with T2DM who visited the out-patient department or were admitted to Endocrinology and Metabolism Division at Yonsei University Gangnam Severance Hospital between January 2009 and January 2010 were reviewed. Subjects were enrolled if hemoglobin A1c (HbA1c)>7.5% before taking sitagliptin as add-on therapy. Exclusion criteria included any insulin use and dose change of anti-diabetic medication in study period.
Study design
A total of 251 subjects were administered an additional 100 mg of sitagliptin together with metformin or metformin+ sulfonylurea. Fasting plasma glucose (FPG) and HbA1c levels were measured in these subjects before and 12 and 24 weeks after administration of sitagliptin. FPG levels were determined using a 747 automatic analyzer (Hitachi, Tokyo, Japan) and a standard glucose oxidase method. HbA1c levels were measured using high performance liquid chromatography.
After 24 weeks, in order to assess the characteristics that affect response to sitagliptin treatment, we defined responders as patients who achieved FPG reduction of >20% or HbA1c reduction of >10% from baseline. The factors affecting the response of sitagliptin treatment were evaluated. The clinical characteristics between the response group and the non-response group included the duration of T2DM, body mass index (BMI), plasma lipid concentrations, and insulin resistance levels.
BMI was calculated using the formula: weight (kg)/height (m2). Total cholesterol, high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) levels were measured by an enzymatic method using a 7,150 autoanalyzer (Hitachi), and insulin and c-peptide were determined by radioimmunoassay. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using FPG and plasma insulin concentrations: fasting insulin (µU/mL)×FPG (mmol/L)/22.5. beta cell function was also calculated using fasting insulin levels and the HOMA-β method: 20×fasting insulin (µU/mL)/(FPG [mmol/L]-3.5).
Statistical analysis
All statistical analysis were performed with SPSS for Windows, version 17.0k (SPSS Inc., Chicago, IL, USA) and descriptive data were expressed as mean±standard deviation or as number. Continuous variables were compared between response group and the non-response group were analyzed using t-tests. More than three group analysis was performed using analysis of covariance (ANOVA), and logistic regression analysis was used to examine the relationships between the variable. P values of less than 0.05 were considered statistically significant.
RESULTS
Baseline clinical characteristics of subjects
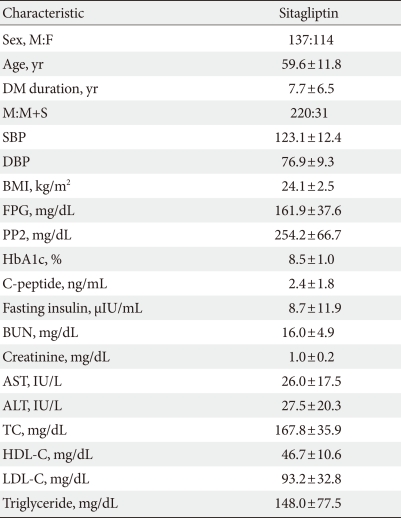
Out of a total of 251 type 2 diabetic patients, 137 were men, and 114 were women. The mean age was 59.59±11.75 years. At baseline the mean value of HbA1c was 8.45±0.96%, FPG was 161.93±37.55 mg/dL, post prandial 2 hour blood glucose (PP2) was 254.21±66.66 mg/dL. Insulin level was 8.70±11.85 µIU/mL and C-peptide was 2.36±1.80 ng/mL. The average BMI was 24.14±2.54 kg/m2. Of the 251 subjects, 220 used sitagliptin together with metformin. Thirty-one patients received sitagliptin together with sulfonylurea and metformin for T2DM treatment (Table 1).
Effect of sitagliptin in plasma glucose and HbA1c
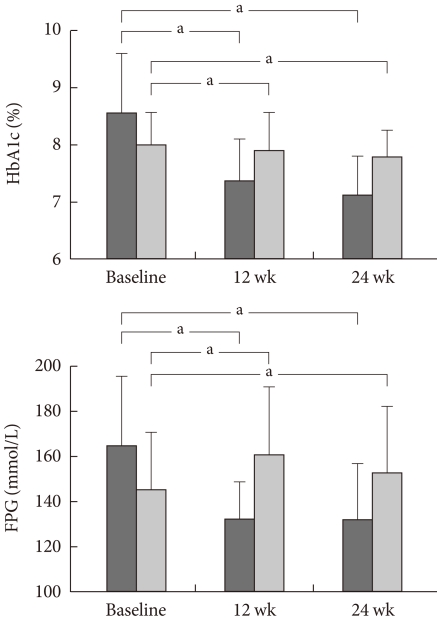
After 12 weeks of additional sitagliptin administration, HbA1c was 7.49±0.94%, FPG was 138.08±32.62 mmol/L, and PP2 was 196.27±64.59 mmol/L. After 24 weeks, HbA1c decreased to 7.25±0.97%, FPG decreased to 136.25±32.02 mmol/L, and PP2 decreased to 190.89±58.53 mmol/L (Fig. 1).
Predictive clinical characteristics of sitagliptin response group
Patients who achieved FPG reduction of >20% or HbA1c reduction of >10% after 24 weeks of sitagliptin administration were classified in the sitagliptin response group. Among 251 subjects of treatment group, 204 (81%) were classified as responders. Biochemical and clinical factors that affected the sitagliptin response were evaluated between the response group and the non-response group. HbA1c reduced from 9.04±5.17% to 7.57±4.07% (ΔHbA1c=1.47±1.10%) in the response group. The response group was younger than the non-response group (58.83±11.57 vs. 62.87±12.09, P=0.03) and the BMI of response group was also lower (23.70±2.40 vs. 26.00±2.26, P≤0.01). The duration of diabetes was shorter in the response group than the non-response group (6.80±6.14 vs. 10.42±9.23, P=0.14) and HOMA-β was also lower (34.10±42.84 vs. 54.35±94.98, P=0.08) but there was no statistically significant (Table 2). After the t-test age, duration of diabetes, BMI, and HOMA-β were treated as independent variables, and regression analysis showed that there was no significant relationship between them, excluding BMI (P<0.05) which was discovered to be a dependent variable (Table 3). There was no significant difference between metformin+sitagliptin group and metformin+sulfonylurea+sitagliptin group.
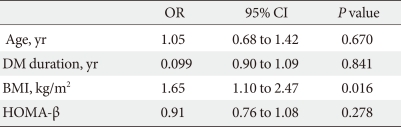
Logistic regression analysis for predictive parameters of clinical efficacy of sitagliptin as dependent variables and its components as independent variables
In order to observe the response to sitagliptin treatment based on age, multigroup analyses were obtained. The ΔHbA1c in the subjects under 50 (n=40) was 1.44±1.04%, in the subjects between 51 and 65 (n=104) was 1.32±1.31%, and in the subjects over 65 (n=70) was 1.00±0.90%. ΔHbA1c level showed a tendency to decrease based on age (Fig. 2A), but this trend was not statistically significant. The results of an analysis of the effect of sitagliptin based on duration of T2DM showed greater effectiveness in subjects treated lower than 5 years (n=69) with a ΔHbA1c of 1.33±1.12% compared subjects with longer duration of T2DM (n=56, 1.25±1.36%). However, this difference was not statistically significant (Fig. 2B). When the effects of sitagliptin were analyzed based on BMI, the ΔHbA1c in the BMI ≤25 kg/m2 group (n=152) was 1.41±1.07%, the 25 kg/m2 <BMI ≤30 kg/m2 group (n=30) was 0.77±1.23%, the BMI >30 kg/m2 group (n=27) was 0.90±1.34%. Subjects with lower BMI were greater response in sitagliptin treatment (P=0.04) (Fig. 2C). The ΔHbA1c based on HOMA-β was 1.32±1.12% when HOMA-β≤22.3 (n=72), 1.27±1.05% when 22.3<HOMA-β≤48.2 (n=34), and 0.99±0.67% when HOMA-β>48.2 (n=25) (P=0.042). The sitagliptin response was higher in subjects with beta cell secretory dysfunction (Fig. 2D).
DISCUSSION
In this study, we analyzed 251 subjects who were administered additional sitagliptin because of poor glycemic control with previous oral hypoglycemic agents. And the factors affecting the response of sitagliptin treatment in Korean T2DM were evaluated. The HbA1c lowering effect of sitagliptin was 1.55±1.01%, and in non-obese subjects and subjects with decreased insulin secretion the response to sitagliptin was very promising. Asian patients are less likely to be obese than Western patients. It is suggested that Koreans with reduced pancreatic insulin secretion would be more effective in the sitagliptin treatment. Insulin secretion is relatively preserved during the initial stages of type 2 diabetes. However, as the duration of diabetes longer, insulin deficiency progresses as a result of beta cell dysfunction.
In the other studies, it is reported that strict blood glucose control can delay the progression of microvascular and macrovascular complications [6]. However, in a multicenter study of Korean hospitals, out of 5,652 T2DM patients who maintained a glycemic control target of HbA1c <7.0% were 36.7%, that considered to have unsatisfactory levels of glycemic control [12]. In addition, according to UK Prospective Diabetes Study (UKPDS) data, out of 826 patients who were newly diagnosed with T2DM and were being treated with sulfonylurea 53% had poor glycemic control and required insulin injections after six years. Based on these results active insulin treatment is recognized as a necessity in type 2 diabetic patients and the research interest in insulin injection time intervals and in insulin types is increasing. However, it has been reported in several studies that continuous high doses of insulin are associated with increased risk of cancer [13-15].
The goal for T2DM treatment is to administer sufficient amounts of insulin in vivo to effectively control plasma glucose without causing severe hypoglycemia. Therefore drugs with different mechanisms of action are needed. For the last 5 years novel drugs have been developed and are currently being used in many countries including Korea. These drugs include incretin mimetics like GLP-1 analogues and DPP-4 inhibitors. Sitagliptin is an orally active, potent and selective DPP-4 inhibitor in development for the treatment of T2DM. Sitagliptin acts through increasing active incretin hormone concentrations. Following ingestion of a meal, incretins, including GLP-1 and glucose-dependent insulinotrophic polypeptide (GIP) attenuate the post-meal rise in glucose concentration and reduce fasting glucose concentrations. Both GLP-1 and GIP are rapidly inactivated by the enzyme DPP-4. In patients with type 2 diabetes, treatment with single doses of sitagliptin provided sustained 24 hour inhibition of DPP-4 enzyme activity and increased active GLP-1 and GIP concentrations.
A 0.6% reduction of HbA1c was observed in an 18-week study of 100 mg sitagliptin monotherapy vs. placebo [16]. In a 24 week study of 100 mg and 200 mg sitagliptin monotherapy vs. placebo involving 741 type 2 diabetic patients the <8% HbA1c level group had 0.57% reduction in HbA1c, and the over 9% group had 1.52% reduction [10]. Drug effectiveness was much higher in the elevated HbA1c group. Although many combination therapies involving sitagliptin have been designed, HbA1c decreased 0.6% when sitagliptin was administered compared with a placebo in a 24 week study involving 702 patients who were not controlled with metformin [17]. In a 24-week study enrolled 353 patients who were not controlled with pioglitazone, HbA1c decreased 0.7% when compared with a placebo [18]. This amount may exceed the moderate hypoglycemic effects of existing drugs, but its predictors of effects to sitagliptin are not well understood. Therefore, we evaluated the predictive clinical parameters for the therapeutic benefits of sitagliptin when added to an ongoing metformin or sulfonylurea therapy in Korean T2DM subjects.
In this study 251 subjects who received 100 mg sitagliptin in combination with sulfonylurea and metformin for 24 weeks exhibited a mean decrease in HbA1c of 1.23±1.15%. The responders were higher baseline HbA1c level compared with non-responders. So a more effect was expected in patients with relatively high baseline HbA1c levels in sitagliptin treatment. Based on HbA1c and FPG levels before and after treatment and in comparisons of the clinical characteristics and biochemical parameters of the response group and the non-response group, the response group's BMI and HOMA-β levels were significantly lower. Thus greater effects of sitagliptin are expected in Korean diabetic patients who have quantitative pancreatic deficiencies [7].
Sitagliptin blocks the degradation of endogenous and exogenous GLP-1 and increases insulin secretion and action. In addition, it inhibits the secretion of glucagon, thereby decreasing plasma glucagon values, which in turn reduces blood glucose and improves glucose tolerance. In this study, more than 81% of patients were found to have improved glucose tolerance. These results suggest that sitagliptin treatment exerts significant effects in patients who have decreasing secretory capacity in pancreatic beta cells and that DPP-4 inhibitors may also improve beta cell function [19]. According to a previous animal study, sitagliptin improved beta cell function and increased insulin secretion [20]. The administration of DPP-4 inhibitor decreases blood glucose, decreases glucagon, and improves beta cell function in humans [21].
This study has several limitations. First, since this was a retrospective study the accuracy of our results may have been compromised. Second, although only Korean subjects were used in this study and there was no control group. Third, diet and exercise were not monitored while the participants of this study were receiving treatment. However despite these limitations, in Korean T2DM subjects, sitagliptin responders had lower BMI and were younger compared to non-responders. Further prospective randomized controlled studies are needed to obtain more detailed information.