Lack of Association between Serum Cystatin C Levels and Coronary Artery Disease in Diabetic Patients
Article information
Abstract
Background
Serum cystatin C level is a more sensitive marker of renal dysfunction than serum creatinine level. Serum cystatin C level was recently reported to predict the development of cardiovascular disease. This study was performed to evaluate whether the cystatin C level is associated with coronary artery disease (CAD), independent of diabetic nephropathy.
Methods
We conducted a case-control study to assess the relationship between serum cystatin C level and coronary artery disease in diabetic patients. Among 460 diabetic patients, 38 diabetic patients had CAD. The control group consisted of 38 diabetic patients who were matched to cases by age, sex, and presence/absence of diabetic nephropathy. Serum cystatin C level was measured in stored samples.
Results
Serum cystatin C level was significantly higher in patients with diabetic nephropathy, both in CAD and non-CAD patients. However, serum cystatin C level did not differ between CAD and non-CAD patients, regardless of diabetic nephropathy.
Conclusion
Serum cystatin C level is a marker of renal dysfunction, but not coronary artery disease, in diabetic patients.
INTRODUCTION
The main cause of death in diabetic patients is cardiovascular disease. It has been reported that two out of every three diabetic patients die of heart failure, myocardial infarction and stroke [1]. Loss of kidney function is a major risk factor for cardiovascular disease [2,3]. Renal dysfunction due to diabetic nephropathy increases the risk of cardiovascular disease, which is the main cause of death in diabetic patients [4]. The current gold standard for estimation of renal function is the glomerular filtration rate (GFR), but this test is time consuming and expensive, thus precluding its routine use in clinical settings. Estimation of GFR from prediction equations based on serum creatinine measurements (Cockcroft-Gault or Modification of Diet in Renal Disease equations) is imperfect [5]. Therefore, researchers have tried to develop more accurate markers for renal function, and serum cystatin C has recently emerged as a target of interest.
Cystatin C is a cysteine protease inhibitor with a low-molecular weight (13 kD) that is produced by all nucleated cells at a constant rate. It is freely filtered across the glomerular membrane and is almost completely reabsorbed and catabolized in proximal renal tubular cells [6]. Unlike serum creatinine, cystatin C is not influenced by age, sex, muscle mass, exercise or diet. Therefore, the serum cystatin C level is a superior marker for the evaluation of renal function compared to other markers such as serum creatinine or creatinine clearance [7-9]. Serum cystatin C level has also been reported to have a higher sensitivity and accuracy than serum creatinine for detecting changes in GFR in diabetic patients [10,11].
It has been reported that serum cystatin C level is a stronger predictor of mortality and cardiovascular events than serum creatinine or estimated GFR in elderly patients with chronic renal disease or coronary heart disease [12-14]. However, few data exist on the relationship between serum cystatin C level and cardiovascular disease in diabetic patients with or without diabetic nephropathy. The aim of the present study was to evaluate whether serum cystatin C level is associated with coronary artery disease (CAD) independent of diabetic nephropathy.
METHODS
Study design
This was a retrospective case-control study. Among 460 diabetic patients who visited the Diabetes Center of Asan Medical Center for evaluation of micro- and macrovascular complications, 38 diabetic patients with CAD were included in the study. The control group consisted of 38 diabetic patients who were matched by age, sex, and presence/absence of diabetic nephropathy. After obtaining written informed consent from each individual and the approval of our institutional review committee, we obtained blood samples from each patient and recorded patient age, sex, blood pressure, duration of diabetes, and other clinical information.
Definition of coronary artery diseases
Patients with CAD (CAD group) were defined as patients who have a past history of stable angina, acute coronary syndrome (myocardial infarction and unstable angina), coronary bypass surgery or percutaneous coronary intervention. Patients without CAD (non-CAD group) were defined as having no past history of coronary artery disease or suggestive symptoms with a normal electrocardiogram.
Definition of diabetic nephropathy
Urinary albumin excretion (UAE) was measured by radioimmunoassay from timed overnight urine collections. Individuals were considered normoalbuminuric if the UAE value was under 20 µg/min. Microalbuminuria was defined as UAE 20 to 200 µg/min on at least two of the three measurements. Overt proteinuria was defined as UAE > 200 µg/min. Azotemia was defined as serum creatinine > 1.5 mg/dL or estimated GFR < 60 mL/min/1.73 m2.
Analysis of serum cystatin C
The serum cystatin C level in the stored plasma samples was measured by particle-enhanced immunonephelometry using the N Latex Cystatin C kit (Dade Behring, Marburg, Germany) and the level was expressed as mg/dL.
Statistical analysis
Values are expressed as the means ± standard deviation. Statistical analysis was performed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). The paired t-test or Wilcoxon signedrank test and McNemar's test were used to assess differences in continuous variables and categorized variables, respectively. The differences in serum cystatin C levels according to the number of stenotic vessels in 38 diabetic patients with CAD were analyzed by Kruskal-Wallis analysis. Conditional logistic regression analysis was performed to determine the risk factor for CAD. Differences were classified as significant at P < 0.05.
RESULTS
Clinical characteristics of CAD and non-CAD subjects in diabetic patients
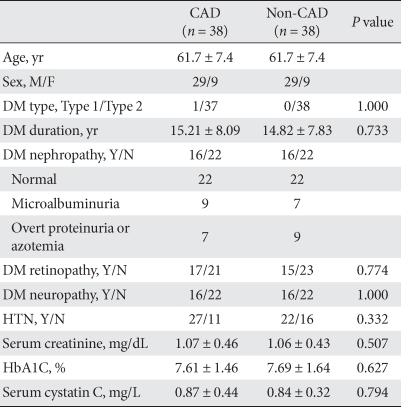
Clinical characteristics between 38 CAD patients and 38 non-CAD patients are shown in Table 1. The mean age of the two groups was 61.7 years and most patients had type 2 diabetes mellitus, except for one patient who had type 1 diabetes mellitus. The mean duration of diabetes did not differ between the CAD and non-CAD groups (15.21 ± 8.09 years vs. 14.82 ± 7.83 years, respectively, P = 0.733). The prevalence of diabetic retinopathy, diabetic neuropathy and hypertension did not differ between the two groups. Serum cystatin C level did not differ between the CAD and non-CAD groups (0.87 ± 0.44 mg/L vs. 0.84 ± 0.32 mg/L, respectively, P = 0.794).
Serum cystatin C level based on diabetic nephropathy in CAD and non-CAD group
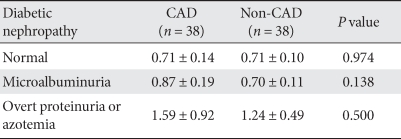
The two groups were divided into three subgroups each, i.e., normoalbuminuric, microalbuminuria, overt proteinuria and azotemia groups, and the serum cystatin C level was compared among the groups. The serum cystatin C level increased with a worsening of diabetic nephropathy, but did not differ between the CAD and non-CAD groups (Table 2).
Serum cystatin C level according to the number of stenotic vessels in the CAD group
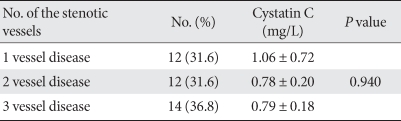
When the CAD group was divided into three subgroups, i.e., one vessel disease, two vessel disease, and three vessel disease, the serum cystatin C level according to the number of stenotic vessels did not show significant differences (Table 3).
Risk factor for CAD
Conditional univariate logistic regression analysis was performed to determine the risk factors for CAD. Neither cystatin C level nor HbA1C, duration of diabetes, serum creatinine, or hypertension was associated with CAD (Table 4).
DISCUSSION
In this study, we did not detect a relationship between serum cystatin C level and CAD in diabetic patients. This result is in conflict with results from previous studies, which reported that cystatin C level is a predictor of cardiovascular disease and mortality. In our study, we selected diabetic patients with and without CAD who were matched for age, sex and presence of diabetic nephropathy. Serum cystatin C level was higher in patients with diabetic nephropathy, but no differences were found between CAD and non-CAD groups. Therefore serum cystatin C level is associated with diabetic nephropathy, but is not associated with CAD.
Cystatin C, a cationic low-molecular weight protein (13 kD), is freely filtered across the glomerular membrane and is almost completely reabsorbed in proximal renal tubular cells [15]. Unlike serum creatinine, for which the serum concentration is strongly affected by muscle mass, cystatin C is produced by all nucleated cells at a constant rate and its serum level is relatively unaffected by age, sex, body composition, diet, and exercise [16,17]. Based on these characteristics, cystatin C has been extensively studied as a potential alternative for creatinine as a serum marker of kidney function. Many studies have been shown that serum cystatin C level is superior to serum creatinine as an estimate for GFR, and can be used to detect mild renal function impairment and rapid changes in GFR. Also, cystatin C level is superior to creatinine for the estimation of GFR under conditions in which there is an altered creatinine production [18]. It has long been recognized that renal dysfunction is an important risk factor of cardiovascular disease. Many researchers have tried to identify more accurate markers for renal function and serum cystatin C level has drawn recent attention.
Serum cystatin C levels in patients with chronic renal disease are associated with cardiovascular disease mortality and all cause mortality [19], and were shown to predict cardiovascular death, myocardial infarction and stroke in a prospective cohort of adults aged ≥ 65 years [12]. Serum cystatin C in patients with CAD is associated with total mortality, cardiovascular events and heart failure [20], and was also found to be associated with total mortality in a follow up study of patients with acute coronary syndrome [13]. Moreover, a study in elderly patients without chronic renal disease showed that serum cystatin C was associated with the risk of total mortality, cardiovascular disease mortality, myocardial infarction and stroke [21]. In a study of an adult population without proteinuria and with GFR > 60 mL/min/1.73 m2, serum cystatin C level was associated with increased incidence of cardiovascular disease [22]. Finally, the serum cystatin C level in patients with CAD who had normal or mildly reduced kidney function predicted cardiovascular disease mortality after adjustment for potential confounders, including classical risk factors and N-terminal pro B-type natriuretic peptide (NT-pro BNP) [14].
The mechanism by which serum cystatin C level and cardiovascular disease are related has not been established. However, depending on the association of serum cystatin C and hsCRP levels, cystatin C level was suggested to be associated with inflammation [12,23]. It was also proposed that cystatin C has a direct effect on atherosclerosis and inflammation [24]. Cystatin C is the most potent endogenous inhibitor of cysteine proteinases such as cathepsins, elastase and papain. Because cysteine proteinases have a prominent role in protein catabolism and the cleavage of membrane and extracellular matrix proteins, cystatin C probably has a central regulatory function in the extracellular compartment. Consequently, imbalance between the proteases and inhibitors may affect the cardiovascular system. In addition, cystatin C is probably involved in the regulation of inflammatory processes, where it inhibits polymorphonuclear cell chemotaxis, O2-release and phagocytosis [25]. Because cystatin C is an inhibitor of cathepsin, its concentration increases as compensation for elevated elastolytic activity [26]. Cytokines related with atherosclerosis stimulate the production of cathepsin and increase the cystatin C concentration. Despite the above mentioned mechanisms, there is no evidence that elevation of serum cystatin C in actual clinical conditions is associated with cardiovascular disease by inflammation.
Our results are in apparent contrast with those of previous studies. In diabetic patients, most previous studies have primarily focused the relationship with impaired renal function. A recent study reported a statistically important relationship between serum cystatin C level and CAD in type 1 diabetic patients, but this study had some limitations [27]; surrogate markers of CAD were used, rather than CAD events or death. More importantly, this study was limited because they did not consider UAE. In another study, serum cystatin C level predicted the elevation of hsCRP [28]. However, as stated above, this result also did not show a direct association with cardiovascular disease and cystatin C. A recent study showing that serum cystatin C is associated with the risk for cardiovascular disease in patients with type 2 diabetes mellitus [29] was also limited because it assessed estimated risk instead of recording cardiovascular events.
In our study, we did not find an association between serum cystatin C level and CAD. Several other studies have reported similar results. In patients without CAD, serum cystatin C predicted chronic renal disease, but not CAD [30]. In a study of middle-aged subjects, carotid atherosclerosis was found to be associated with microalbuminuria, but not serum cystatin C level [11]. Therefore, in most studies reporting serum cystatin C as a predictor of the risk for cardiovascular disease, it is not clear whether cardiovascular disease was a direct effect of elevated level of cystatin C or indirect effect of deterioration of renal function.
In conclusion, our results suggest that serum cystatin C levels are useful to assess renal function, but that they are not a suitable marker for CAD. However, our study is limited in that it is a retrospective study with a small sample size. Further prospective studies with larger sample sizes are needed to evaluate the relationship between serum cystatin C and cardiovascular disease.