- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 43(6); 2019 > Article
-
Short CommunicationClinical Diabetes & Therapeutics Three Months Monitored Metabolic Fitness Modulates Cardiovascular Risk Factors in Diabetic Patients
-
Ilenia Cirilli1
 , Sonia Silvestri2,3, Fabio Marcheggiani2, Fabiola Olivieri4,5, Roberta Galeazzi6, Roberto Antonicelli7, Rina Recchioni4, Fiorella Marcheselli4, Tiziana Bacchetti2, Luca Tiano2
, Sonia Silvestri2,3, Fabio Marcheggiani2, Fabiola Olivieri4,5, Roberta Galeazzi6, Roberto Antonicelli7, Rina Recchioni4, Fiorella Marcheselli4, Tiziana Bacchetti2, Luca Tiano2 , Patrick Orlando2
, Patrick Orlando2 -
Diabetes & Metabolism Journal 2019;43(6):893-897.
DOI: https://doi.org/10.4093/dmj.2018.0254
Published online: June 27, 2019
1Department of Clinical Dental Sciences, Polytechnic University of Marche, Ancona, Italy.
2Department of Life and Environmental Sciences, Polytechnic University of Marche, Ancona, Italy.
3Biomedfood s.r.l., Spinoff of Polytechnic University of Marche, Ancona, Italy.
4Center of Clinical Pathology and Innovative Therapy, Italian National Research Center on Aging (IRCCS INRCA), Ancona, Italy.
5Department of Clinical and Molecular Sciences, DISCLIMO, Polytechnic University of Marche, Ancona, Italy.
6Clinical and Molecular Diagnostic Laboratory, IRCCS INRCA National Institute, Ancona, Italy.
7Cardiology Unit, IRCCS INRCA, Ancona, Italy.
- Corresponding author: Luca Tiano. Department of Life and Environmental Sciences, Polytechnic University of Marche, via Ranieri 61, Ancona, Italy. l.tiano@univpm.it
Copyright © 2019 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Cardiovascular diseases represent the leading cause of death and moderate physical exercise is associated with a reduction in cardiovascular risk. The aim of the study was to evaluate the correlation between the amount of exercise recorded daily by a wearable gravitometer for 3 months and selected biochemical and clinical parameters. Nineteen sedentary type 2 diabetics were recruited and distributed into three homogenous groups, low, medium, and high exercise, according to the level of physical exercise monitored and expressed as MOVEs. Data showed an inverse correlation between MOVEs and oxidative stress indexes and a significant improvement in paraoxonase-1 activities and endothelial functionality. Decrease of visceral/total adipose tissue ratio, systolic blood pressure and a down-regulation of the inflammatory microRNA-146a in high exercise group were observed. Finally, a decrease of glycosylated hemoglobin and an up-regulation of the angiogenic microRNA-130a in medium exercise one was obtained. In this study, precise daily monitoring permitted to underline the importance of the amount of physical activity to counteract some cardiovascular risk factors persisting in diabetes. Finally, it identifies new microRNA biomarkers for future investigation on the same topic.
- Cardiovascular diseases are the most prevalent cause of mortality and morbidity in diabetic populations associated with chronic endothelial dysfunction [1] characterized by decreased bioavailability of nitric oxide (NO) due both to reduced endothelial nitric oxide synthase (e-NOS) activity and NO inactivation by reactive oxygen species (ROS) produced in excess under oxidative stress conditions associated with diabetes. Consequently, endothelial dysfunction together with obesity, hypertension and dyslipidemia represent major cardiovascular risk factors in diabetes [2].
- Physical activity (PA) could be an important therapy in diabetes treatment being able to influence cardiovascular risk factors, improving endothelial functionality and lowering oxidative stress. In fact, it enhance NO production at the endothelial level by promoting blood-flow mediated shear stress [3]. Moreover, regular PA stimulates endogenous antioxidant defense systems improving the cellular redox status [4].
- However it is difficult to engage patients in a radical lifestyle change involving a structured PA routine, especially in the case of sedentary subjects. Besides exercise sessions, the overall daily workload should be considered in relation to its influences in lowering cardiovascular risk factors in diabetic patients.
- The present study was designed to evaluate the hematochemical, clinical, and biochemical effects of PA measured as recorded amount of activity rather than as scheduled and/or perceived amount of exercise in diabetics. microRNAs expression modulation in circulating angiogenic cells (CACs) was also evaluated as an innovative biomarker of vascular senescence in age-related diseases including diabetes.
INTRODUCTION
- Participants and study design
- This prospective observational study was conducted on nineteen naïve type 2 diabetes mellitus patients not doing any type of structured physical exercise activity (13 males and six females; aged 62±2 years; body mass index, 30±1 kg/m2; glycosylated hemoglobin [HbA1c], 7.6%±0.25%), recruited at the Diabetology Unit of the Italian National Research Center on Aging (INRCA) Hospital of Ancona, Italy. Inclusion criteria were age 50 to 70 and ability to perform at least 30 minutes walking without assistance. Exclusion criteria included physical inhabilities, neurodegenerative diseases and severe cardiovascular events.
- The patients were instructed on the benefits of regular PA participating in a counseling activity based on the guidelines of the Italian Standard for Diabetes Mellitus care and American Diabetes Association recommendations and on the use of the portable gravitometer (MyWellness Key; Technogym, Cesena, Italy) used to record daily PA performed for 3 months. Clinical, hematochemical, and biochemical indexes were analyzed at the start and at the end of the study under fasting conditions.
- The study was performed in accordance with the Helsinki Declaration of human studies and was approved by the Ethics Committee of INRCA (CER 2016-0614 IN). A written informed consent was obtained by all participants prior their inclusion in the study.
- Evaluation of physical activity: MyWellness Key
- PA was recorded through MyWellness Key [5], a wearable gravitometer that measures vertical acceleration determining the time and intensity [6]. MyWellness Key quantifies in real time during the day the volume of PA using MOVE units [7] an adimensional index similar to the metabolic equivalent of task for minute (MET-minutes) (2.5 MOVE=1 MET-minutes) [89].
- A dose of 500 to 1,000 MET-minutes (equivalent to 1,250 to 2,500 MOVEs) per week, is considered a moderate PA sufficient to produce health benefits [10]. MOVEs can be used to identify different levels of daily PA, in particular: light exercise below 499 MOVEs, moderate exercise from 500 to 749 MOVEs, high intensity exercise from 750 to 999 MOVEs and very high intensity over 1,000 MOVEs [9].
- Hematochemical evaluation
- Lipid profile (triglycerides, high density lipoprotein cholesterol [HDL-C], low density lipoprotein cholesterol [LDL-C], and total cholesterol) and HbA1c were evaluated.
- Clinical evaluation
- The clinical parameters considered were systolic and diastolic blood pressure, body composition using dual-energy X-ray absorptiometry technique and endothelial function by using the endothelial-dependent vasodilation of finger arteries measured by peripheral arterial tonometry which permits to evaluate both reactive hyperemia index and augmentation index (AI) [11].
- Biochemical evaluation
- Paraoxonase-1 (PON-1) paraoxonase and arylesterase activities [12] and oxidized LDL levels by using enzyme-linked immunosorbent assay (ELISA) test (Oxidized LDL ELISA kit; Mercodia, Uppsala, Sweden) were evaluated in plasma. CACs isolation was performed from peripheral blood and microRNA-21, -126, -130a, and -146a were evaluated [13]. Expression levels are reported as relative expression according to the 2-ΔCt method and cell microRNA-39 was used as spike in.
- Peripheral blood mononuclear cells were isolated to quantify DNA damage by using the alkaline version of the Comet Assay [14] and both intracellular ROS levels and mitochondrial membrane potential (MMP) flow-cytometrically (Guava InCyte software; Millipore, Burlington, MA, USA) [15] using 2-7-dichlorodihydro-fluorescein diacetate (DCFH-DA) [12] and 5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) [14] fluorescence probes, respectively.
- Statistical analysis
- Average data were calculated for the three groups according to the amount of PA recorded and, for each one, data as mean±standard error of mean are reported. Significance of differences between before and after 3 months of trial and among groups were calculated using paired and unpaired Student's t-test. Pearson's correlation coefficient was used to evaluate the correlations with the daily exercise. P≤0.01 and P≤0.05 are considered statistically highly significant and significant.
METHODS
- Participants and physical activity
- The patients were distributed according to the level of daily PA recorded by MyWellness Key and they were divided into three homogenous groups, according to MOVE [10]: high exercise (HE; 860±51 MOVEs), medium exercise (ME; 674±14 MOVEs) and low exercise (LE; 462±51 MOVEs) groups. No significant differences were observed at baseline among groups in relation to age distribution, anthropometric characteristics and values of HbA1c (data not shown).
- Hematochemical analysis
- Lipid profile remained unchanged following the trial independently of the amount of PA. On the contrary, all groups presented a reduction in the levels of HbA1c, although the variation was significant only in ME group (P=0.03) (Table 1).
- Clinical analysis
- Although no significant differences in terms of lean and total adipose tissues were observed (data not shown), in HE group visceral adipose tissue showed a decreasing trend (P=0.08) that resulted significant when normalized by total adipose tissue (P=0.03) (Table 1). Simultaneously, more active patients showed an improvement of endothelial functionality highlighted by a considerable decrease in the percent variation of AI compared to LE group close to significance (P=0.06). This data is also supported by a significant negative correlation between average daily MOVEs and AI % variation (R2=0.247, P=0.04). Finally, a slightly decrease of systolic blood pressure was observed in the HE (pre: 135±5 mm Hg; post: 127±5 mm Hg; P=0.08).
- Biochemical analysis
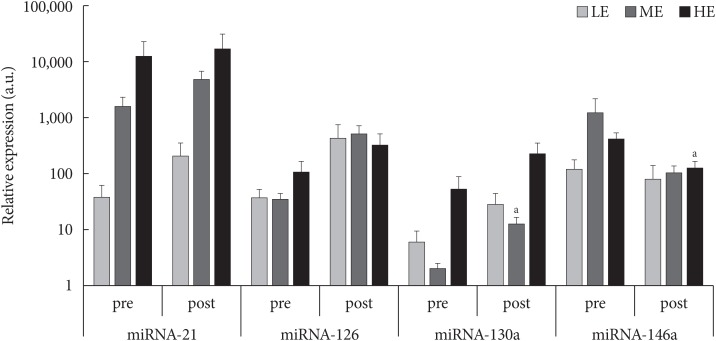
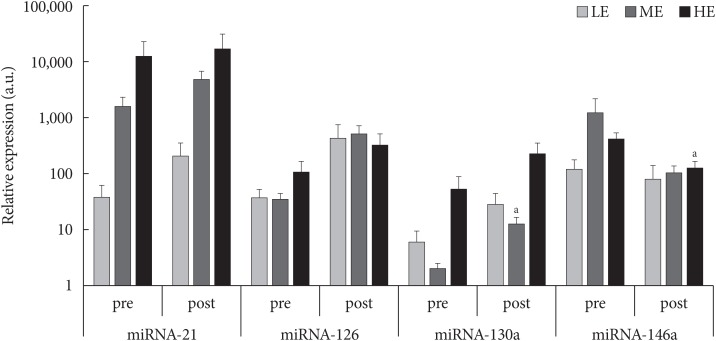
- PON-1 paraoxonase and arylesterase enzymatic activities were significantly modulated by PA particularly in the more active patients, while in the ME group only paraoxonase activity was enhanced (Table 1). Flow-cytometric analyses highlighted no significant differences after 3-month of monitored intervention in terms of intracellular ROS production and MMP (data not shown). However, DNA damage, proportional to the comet tail intensity, differs significantly among the groups of less active and more active patients (P=0.03) (Table 1) and it was inversely correlated with the amount of PA (R2=0.379, P=0.01). Similarly, a slight negative correlation between average daily MOVEs and oxidized LDL/total LDL after 3 months of monitored activity was observed (R2=0.244, P=0.06). Finally, as shown in Fig. 1, among microRNA expression levels evaluated, microRNA-130a was up-regulated in ME group (P=0.05) whereas microRNA-146a was down-regulated in the HE one (P=0.05).
RESULTS
- The present study evaluates the effects of PA recorded for 3 months in diabetics on hematochemical, clinical, and biochemical parameters. The inverse correlation between daily MOVEs and both oxidized LDL level and the extent of oxidative DNA damage, underline the important role of PA in counteracting oxidative stress in conditions of redox imbalance frequent in diabetes. These effects are likely due to a dose-dependent hormetic adaptive response.
- An increase in PON-1 paraoxonase and arylesterase activities was observed in all experimental groups but a significant level was reached only in the most active patients showing an improvement in HDL-C with anti-atherogenic properties. This might have concurred to the observed dose-dependent decrease in plasma oxidized LDL level.
- Notably, exercise dose significantly correlated with improvement in vascular stiffness expressed as AI and decrease in oxidative damage. Taken together, both correlations support the protective antioxidant role of exercise toward endothelium-derived NO. In fact, exercise-induced antioxidant defense likely protect NO limiting its oxidation by circulating ROS and therefore promoting its bioavailability [16].
- PA in the most active group was also able to positively influence other cardiovascular risk factors such as visceral/total adipose tissue ratio, systolic blood pressure, although not significantly, and to down-regulate microRNA-146a, a marker of a senescence-associated pro-inflammatory status in vascular cells [17].
- However, ME group benefited of PA both in terms of HbA1c decrease and microRNA-130a up-regulation, both considered relevant cardiovascular risk indexes [1819]. Finally, unaltered plasma lipid profile following PA is not surprising, taking into consideration both the normal baseline values of enrolled patients and the unclear role of training in this respect as showed by Kelley and Kelley [20].
- In conclusion, the innovative aspect of the study is represented by the precise monitoring of all daily PA performed by patients, highlighting the inverse correlation between the amount of MOVEs and some cardiovascular risk factors associated with diabetes.
- Moreover, the present study highlights the relevance of the novel methodological approaches proposed and identifies interesting new microRNA biomarkers that warrants future investigation on the role of PA in lowering cardiovascular risk in diabetic patients.
DISCUSSION
-
Acknowledgements
- None
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS:
NOTES
- 1. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964-967. ArticlePubMed
- 2. Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 2015;6:1246-1258. ArticlePubMedPMC
- 3. Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 2004;561:1-25. ArticlePubMedPMC
- 4. Gomez-Cabrera MC, Ferrando B, Brioche T, Sanchis-Gomar F, Vina J. Exercise and antioxidant supplements in the elderly. J Sport Health Sci 2013;2:94-100.Article
- 5. McGinley SK, Armstrong MJ, Khandwala F, Zanuso S, Sigal RJ. Assessment of the MyWellness Key accelerometer in people with type 2 diabetes. Appl Physiol Nutr Metab 2015;40:1193-1198. ArticlePubMed
- 6. Sieverdes JC, Wickel EE, Hand GA, Bergamin M, Moran RR, Blair SN. Reliability and validity of the Mywellness Key physical activity monitor. Clin Epidemiol 2013;5:13-20. ArticlePubMedPMC
- 7. Bergamin M, Ermolao A, Sieverdes JC, Zaccaria M, Zanuso S. Validation of the Mywellness Key in walking and running speeds. J Sports Sci Med 2012;11:57-63. PubMedPMC
- 8. Jette M, Sidney K, Blumchen G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin Cardiol 1990;13:555-565. ArticlePubMed
- 9. Mywellness. MyWellness Key cited 2019 May 1. Available from: https://www.mywellness.com.
- 10. Office of Disease Prevention and Health Promotion, U.S. Department of Health and Human Services: Physical Activity Guidelines Advisory Committee Report 2008 cited 2019 May 1. Available from: http://www.health.gov/PAGuidelines/Report.
- 11. Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol 2002;17:543-551. ArticlePubMed
- 12. Orlando P, Silvestri S, Galeazzi R, Antonicelli R, Marcheggiani F, Cirilli I, Bacchetti T, Tiano L. Effect of ubiquinol supplementation on biochemical and oxidative stress indexes after intense exercise in young athletes. Redox Rep 2018;23:136-145. ArticlePubMedPMC
- 13. Olivieri F, Spazzafumo L, Bonafe M, Recchioni R, Prattichizzo F, Marcheselli F, Micolucci L, Mensa E, Giuliani A, Santini G, Gobbi M, Lazzarini R, Boemi M, Testa R, Antonicelli R, Procopio AD, Bonfigli AR. MiR-21-5p and miR-126a-3p levels in plasma and circulating angiogenic cells: relationship with type 2 diabetes complications. Oncotarget 2015;6:35372-35382. ArticlePubMedPMC
- 14. Silvestri S, Orlando P, Armeni T, Padella L, Bruge F, Seddaiu G, Littarru GP, Tiano L. Coenzyme Q10 and α-lipoic acid: antioxidant and pro-oxidant effects in plasma and peripheral blood lymphocytes of supplemented subjects. J Clin Biochem Nutr 2015;57:21-26. ArticlePubMedPMC
- 15. Skarabahatava AS, Lukyanenko LM, Slobozhanina EI, Falcioni ML, Orlando P, Silvestri S, Tiano L, Falcioni G. Plasma and mitochondrial membrane perturbation induced by aluminum in human peripheral blood lymphocytes. J Trace Elem Med Biol 2015;31:37-44. ArticlePubMed
- 16. Kojda G, Hambrecht R. Molecular mechanisms of vascular adaptations to exercise. Physical activity as an effective antioxidant therapy? Cardiovasc Res 2005;67:187-197. ArticlePubMed
- 17. Olivieri F, Lazzarini R, Recchioni R, Marcheselli F, Rippo MR, Di Nuzzo S, Albertini MC, Graciotti L, Babini L, Mariotti S, Spada G, Abbatecola AM, Antonicelli R, Franceschi C, Procopio AD. MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling. Age (Dordr) 2013;35:1157-1172. ArticlePubMedPDF
- 18. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405-412. ArticlePubMedPMC
- 19. Meng S, Cao J, Zhang X, Fan Y, Fang L, Wang C, Lv Z, Fu D, Li Y. Downregulation of microRNA-130a contributes to endothelial progenitor cell dysfunction in diabetic patients via its target Runx3. PLoS One 2013;8:e68611. ArticlePubMedPMC
- 20. Kelley GA, Kelley KS. Effects of aerobic exercise on lipids and lipoproteins in adults with type 2 diabetes: a meta-analysis of randomized-controlled trials. Public Health 2007;121:643-655. ArticlePubMedPMC
REFERENCES
Relative expression (a.u.) of microRNA (miRNA)-21, -126, -130a, and -146a in low exercise (LE), medium exercise (ME), high exercise (HE) groups before (pre) and after (post) 3-month monitored exercise. aP<0.05 significant compared to study entry.
Hematochemical, clinical and biochemical data in LE, ME, HE groups before (pre) and after (post) 3-month monitored exercise
Figure & Data
References
Citations

- Emerging roles of microRNAs as diagnostics and potential therapeutic interest in type 2 diabetes mellitus
Dharmsheel Shrivastav, Desh Deepak Singh
World Journal of Clinical Cases.2024; 12(3): 525. CrossRef - Effects of Seven Weeks of Combined Physical Training on High-Density Lipoprotein Functionality in Overweight/Obese Subjects
Tiziana Bacchetti, Camilla Morresi, Gianna Ferretti, Anders Larsson, Torbjörn Åkerfeldt, Michael Svensson
Metabolites.2023; 13(10): 1068. CrossRef - Physical Exercise Protects Against Endothelial Dysfunction in Cardiovascular and Metabolic Diseases
Juan Gao, Xue Pan, Guoping Li, Emeli Chatterjee, Junjie Xiao
Journal of Cardiovascular Translational Research.2022; 15(3): 604. CrossRef - Effects of Exercise Training on the Paracrine Function of Circulating Angiogenic Cells
William S. Evans, Ryan M. Sapp, Katherine I. Kim, James M. Heilman, James Hagberg, Steven J. Prior
International Journal of Sports Medicine.2021; 42(12): 1047. CrossRef - Chronic and Transient Hyperglycemia Induces Changes in the Expression Patterns of IL6 and ADIPOQ Genes and Their Associated Epigenetic Modifications in Differentiating Human Visceral Adipocytes
Adam Wróblewski, Justyna Strycharz, Ewa Świderska, Aneta Balcerczyk, Janusz Szemraj, Józef Drzewoski, Agnieszka Śliwińska
International Journal of Molecular Sciences.2021; 22(13): 6964. CrossRef - The Potential Role of MicroRNA in Diabetic Cardiomyopathy
Jin Hwa Kim
Diabetes & Metabolism Journal.2020; 44(1): 54. CrossRef

 KDA
KDA PubReader
PubReader Cite
Cite