Concern about the Safety of Bisphenol A Substitutes
Article information
Bisphenol A (BPA) is a base material for the production of polycarbonate plastic and epoxy resin, and it is one of the most produced chemicals [1]. BPA is a well-known endocrine disruptor and has a weak estrogenic effect. The adverse effects of BPA are largely related to its estrogenic activity [23], and they can disturb reproductive function. In addition, recent epidemiologic evidence has also shown that BPA is implicated in cardiovascular disease, type 2 diabetes mellitus, and obesity: high serum or urine BPA levels have been positively associated with diabetes mellitus [45], cardiovascular disease [4], obesity, and insulin resistance [6]. Therefore, BPA has been regulated in many countries. BPA was banned in baby bottles in Canada in 2008, in France in 2010, and in the European Union in 2011 [7]. Such regulations have led to the development of substitutes such as bisphenol S (BPS) and bisphenol F (BPF).
These BPA substitutes-based products are consumed under the label of “BPA-free.” This term gives the impression that the products are safe, but the safety of the substitutes is not fully verified [89]. Because of structural similarities with BPA (Fig. 1), these alternatives also show endocrine disruption effects like BPA, and many studies on adverse health effects of these alternatives are being reported.
In this issue of the Diabetes and Metabolism Journal, Liu et al. [10] evaluated the associations of BPA, BPF, and BPS with obesity in children and adolescents using data from the U.S. National Health and Nutrition Examination Survey 2013 to 2014. They found that exposure to BPF was positively associated with higher risk of obesity in children and adolescents, especially in boys. In terms of BPS, they did not observe significant associations with either general obesity or abdominal obesity. In their previous article, BPF or BPS was not significantly associated with obesity in U.S. adults [11]. BPS and BPF may have less harmful impact on health than BPA. However, as noted by the authors, since BPS or BPF has not replaced BPA for a long time, no significant results could be obtained due to their relative low exposure.
In a systematic review regarding comparison of the hormonal activity of BPA substitutes, the hormonal activities of BPS and BPF were in the same order of magnitude and of similar action as BPA (estrogenic, anti-estrogenic, androgenic, and anti-androgenic) in vitro and in vivo [9]. BPS and BPF showed altered organ weights, reproductive end points, and enzyme expression. In a recent study, the agonistic and/or antagonistic activities of BPA and its eight analogues (BPAF, BPAP, BPB, BPE, BPF, BPP, BPS, and BPZ) against human nuclear receptors (estrogen receptors [ERs], androgen receptor, glucocorticoid receptor, pregnane X receptor, and constitutive androstane receptor) were characterized via in vitro transactivation assays (Fig. 2) [12]. All the test compounds, except for BPP, showed both ERα- and ERβ-agonistic activities, with BPAF being the most potent. BPF and BPS were found to show weaker estrogenic activities than BPA. Their results suggested that BPA analogues demonstrate multiple effects via human nuclear receptors in a similar manner to BPA, and several analogues might have more potent endocrine-disrupting activity than BPA.
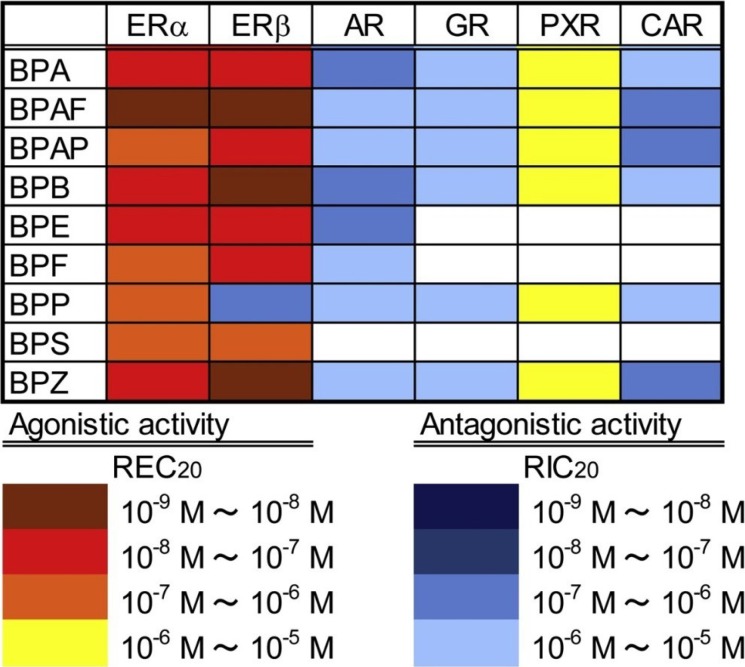
Profiling of bisphenol A (BPA) and eight its analogues on transcriptional activity via human nuclear receptors. Adapted from Kojima et al. [12], with permission from Elsevier. ERα, human estrogen receptors α; ERβ, human estrogen receptors β; AR, androgen receptor; GR, glucocorticoid receptor; PXR, pregnane X receptor; CAR, constitutive androstane receptor; REC20, the concentrations of the compound equal to 20% of the maximal response of the positive control intensity; RIC20, the concentrations of the test compounds showing 20% inhibition of the activities induced by the endogenous hormones or vehicle control.
In Korea, BPS are used for thermal receipt papers and BPF as a water pipe coating agent instead of BPA [13]. In the study measuring the concentrations of BPA, BPS, and BPF in the rivers of Japan, Korea, China, and India, BPF was the major bisphenol in rivers and the levels exceeded those of BPA. In the Han River in Korea, median concentrations of BPA, BPS, and BPF were 144, 41, and 555 ng/L, respectively. These were much higher compared with Nakdong River or Yeongsan River, where no BPF was detected [14]. This could be associated with the use of BPF-coated water pipes in Seoul [13]. In 2012, Liao et al. [14] analyzed BPS concentrations in 315 urine samples collected from the general populations in the United States and seven Asian countries including Korea. The urinary BPS concentration in Korea was very low (0.030 ng/mL) compared with Japan (1.18 ng/mL), United States (0.299 ng/mL) or China (0.226 ng/mL). However, the results are likely to change as BPS is replacing BPA for thermal receipt papers.
Because BPA substitutes such as BPS and BPF have similar structures to BPA, they appear to have similar metabolism, potencies, and action to BPA. In addition, they may pose similar potential health hazards as BPA. Continued biomonitoring of these bisphenols in populations and thorough investigations on their health effects in humans are needed. Manufacturers should continue to seek alternative safe materials rather than merely replacing BPA with bisphenol analogs.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.

