- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 43(3); 2019 > Article
-
Original ArticleEpidemiology Diabetes Mellitus and Cause-Specific Mortality: A Population-Based Study
-
Sen Li1,2
 , Jiaxin Wang1
, Jiaxin Wang1 , Biao Zhang3, Xinyi Li4, Yuan Liu5
, Biao Zhang3, Xinyi Li4, Yuan Liu5 -
Diabetes & Metabolism Journal 2019;43(3):319-341.
DOI: https://doi.org/10.4093/dmj.2018.0060
Published online: April 19, 2019
1School of Life Sciences, Beijing University of Chinese Medicine, Beijing, China.
2Department of Physiology, LKS Faculty of Medicine, University of Hong Kong, Hong Kong.
3Department of Epidemiology and Statistics, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, School of Basic Medicine, Peking Union Medical College, Beijing, China.
4School of Management, Beijing University of Chinese Medicine, Beijing, China.
5Department of Biostatistics and Bioinformatics, Winship Cancer Institute, Emory University, Atlanta, GA, USA.
- Corresponding author: Sen Li. School of Life Sciences, Beijing University of Chinese Medicine, No. 11, Bei San Huan Dong Lu, Chaoyang District, Beijing 100029, China. senli@connect.hku.hk
- *Sen Li and Jiaxin Wang contributed equally to this study as first authors.
Copyright © 2019 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- To investigate whether diabetes contributes to mortality for major types of diseases.
-
Methods
- Six National Health and Nutrition Examination Survey data cycles (1999 to 2000, 2001 to 2002, 2003 to 2004, 2005 to 2006, 2007 to 2008, and 2009 to 2010) and their linked mortality files were used. A population of 15,513 participants was included according to the availability of diabetes and mortality status.
-
Results
- Participants with diabetes tended to have higher all-cause mortality and mortality due to cardiovascular disease, cancer, chronic lower respiratory diseases, cerebrovascular disease, influenza and pneumonia, and kidney disease. Confounder-adjusted Cox proportional hazard models showed that both diagnosed diabetes category (yes or no) and diabetes status (diabetes, prediabetes, or no diabetes) were associated with all-cause mortality and with mortality due to cardiovascular disease, chronic lower respiratory diseases, influenza and pneumonia, and kidney disease. No associations were found for cancer-, accidents-, or Alzheimer's disease-related mortality.
-
Conclusion
- The current study's findings provide epidemiological evidence that diagnosed diabetes at the baseline is associated with increased mortality risk due to cardiovascular disease, chronic lower respiratory diseases, influenza and pneumonia, and kidney disease, but not with cancer or Alzheimer's disease.
- Diabetes mellitus is a common chronic disease and has multiple complications, which contribute to the global health-care burden. Diabetes is the seventh leading cause of death in the United States. The International Diabetes Federation has predicted that the number of people with diabetes worldwide will increase by 50%, from 366 million in 2011, by 2030 [1]. This increase in diabetes prevalence is presumably the result of population aging, increasing prevalence of obesity, and longer survival of people with diabetes [2].
- Diabetes is characterized by the chronic hyperglycemia-induced triad of symptoms (polydipsia, polyuria, and polyphagia) caused by elevated blood glucose level and metabolic dysregulation. Untreated diabetes leads to multiorgan and systemic injury, including to the heart, kidneys, nerves, and blood vessels, which impair the quality of life and increase the death rate caused by diabetes complications [3]. Some of these complications play a well-defined role in increasing the mortality of people with diabetes. For instance, people with diabetes have a twofold increased risk for cardiovascular mortality [4]. Diabetes also increases the risk of chronic kidney disease (affects 30% to 40% of individuals with diabetes) which is a major predictor of long-term mortality [5]. Whether diabetes can contribute to mortality caused by other major types of diseases, such as cancer and Alzheimer's disease (AD), remains a topic of debate because of limited epidemiological evidence. We investigated this issue using National Health and Nutrition Examination Survey (NHANES) 1999 to 2010 and its publicly available linked mortality file (LMF). Here, we report the associations between diabetes and mortality due to eight underlying causes of death, including cardiovascular disease (CVD), cancer, chronic lower respiratory disease (CLRD), accidents, cerebrovascular disease (CeVD), AD, influenza and pneumonia, and kidney disease.
INTRODUCTION
- Study population
- As a nationwide complex survey, NHANES collects health and nutrition data from the noninstitutional civilian United States population. Continuous NHANES data have been released by the National Center for Health Statistics (NCHS) every 2 years for public use since 1999. NHANES has been approved by the National Health Statistics Institutional Review Board. The current study used data from six NHANES survey cycles (1999 to 2010) as well as the publicly available NHANES (1999 to 2010) LMF to identify possible associations between diabetes status and mortality due to various causes, including CVD, cancer, CLRD, accidents, CeVD, AD, influenza and pneumonia, and kidney disease. National Death Index death certificate records were linked with NHANES LMF to identify the leading causes of death.
- This study was restricted to participants aged ≥40 years at the baseline. Of the 19,968 participants aged ≥40 years, 18,588 (93.1%) had information about diabetes status at the time of examination. We excluded 3,075 participants who had inadequate information of follow-up or other variables, which yielded a final population of 15,513 participants in this study (Supplementary Fig. 1). The median follow-up was 66 months and 2,042 all-cause deaths were recorded during follow-up.
- Diabetes is generally divided into type 1 (insufficient insulin production) and type 2 (insulin resistance), where type 2 diabetes mellitus represents the most prevalent type. Most of the 2,396 diabetic participants in the current study had type 2 diabetes mellitus and only 98 (4.09%) had been diagnosed with type 1 diabetes mellitus using the criteria diabetes diagnosis before age 40 years and currently using only insulin [6]. Seventeen participants (0.71%) could not be categorized because of insufficient information. The results of the analyses were essentially the same when excluding the 115 type 1 diabetes mellitus/uncategorized participants from the population; therefore, they were included in the study. However, given that a higher percentage of diabetic participants had type 2 diabetes mellitus, the study results represented mainly that type of diabetes.
- Cause-specific mortality
- Deaths due to numerous causes were identified according to the leading causes of death included in the publicly available NHANES LMF, which are based on the International Statistical Classification of Diseases, Injuries, and Causes of Death (ICD-10) guidelines. The nine leading causes of death are consistent with the following ICD-10 codes: CVD (I00–I09, I11, I13, I20–I51), cancer (C00–C97), CLRD (J40–J47), accidents (V01–X59, Y85–Y86), CeVD (I60–I69), AD (G30), influenza and pneumonia (J09–J18), and kidney disease (N00–N07, N17–N19, N25–N27). Participants with no record of death were deemed as alive and were censored at the end of follow-up (December 31, 2011). For the analysis of specific cause-related mortality, follow-up participants with other leading causes of death were censored at the age of death.
- Diagnosed diabetes category and diabetes status
- Diagnosed diabetes was categorized as yes or no based on the question, “Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Diabetes status was based on the category of diagnosed diabetes and blood glycosylated hemoglobin (HbA1c) level, and was defined as diabetes (with diagnosed diabetes or HbA1c ≥6.5%), prediabetes (without diagnosed diabetes and HbA1c 5.7% to 6.4%) or no diabetes (without diagnosed diabetes and HbA1c <5.7%). To measure blood HbA1c level, three instruments from two laboratories were used during 1999 to 2010. For NHANES 1999 to 2004, HbA1c level was measured at the University of Missouri in Columbia using a Primus CLC330 analyzer (Primus Corp., Kansas City, MO, USA). For NHANES 2005 to 2006 and 2007 to 2010, HbA1c levels were measured at the University of Minnesota using Tosoh A1C 2.2 Plus (Tosoh Medics, San Francisco, CA, USA) and Tosoh A1C G7, respectively. Cross-over studies for laboratory method were performed each time when changing instruments. The detailed methodology can be found in the NHANES Laboratory Procedures Manual. Population-attributable risk percentage (PAR%) for diabetes at the baseline was calculated for cause-specific mortality using the following equation:
- where P refers to the prevalence of sampled persons with diagnosed diabetes and HR is the hazard ratio calculated using the Cox proportional-hazards model.
- Other variables
- The associations between diagnosed diabetes/diabetes status and all-cause/cause-specific mortality were adjusted for a series of potential confounding factors: sex (male or female); age in years at the baseline examination (40 to 49, 50 to 59, 60 to 69, or ≥70 years); race (non-Hispanic white, non-Hispanic black, or others); educational level (less than high school, high school, or more than high school); poverty income ratio (PIR; <1, ≤1 and less than the median, or greater than the median, where the medians was computed based on PIR ≥1 for each of the six data cycles); body mass index (BMI; <25 or ≥25 kg/m2, where BMI ≥25 kg/m2 indicates overweight based on the National Institutes of Health's health guidelines); smoking status (yes or no, based on the question, “Have you smoked at least 100 cigarettes in your entire life?”); alcohol use (yes or no, based on the question, “In any 1 year, have you had at least 12 drinks of any type of alcoholic beverage?”); hypertension status (yes or no, based on the question, “Have you ever been told by a doctor or other health professional that you had hypertension, also called high blood pressure?”); and physical activity (yes or no; participants reporting any vigorous or moderate activities were considered active).
- Statistical analysis
- Sample weighting was used to account for the complex sampling design following the NHANES Analytic and Reporting Guidelines. The weighted characteristics were calculated based on the overall data, and data were stratified by the diagnosed diabetes category (yes or no) and diabetes status (diabetes, prediabetes, no diabetes). Possible statistical differences for variables were examined using the Rao-Scott chi-square test. The association between diagnosed diabetes/diabetes status and cause-specific death rate was studied using Cox proportional-hazards regression using the “proportional hazards regression (PHREG)” procedure. The HR and 95% confidence interval for the risk of all-cause or specific cause-related mortality for diabetic participants were calculated by comparing with the population without diabetes. Ptrend was also calculated for diabetes status categories. In the adjusted hazard model, age was used as a continuous variable for confounder adjustments. In analyses of all-cause and each of the eight cause-specific mortalities, the proportional-hazards assumption for diagnosed diabetes category and diabetes status was verified using the Kolmogorov-type supremum test. Directly adjusted Kaplan-Meyer curves were plotted using the “direct adjusted (DIRADJ)” option in SAS software version 9.4 (SAS Institute, Cary, NC, USA) and SAS was used for all statistical analyses.
- Ethics approval and consent to participate
- Data analysed in this study were obtained from NHANES. Protocols involved were approved by the NCHS Research Ethics Review Board (ERB) (protocol #98-12 and #2005-06), and consent from all participants was documented.
- Availability of data and material
- The data used in this study is from NHANES 1999 to 2010 and corresponding mortality follow-up study. Data are publicly available and can be downloaded from NHANES website: http://www.cdc.gov/nchs/nhanes.htm.
METHODS
- The demographic data for the overall study population and subpopulations according to diagnosed diabetes category or diabetes status are shown in Table 1. The sample sizes were 2,396 and 13,117 for the diagnosed diabetes categories yes and no, respectively. For diabetes status, the sample sizes were 2,909, 3,770, and 8,834 for diabetes, prediabetes, and no diabetes, respectively. Compared with the subpopulation without diabetes, diabetic participants were more likely to be men, ≥60 years old, of non-White ethnicity, with less than a high school education, with income below the median, to have a BMI ≥25 km/m2, to have a history of hypertension, and to be physically inactive. Participants with diabetes also tended to have higher all-cause mortality and mortality due to CVD, cancer, CLRD, CeVD, influenza and pneumonia, and kidney disease. All-cause and cause-specific mortality rate was calculated according to the glycemic control status and duration of diabetes in diabetic participants (Supplementary Tables 1 and 2).
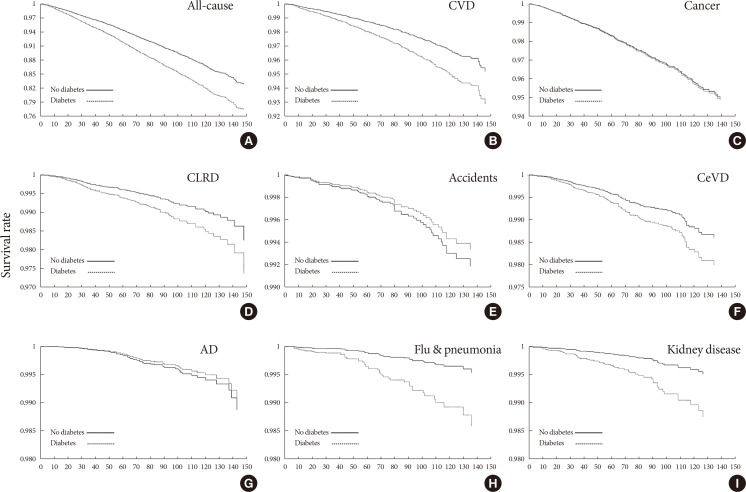
- HRs from the Cox proportional-hazard models for all-cause and eight cause-specific mortalities were calculated using the diagnosed diabetes category (yes or no) as an independent variable (Table 2). All-cause mortality and mortality due to CVD, CLRD, CeVD, influenza and pneumonia, and kidney disease were consistently associated with diagnosed diabetes both before and after multivariable adjustment. However, these associations were not significant for mortality related to cancer, accidents, or AD. Sex-stratified analysis suggested that baseline diabetes was associated with mortality due to CeVD in men, but not women after adjusting for potential confounders (Supplementary Table 3). Information on history of cancer, CVD, CeVD, and lung disease at the baseline are shown in Supplementary Table 4, and the results from the Cox model after adjusting for the abovementioned major types of disease at the baseline as confounding variables are shown in Supplementary Table 5. Adjusting for the NHANES data cycle in the Cox proportional-hazards model produced similar results (Supplementary Table 6). As an important indicator of public health impact, PAR represents the proportion of cause-specific mortality that could be attributed to baseline diabetes. Compared with nondiabetic participants, the PAR% values for those with diagnosed diabetes for all-cause mortality and mortality related to CVD, CLRD, CeVD, influenza and pneumonia, and kidney disease were 7.00%, 8.23%, 6.04%, 5.64%, 22.09%, and 18.14%, respectively. However, we note that the diabetes prevalence rate used to calculate PAR% was derived from the population in the present study rather than the community. Kaplan-Meier curves for diagnosed diabetes category were used to estimate 10-year survival (Fig. 1).
- The HRs for all-cause and eight cause-specific mortalities are presented in Table 3; diabetes status (diabetes, prediabetes, no diabetes) was set as an independent variable. Trend analysis indicated that diabetes status was significantly associated with all-cause mortality and mortality related to CVD, CLRD, influenza and pneumonia, and kidney disease. The association between diabetes status and cancer- or CeVD-specific mortality was nonsignificant after adjusting for covariates, and no such association was found for accident- and AD-related mortality. Kaplan-Meier survival curves for diabetes status are shown in Fig. 2.
RESULTS
- In the present study, we analyzed data from six NHANES survey cycles (1999 to 2010) and the mortality follow-up data to identify associations between diabetes and mortality due to specific causes. Our findings provide epidemiological evidence that diabetes contributes to mortality due to major types of diseases. According to studies published in the 1990s, the life expectancy of people with diabetes is generally 7.5 years less than that of nondiabetic people and this life-shortening effect of diabetes is greater in people who develop diabetes at a younger age [7]. Our analysis showed a 10% higher all-cause mortality in the diabetic subpopulation compared with the nondiabetic subpopulation. Moreover, the percent of diabetic participants with a lower level of education (30.62%) or PIR (14.92%) was higher than those of non-diabetic participants (17.62% or 9.37%, respectively). Mortality rates among people with diabetes also vary according to individual income and educational level [8]. Our analysis showed that adults with diagnosed diabetes were more likely to have a BMI >25 kg/m2 compared with nondiabetic participants (85.70% and 69.73%, respectively), which is consistent with the National Health Interview Survey data (2000 to 2009), indicating that obesity correlates positively with the occurrence of diabetes [9]. Given that hypertension is a common risk factor for diseases such as peripheral arterial disease [10], we included high blood pressure status as a confounder in our analysis. The weighted percentage of participants with hypertension was much higher in the diabetic than in the nondiabetic subpopulation (68.18% and 36.03%, respectively) and shows the high rate of co-occurrence of these two diseases. People with diabetes are also more likely to be physically inactive [10], and behavioral intervention strategies have been implemented to help people with diabetes maintain a physically active lifestyle [11]. Similarly, our analysis showed that diabetic participants were 14.86% less likely to be physically active.
- CVD is a known complication of diabetes. A mechanistic study in diabetic rats showed that cardiac function changes gradually during the progression of diabetes and that these changes are closely related to alterations in two groups of proteins, neurotrophic cascade protein (NTF4) and electron transport chain cascade protein (ETFB) [12]. Several epidemiological studies have investigated the elevated mortality rate due to CVD in people with diabetes. Evidence from the Cardiovascular Prevention from Observational Cohorts in JAPAN (EPOCH-JAPAN) study showed that diabetes is a significant risk factor for all-cause and CVD-specific mortality and is associated with a two- to four-fold increased risk of cardiovascular death [4]. Seventy percent of diabetes-related mortality is attributed to CVD [13]. Given this high death rate, the use of prognostic factors, such as serum 25-hydroxyvitamin D3 (25(OH)D3), has been proposed for CVD prevention in diabetic patients [14]. Our data indicated a 1.81-fold elevated CVD mortality risk in participants with diagnosed diabetes, and the trend analysis indicated that diabetes status (no diabetes, prediabetes, and diabetes) was significantly (Ptrend<0.01) associated with CVD-specific mortality.
- According to the publicly available NHANES LMF, as an underlying cause of death, CLRD comprises mainly chronic bronchitis, emphysema, and asthma. Characterized by long-term breathing problems and poor airflow, chronic obstructive pulmonary disease (COPD) represents a severe respiratory disease, and chronic bronchitis and emphysema are the most common forms of COPD. A retrospective longitudinal cohort study in northern California reported a significantly higher incidence of COPD in people diagnosed with diabetes [15]. Diabetic people are more sensitive to complicated lower respiratory tract infections, and a nearly half of them develop emphysema or asthma as a frequent comorbidity [16]. Respiratory function tests confirm the adverse effects of diabetes on the respiratory system [17], which may reflect pathological changes observed in the diabetic lung, such as vascular hyalinosis and septal degeneration [17]. Similar to people with COPD, diabetic people also have a higher prevalence of asthma [18]. This evidence suggests that diabetic people are a high-risk population for CLRD-mediated morbidity and mortality. Our analyses showed an association between diabetes and CLRD mortality (P=0.05) when using diabetes as a dichotomous variable (Table 2). Trend analysis for diabetes status showed significant results (Ptrend<0.01), and the adjusted HRs for participants with prediabetes and diabetes were 1.36 and 1.89, respectively, compared with those without diabetes as the reference (Table 3).
- Seasonal influenza is a global health burden among the general population and increases the risk of mortality in winter. Adults with diabetes are at high risk for influenza-mediated morbidity and mortality [19]. People with diabetes are also more likely to have an impaired immune response to influenza vaccine and are thus more sensitive to influenza-related complications [19]. Dysfunction of the immune system in diabetic people may be attributed to an abnormal CD4/CD8 lymphocyte ratio and malfunction of natural killer cells and monocytes [20]. Epidemiological studies suggest that people with diabetes, especially those with cardiac and renal complications, are at high risk of death due to influenza and pneumococcal disease [20]. Analysis of Canadian administrative data showed greater susceptibility to influenza in adults with diabetes and a 6% higher rate of influenza-attributable all-cause hospitalization in diabetic people of working age (aged <65 years) [21]. Similar as for influenza, analyses of 97 prospective studies revealed a higher risk for mortality due to pneumonia and other infectious diseases (1.67- and 2.39-fold, respectively) [22]. Moreover, an observational study of a prospective cohort of immunocompetent adults with community-acquired pneumonia reported different clinical features between pneumonia patients with and without diabetes. This study also reported that additional risk factors for mortality, such as bacteremia and septic shock, are found in patients with both pneumonia and diabetes [23]. In that study, patients with diabetes generally had more severe pneumonia compared with nondiabetic patients [23], which may lead to an elevated death rate in people with diabetes. Consistent with this evidence, we found a 3.56-fold elevated risk for influenza and pneumonia-specific mortality in participants with diagnosed diabetes after adjustment for confounders.
- Diabetes is a known risk factor for CeVD, and CeVD represents one of the leading causes of morbidity and mortality in people with diabetes. Adjusted models in the Cox proportional-hazards regression analysis of the diagnosed diabetes category (Table 2) revealed a significant association between baseline diabetes and CeVD mortality (P=0.05). Trend analysis suggested that the association between diabetes status (diabetes, prediabetes, no diabetes) and mortality due to CeVD was nonsignificant (Table 3), which is presumably because prediabetes or less severe diabetes is not associated with vascular pathology in the brain as revealed by magnetic resonance imaging [24]. Moreover, our sex-stratified analysis suggested that baseline diabetes was associated with mortality due to CeVD in men, but not in women (Supplementary Table 3), which may also have contributed to the overall weak association between diabetes and CeVD-specific mortality.
- Kidney disease, as defined by increased urine albumin excretion and/or impaired glomerular filtration rate, is a known complication of diabetes. Ten-year cumulative mortality analysis using NHANES III (1988 to 1994) data revealed that kidney disease contributes predominantly to the elevated mortality in diabetic people [25]. A population-based case-control study concluded that high HbA1c level may contribute to deteriorating kidney function and may thereby increase mortality due to kidney disease [2]. An animal model suggested that upregulation of the release of proinflammatory cytokines and transforming growth factor-β1 signaling may be the mechanisms underlying diabetic nephropathy [26]. Consistent with this evidence, we observed a threefold increased risk of kidney disease-mediated mortality in people with diagnosed diabetes.
- Diabetes treatment by insulin may lead to hypoglycemia as a common side effect. Low blood glucose level has been linked to impaired cognitive function and may contribute to accidents due to deteriorated driving performance [27]. However, hypoglycemia-induced accidents are extremely rare and cannot serve as material consideration for the accidents during driving [28]. A population register-based study showed a similar risk for road traffic collisions in insulin-treated patients and nondiabetic individuals [29]. We included mortality due to accidents in our analysis, although this was not limited to transport accidents, and our data show clearly that diabetes status was not associated with accident-specific mortality.
- Cancer and diabetes share many common risk factors, such as obesity and physical inactivity. Although the potential role of poorly controlled hyperglycemia and hyperinsulinemia in carcinogenesis has been proposed in people with diabetes, the relationship between diabetes and cancer-specific mortality is inconsistent [30]. A retrospective study in China reported a significantly increased overall mortality risk of cancer in people with diabetes [31]. Data from the Strong Heart Study in the United States show that diabetes increases the risk of cancer mortality by 1.27-fold in American Indians [32]. Data analyses from a French cohort suggest that diabetes is not associated with cancer mortality in patients with end-stage renal disease [33]. For specific types of cancer, a consistent positive association between baseline diabetes and mortality due to liver, pancreas, or bladder cancer has been reported, although the relationship between diabetes and endometrial cancer mortality is inconsistent [30]. Moreover, diabetes is not associated with kidney cancer mortality [30]. For other site-specific cancers, a positive association between diabetes and breast cancer-specific mortality has been found in Black women, and this excess breast cancer-mediated death caused by diabetes is with racial disparities [34]. Another study suggested that diabetes is not associated with mortality due to breast cancer, regardless of diabetes treatment and duration [35]. For male-specific cancer, diabetes has been reported to be associated with a reduced incidence of prostate cancer in several studies [36]. A Japan cohort study showed an association between diabetes and colorectal cancer mortality only in women [37]. Thus, the evidence of a possible association between diabetes and mortality due to specific types of cancer is inconsistent. The crude models in the Cox proportional-hazards regression analysis of diabetes status (Table 3) in our study suggest that baseline diabetes was significantly associated with increased risk of cancer mortality. However, this association disappeared after multivariable adjustment, which is consistent with a recent study [38]. We did not analyze site-specific cancer mortality because such information is not publicly available in the NHANES (1999 to 2010) LMF to protect the confidentiality of NHANES participants.
- As another age-related disease, AD has been linked to diabetes in several studies that have shown an increased risk of AD development in diabetes patients. The Tel Aviv Brain Acute Stroke Cohort (TABASCO) study showed an independent effect of diabetes on brain atrophy and the risk of cognitive decline in survivors of stroke/transient ischemic attack [39]. However, the results of clinicopathological investigations suggest that AD patients with diabetes have the same levels of amyloid-β and neurofibrillary tangles as nondiabetic AD patients [40]. Most of the papers analyzed in a systematic review showed no significant association between diabetes and cognitive decline in AD patients [41]. Diabetes was not associated with AD after controlling for CeVD in a study based on a nationally representative database of aged United States Medicare beneficiaries [42]. Therefore, the relationship between diabetes and AD remains uncertain. In our study, baseline diabetes was not associated with increased risk of AD mortality in the United States general population.
- In summary, diabetes plays a well-defined role in elevating mortality due to several major diseases while whether diabetes can contribute to mortality of other diseases, such as cancer and AD, is still a topic of debate. We examined the associations between diabetes and mortality due to eight underlying causes of death. We found epidemiological evidence that diabetes diagnosed at the baseline was associated with increased mortality risk due to CVD, CLRD, influenza and pneumonia, and kidney disease, but not cancer or AD. These findings remained consistent even after including participants with insufficient information on confounding variables (Supplementary Table 7). As an indicator of the proportion of cause-specific mortality attributable to baseline diabetes, the PAR% was calculated using HRs from the Cox models [43]. However, we note that HR may not be identical to relative risk when calculating PAR, especially at longer follow-up time points.
- Our study has several limitations. First, the follow-up was relatively short, especially for the NHANES data cycle 2009 to 2010, in which participants were followed for only 2 years. Second, only one certifying physician-marked leading cause of death was recorded in the death certificate even though some deaths may be attributed to multiple causes. Third, several diseases are with few events in the analysis of cause-specific mortality, and we have to include a limited number of confounding variables to prevent biased adjusted HRs. Our work has several strengths. The NHANES data are generalizable to the noninstitutional civilian United States population. By using the most recent NHANES and its mortality data, this study included all available leading causes of death-mediated mortality and examined the association with baseline diabetes. Our findings provide new information about whether diabetes contributes to mortality due to the major types of diseases.
- In conclusion, we studied the association between diabetes diagnosed at baseline and mortality due to various causes using NHANES (1999 to 2010) and its mortality follow-up study. We found positive associations between diagnosed diabetes and mortality due to CVD, CLRD, influenza and pneumonia, and kidney disease; baseline diabetes did not contribute to cancer- and AD-specific mortality.
DISCUSSION
-
Acknowledgements
- This study is supported by the National Natural Science Foundation of China (Grant No. 81703942), Science Fund for Distinguished Young Scholars in BUCM (Grant No. BUCM-2019-JCRC004) and BUCM research program (to Sen Li).
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS:
NOTES
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Supplementary Table 2
Supplementary Table 3
Supplementary Table 4
Supplementary Table 5
Supplementary Table 6
Supplementary Table 7
Supplementary Fig. 1
- 1. Cheng LJ, Chen JH, Lin MY, Chen LC, Lao CH, Luh H, Hwang SJ. A competing risk analysis of sequential complication development in Asian type 2 diabetes mellitus patients. Sci Rep 2015;5:15687ArticlePubMedPMCPDF
- 2. Nicholas J, Charlton J, Dregan A, Gulliford MC. Recent HbA1c values and mortality risk in type 2 diabetes. Population-based case-control study. PLoS One 2013;8:e68008. ArticlePubMedPMC
- 3. Liu Z, Fu C, Wang W, Xu B. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients: a cross-sectional hospital based survey in urban China. Health Qual Life Outcomes 2010;8:62ArticlePubMedPMCPDF
- 4. Hirakawa Y, Ninomiya T, Kiyohara Y, Murakami Y, Saitoh S, Nakagawa H, Okayama A, Tamakoshi A, Sakata K, Miura K, Ueshima H, Okamura T. Evidence for Cardiovascular Prevention From Observational Cohorts in Japan Research Group (EPOCH-JAPAN). Age-specific impact of diabetes mellitus on the risk of cardiovascular mortality: an overview from the evidence for Cardiovascular Prevention from Observational Cohorts in the Japan Research Group (EPOCH-JAPAN). J Epidemiol 2017;27:123-129. ArticlePubMedPMC
- 5. Salinero-Fort MA, San Andres-Rebollo FJ, de Burgos-Lunar C, Abanades-Herranz JC, Carrillo-de-Santa-Pau E, Chico-Moraleja RM, Jimenez-García R, Lopez-de-Andres A, Gomez-Campelo P. MADIABETES Group. Cardiovascular and all-cause mortality in patients with type 2 diabetes mellitus in the MADIABETES Cohort Study: association with chronic kidney disease. J Diabetes Complications 2016;30:227-236. ArticlePubMed
- 6. Looker AC, Eberhardt MS, Saydah SH. Diabetes and fracture risk in older U.S. adults. Bone 2016;82:9-15. ArticlePubMed
- 7. Nwaneri C, Bowen-Jones D, Cooper H, Chikkaveerappa K, Afolabi BA. Falling mortality rates in type 2 diabetes mellitus in the Wirral Peninsula: a longitudinal and retrospective cohort population-based study. Postgrad Med J 2012;88:679-683. ArticlePubMedPDF
- 8. Rawshani A, Svensson AM, Zethelius B, Eliasson B, Rosengren A, Gudbjornsdottir S. Association between socioeconomic status and mortality, cardiovascular disease, and cancer in patients with type 2 diabetes. JAMA Intern Med 2016;176:1146-1154. ArticlePubMed
- 9. Liu L, Simon B, Shi J, Mallhi AK, Eisen HJ. Impact of diabetes mellitus on risk of cardiovascular disease and all-cause mortality: evidence on health outcomes and antidiabetic treatment in United States adults. World J Diabetes 2016;7:449-461. ArticlePubMedPMC
- 10. Ito WD, Lund N, Sager H, Becker W, Wenzel U. Differential impact of diabetes mellitus type II and arterial hypertension on collateral artery growth and concomitant macrophage accumulation. Vasa 2015;44:31-41. ArticlePubMed
- 11. Balducci S, D'Errico V, Haxhi J, Sacchetti M, Orlando G, Cardelli P, Vitale M, Bollanti L, Conti F, Zanuso S, Nicolucci A, Pugliese G. Italian Diabetes and Exercise Study 2 (IDES_2) Investigators. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care 2017;40:1444-1452. PubMed
- 12. Karthik D, Vijayakumar R, Pazhanichamy K, Ravikumar S. A proteomics approach to identify the differential protein level in cardiac muscle of diabetic rat. Acta Biochim Pol 2014;61:285-293. ArticlePubMedPDF
- 13. Wan EY, Fung CS, Fong DY, Lam CL. Association of variability in hemoglobin A1c with cardiovascular diseases and mortality in Chinese patients with type 2 diabetes mellitus: a retrospective population-based cohort study. J Diabetes Complications 2016;30:1240-1247. ArticlePubMed
- 14. Samefors M, Scragg R, Lanne T, Nystrom FH, Ostgren CJ. Association between serum 25(OH)D(3) and cardiovascular morbidity and mortality in people with type 2 diabetes: a community-based cohort study. Diabet Med 2017;34:372-379. ArticlePubMedPDF
- 15. Ehrlich SF, Quesenberry CP Jr, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 2010;33:55-60. ArticlePubMedPDF
- 16. Venmans LM, Bont J, Gorter KJ, Verheij TJ, Rutten GE, Hak E. Prediction of complicated lower respiratory tract infections in older patients with diabetes. Br J Gen Pract 2008;58:564-568. ArticlePubMedPMC
- 17. Colbay G, Cetin M, Colbay M, Berker D, Guler S. Type 2 diabetes affects sleep quality by disrupting the respiratory function. J Diabetes 2015;7:664-671. ArticlePubMed
- 18. Klekotka RB, Mizgała E, Krol W. The etiology of lower respiratory tract infections in people with diabetes. Pneumonol Alergol Pol 2015;83:401-408. ArticlePubMed
- 19. Lau D, Eurich DT, Majumdar SR, Katz A, Johnson JA. Effectiveness of influenza vaccination in working-age adults with diabetes: a population-based cohort study. Thorax 2013;68:658-663. ArticlePubMedPMC
- 20. American Diabetes Association. Immunization and the prevention of influenza and pneumococcal disease in people with diabetes. Diabetes Care 2000;23(Suppl 1):S91-S93. ArticlePubMedPDF
- 21. Lau D, Eurich DT, Majumdar SR, Katz A, Johnson JA. Working-age adults with diabetes experience greater susceptibility to seasonal influenza: a population-based cohort study. Diabetologia 2014;57:690-698. ArticlePubMedPDF
- 22. Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njolstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829-841. ArticlePubMedPMC
- 23. Di Yacovo S, Garcia-Vidal C, Viasus D, Adamuz J, Oriol I, Gili F, Vilarrasa N, García-Somoza MD, Dorca J, Carratala J. Clinical features, etiology, and outcomes of community-acquired pneumonia in patients with diabetes mellitus. Medicine (Baltimore) 2013;92:42-50. ArticlePubMedPMC
- 24. Schneider ALC, Selvin E, Sharrett AR, Griswold M, Coresh J, Jack CR Jr, Knopman D, Mosley T, Gottesman RF. Diabetes, prediabetes, and brain volumes and subclinical cerebrovascular disease on MRI: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Diabetes Care 2017;40:1514-1521. ArticlePubMedPMCPDF
- 25. Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24:302-308. ArticlePubMedPMC
- 26. Ohno K, Kuno A, Murase H, Muratsubaki S, Miki T, Tanno M, Yano T, Ishikawa S, Yamashita T, Miura T. Diabetes increases the susceptibility to acute kidney injury after myocardial infarction through augmented activation of renal Toll-like receptors in rats. Am J Physiol Heart Circ Physiol 2017;313:H1130-H1142. ArticlePubMed
- 27. Graveling AJ, Frier BM. Driving and diabetes: problems, licensing restrictions and recommendations for safe driving. Clin Diabetes Endocrinol 2015;1:8ArticlePubMedPMCPDF
- 28. Harsch IA, Stocker S, Radespiel-Troger M, Hahn EG, Konturek PC, Ficker JH, Lohmann T. Traffic hypoglycaemias and accidents in patients with diabetes mellitus treated with different antidiabetic regimens. J Intern Med 2002;252:352-360. ArticlePubMed
- 29. Lonnen KF, Powell RJ, Taylor D, Shore AC, MacLeod KM. Road traffic accidents and diabetes: insulin use does not determine risk. Diabet Med 2008;25:578-584. ArticlePubMed
- 30. Renehan AG, Yeh HC, Johnson JA, Wild SH, Gale EA, Moller H;. Diabetes and cancer (2): evaluating the impact of diabetes on mortality in patients with cancer. Diabetologia 2012;55:1619-1632. ArticlePubMedPDF
- 31. Gu Y, Hou X, Zheng Y, Wang C, Zhang L, Li J, Huang Z, Han M, Bao Y, Zhong W, Jia W, Cui S. Incidence and mortality risks of cancer in patients with type 2 diabetes: a retrospective study in Shanghai, China. Int J Environ Res Public Health 2016;13:E559. Article
- 32. Best LG, Garcia-Esquinas E, Yeh JL, Yeh F, Zhang Y, Lee ET, Howard BV, Farley JH, Welty TK, Rhoades DA, Rhoades ER, Umans JG, Navas-Acien A. Association of diabetes and cancer mortality in American Indians: the Strong Heart Study. Cancer Causes Control 2015;26:1551-1560. ArticlePubMedPMCPDF
- 33. Pladys A, Couchoud C, LeGuillou A, Siebert M, Vigneau C, Bayat S. Type 1 and type 2 diabetes and cancer mortality in the 2002-2009 cohort of 39,811 French dialyzed patients. PLoS One 2015;10:e0125089. ArticlePubMedPMC
- 34. Charlot M, Castro-Webb N, Bethea TN, Bertrand K, Boggs DA, Denis GV, Adams-Campbell LL, Rosenberg L, Palmer JR. Diabetes and breast cancer mortality in Black women. Cancer Causes Control 2017;28:61-67. ArticlePubMedPDF
- 35. Luo J, Virnig B, Hendryx M, Wen S, Chelebowski R, Chen C, Rohan T, Tinker L, Wactawski-Wende J, Lessin L, Margolis K. Diabetes, diabetes treatment and breast cancer prognosis. Breast Cancer Res Treat 2014;148:153-162. ArticlePubMedPMCPDF
- 36. Lee J, Giovannucci E, Jeon JY. Diabetes and mortality in patients with prostate cancer: a meta-analysis. Springerplus 2016;5:1548ArticlePubMedPMCPDF
- 37. Tan C, Mori M, Adachi Y, Wakai K, Suzuki S, Suzuki K, Hashimoto Sh, Watanabe Y, Tamakoshi A. Diabetes mellitus and risk of colorectal cancer mortality in Japan: the Japan Collaborative Cohort Study. Asian Pac J Cancer Prev 2016;17:4681-4688. PubMedPMC
- 38. Tsujimoto T, Kajio H, Sugiyama T. Favourable changes in mortality in people with diabetes: US NHANES 1999-2010. Diabetes Obes Metab 2018;20:85-93. PubMed
- 39. Ben Assayag E, Eldor R, Korczyn AD, Kliper E, Shenhar-Tsarfaty S, Tene O, Molad J, Shapira I, Berliner S, Volfson V, Shopin L, Strauss Y, Hallevi H, Bornstein NM, Auriel E. Type 2 diabetes mellitus and impaired renal function are associated with brain alterations and poststroke cognitive decline. Stroke 2017;48:2368-2374. ArticlePubMed
- 40. Kalaria RN. Neurodegenerative disease: diabetes, microvascular pathology and Alzheimer disease. Nat Rev Neurol 2009;5:305-306. ArticlePubMedPDF
- 41. Li J, Cesari M, Liu F, Dong B, Vellas B. Effects of diabetes mellitus on cognitive decline in patients with Alzheimer disease: a systematic review. Can J Diabetes 2017;41:114-119. ArticlePubMed
- 42. Lu ZK, Li M, Yuan J, Wu J. The role of cerebrovascular disease and the association between diabetes mellitus and dementia among aged medicare beneficiaries. Int J Geriatr Psychiatry 2016;31:92-98. ArticlePubMed
- 43. Dodge HH, Chang CC, Kamboh IM, Ganguli M. Risk of Alzheimer's disease incidence attributable to vascular disease in the population. Alzheimers Dement 2011;7:356-360. ArticlePubMedPMCPDF
REFERENCES
Adjusted Kaplan-Meier survival curves for cumulative all-cause and other mortality according to diagnosed diabetes category: (A) all-cause, (B) cardiovascular disease (CVD), (C) cancer, (D) chronic lower respiratory disease (CLRD), (E) accidents, (F) cerebrovascular disease (CeVD), (G) Alzheimer's disease (AD), (H) flu & pneumonia, and (I) kidney disease.
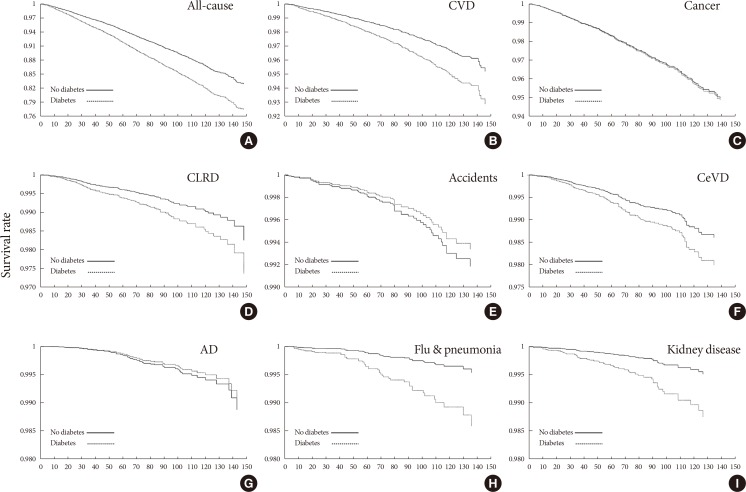
Adjusted Kaplan-Meier survival curves for cumulative all-cause and other mortality according to diabetes status category: (A) all-cause, (B) cardiovascular disease (CVD), (C) cancer, (D) chronic lower respiratory disease (CLRD), (E) accidents, (F) cerebrovascular disease (CeVD), (G) Alzheimer's disease (AD), (H) flu & pneumonia, and (I) kidney disease.

Weighted characteristics of the study population: National Health and Nutrition Examination Survey 1999 to 2010
Hazard ratio for all-cause and other mortality by diagnosed diabetes category: National Health and Nutrition Examination Survey 1999 to 2010
cHR, crude hazard ratio; LL, lower 95% confidence limit; UL, upper 95% confidence limit; aHR, adjusted hazard ratio; CVD, cardiovascular disease; CLRD, chronic lower respiratory diseases; CeVD, cerebrovascular disease; AD, Alzheimer's disease; Flu, influenza.
aModel was adjusted for sex, age, body mass index, smoking status, and alcohol-use status.
Hazard ratio for all-cause and other mortality by diabetes status category: National Health and Nutrition Examination Survey 1999 to 2010
cHR, crude hazard ratio; LL, lower 95% confidence limit; UL, upper 95% confidence limit; aHR, adjusted hazard ratio; CVD, cardiovascular disease; CLRD, chronic lower respiratory disease; CeVD, cerebrovascular disease; AD, Alzheimer's disease; Flu, influenza.
aModel was adjusted for sex, age, body mass index, smoking status, and alcohol-use status.
Figure & Data
References
Citations

- Association of type 2 diabetes mellitus with dementia‐related and non–dementia‐related mortality among postmenopausal women: A secondary competing risks analysis of the women's health initiative
Tyler J. Titcomb, Phyllis Richey, Ramon Casanova, Lawrence S. Phillips, Simin Liu, Shama D. Karanth, Nazmus Saquib, Tomas Nuño, JoAnn E. Manson, Aladdin H. Shadyab, Longjian Liu, Terry L. Wahls, Linda G. Snetselaar, Robert B. Wallace, Wei Bao
Alzheimer's & Dementia.2024; 20(1): 234. CrossRef - Antiglycation potential of metal ions and polyphenolic extract of chickpea on thiol-protease inhibitor: A management for diabetic complications
Mohd Shahnawaz Khan, Sheraz Ahmad Bhat, Monnera Saud Albagmi, Mohammed Arshad, Mohammad Tarique, Bilqees Bano
Saudi Pharmaceutical Journal.2024; 32(1): 101916. CrossRef - GLP-1 receptor agonists, SGLT2 inhibitors and noncardiovascular mortality in type 2 diabetes: Insights from a meta-analysis
Mainak Banerjee, Rimesh Pal, Indira Maisnam, Satinath Mukhopadhyay
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2024; 18(1): 102943. CrossRef - Assessment of the radial peripapillary capillary plexus and retinal nerve fiber layer thickness in diabetic patients in comparison to normal age-matched individuals
Christina S.I. Farag, Heba M.A. El-Saied, Hala M. El-Mofty, Randa M.A.M. El-Mofty
Journal of the Egyptian Ophthalmological Society.2024; 117(1): 43. CrossRef - Fasting GLP-1 Levels and Albuminuria Are Negatively Associated in Patients with Type 2 Diabetes Mellitus
Cheol-Won Jang, Tae Yang Yu, Jin Woo Jeong, Se Eun Ha, Rajan Singh, Moon Young Lee, Seungil Ro
Journal of Personalized Medicine.2024; 14(3): 280. CrossRef - The Benefits and Harms of Lung Cancer Screening in Individuals With Comorbidities
Minal S. Kale, Keith Sigel, Arushi Arora, Bart S. Ferket, Juan Wisnivesky, Chung Yin Kong
JTO Clinical and Research Reports.2024; 5(3): 100635. CrossRef - Risk factor control and cardiovascular events in patients with type 2 diabetes mellitus
Do Kyeong Song, Young Sun Hong, Yeon-Ah Sung, Hyejin Lee, Hidetaka Hamasaki
PLOS ONE.2024; 19(2): e0299035. CrossRef - Liraglutide Attenuates Diabetic Cardiomyopathy via the ILK/PI3K/AKT/PTEN Signaling Pathway in Rats with Streptozotocin-Induced Type 2 Diabetes Mellitus
Shatha M. Alobaid, Rahaf M. Alshahrani, Asma S. Alonazi, Nawal M. Alrasheed, Maha A. Alamin, Tahani K. Alshammari, Anfal F. Bin Dayel, Doaa M. Elnagar, Rana R. Alotaibi, Lama A. Almuthnabi, Dalia H. Almasud, Shahad E. Al-Ammar, Shahad O. Almadhi, Reema A.
Pharmaceuticals.2024; 17(3): 374. CrossRef - Effects of high-intensity interval training and its different protocols on lipid profile and glycaemic control in type 2 diabetes: A meta-analysis
Nandiny Paula Cavalli, Mariana Brondani de Mello, Natiele Camponogara Righi, Felipe Barreto Schuch, Luis Ulisses Signori, Antônio Marcos Vargas da Silva
Journal of Sports Sciences.2024; : 1. CrossRef - Role of renin-angiotensin system/angiotensin converting enzyme-2 mechanism and enhanced COVID-19 susceptibility in type 2 diabetes mellitus
Ashwin Kumar Shukla, Komal Awasthi, Kauser Usman, Monisha Banerjee
World Journal of Diabetes.2024; 15(4): 606. CrossRef - Precision Medicine in Bariatric Procedures
Khushboo Gala, Wissam Ghusn, Andres Acosta
Gastrointestinal Endoscopy Clinics of North America.2024;[Epub] CrossRef - Design of the DAVOS Study: Diabetes Smartphone App, a Fully Automatic Transmission of Data From the Blood Glucose Meter and Insulin Pens Using Wireless Technology to Enhance Diabetes Self-Management—A Study Protocol for a Randomized Controlled Trial
Franziska Grosser, Sandra Herrmann, Maxi Bretschneider, Patrick Timpel, Janko Schildt, Markus Bentrup, Peter E. H. Schwarz
Journal of Diabetes Science and Technology.2023; 17(3): 742. CrossRef - Low diet quality is associated with adverse levels of metabolic health markers and clustering of risk factors in adults with type 2 diabetes
Namrata Sanjeevi, Jeanne H. Freeland‐Graves
Journal of Human Nutrition and Dietetics.2023; 36(1): 31. CrossRef - Need for improving immunization status and preventive care in diabetes mellitus patients
Teresa Gisinger, Alexandra Kautzky-Willer, Michael Leutner
Wiener klinische Wochenschrift.2023; 135(13-14): 336. CrossRef - Nanoparticles encapsulating sesame seeds (Sesamum indicum) oil: Physicochemical, antioxidant and enzymatic inhibition properties
Narimane Lammari, Mehdi Louaer, Ouahida Louaer, Chawki Bensouici, Ahmed Zermane, Abdelhamid Elaissari, Abdeslam Hassen Meniai
Journal of Drug Delivery Science and Technology.2023; 79: 104003. CrossRef - Significant Association between Subclinical Left Cardiac Dysfunction and Liver Stiffness in Metabolic Syndrome Patients with Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease
Alexandru Apostu, Daniel Malita, Sergiu-Florin Arnautu, Mirela-Cleopatra Tomescu, Dan Gaiță, Alina Popescu, Ruxandra Mare, Ramona Gidea, Diana-Aurora Arnautu
Medicina.2023; 59(2): 328. CrossRef - An examination of causal associations and shared risk factors for diabetes and cardiovascular diseases in the East Asian population: A Mendelian randomization study
Yulin Guo, Jie Gao, Yan Liu, Yanxiong Jia, Xiangguang An, Xitao Zhang, Pixiong Su
Frontiers in Endocrinology.2023;[Epub] CrossRef - Synthesis, Cytotoxicity and In Vitro α-Glucosidase Inhibition of New N-Substituted Glitazone and Rhodanine Derivatives
N. R. Tshiluka, M. V. Bvumbi, S. S. Mnyakeni-Moleele
Russian Journal of Bioorganic Chemistry.2023; 49(2): 384. CrossRef - Prevalence of QT prolongation and its risk factors in patients with type 2 diabetes
Khaled Aburisheh, Mohammad F. AlKheraiji, Saleh I. Alwalan, Arthur C. Isnani, Mohamed Rafiullah, Muhammad Mujammami, Assim A. Alfadda
BMC Endocrine Disorders.2023;[Epub] CrossRef - Adapted Diabetes Complications Severity Index and Charlson Comorbidity Index in predicting all-cause and cause-specific mortality among patients with type 2 diabetes
Yu-Wen Hu, Chiu-Mei Yeh, Chia-Jen Liu, Tzeng-Ji Chen, Nicole Huang, Yiing-Jenq Chou
BMJ Open Diabetes Research & Care.2023; 11(2): e003262. CrossRef - Influenza: Diabetes as a risk factor for severe related-outcomes and the effectiveness of vaccination in diabetic population. A meta-analysis of observational studies
Ilaria Dicembrini, Giovanni Antonio Silverii, Alessandra Clerico, Riccardo Fornengo, Giovanni Gabutti, Valeria Sordi, Silvio Tafuri, Ottavia Peruzzi, Edoardo Mannucci
Nutrition, Metabolism and Cardiovascular Diseases.2023; 33(6): 1099. CrossRef - Research progress on classification, sources and functions of dietary polyphenols for prevention and treatment of chronic diseases
Wei Li, Haihong Chen, Bing Xu, Yi Wang, Canyang Zhang, Yong Cao, Xinhui Xing
Journal of Future Foods.2023; 3(4): 289. CrossRef - Greater efficiency of polyherbal drug encapsulated biosynthesized chitosan nano-biopolymer on diabetes and its complications
G. Revathi, S. Elavarasi, K. Saravanan, M. Ashokkumar, Chukwuebuka Egbuna
International Journal of Biological Macromolecules.2023; 240: 124445. CrossRef - Variations in all-cause mortality, premature mortality and cause-specific mortality among persons with diabetes in Ontario, Canada
Laura C Rosella, Kathy Kornas, Ednah Negatu, Limei Zhou
BMJ Open Diabetes Research & Care.2023; 11(3): e003378. CrossRef - The relationship between family support and the level of self care in type 2 diabetes patients
Fatma Gul Zeren, Ozlem Canbolat
Primary Care Diabetes.2023; 17(4): 341. CrossRef - Diabetes mellitus-related hospital admissions and prescriptions of antidiabetic agents in England and Wales: an ecological study
Gayda Abdel Rahman AbuHammad, Abdallah Y. Naser, Loay Khaled Mohammad Hassouneh
BMC Endocrine Disorders.2023;[Epub] CrossRef - Administration of alloxan and streptozotocin in Sprague Dawley rats and the challenges in producing diabetes model
Indah Fajarwati, Dedy Duryadi Solihin, Tutik Wresdiyati, Irmanida Batubara
IOP Conference Series: Earth and Environmental Science.2023; 1174(1): 012035. CrossRef - Impacts of SARS-CoV-2 on diabetes mellitus: A pre and post pandemic evaluation
A H M Nurun Nabi, Akio Ebihara, Hossain Uddin Shekhar
World Journal of Virology.2023; 12(3): 151. CrossRef - Risk Stratification for Herpes Simplex Virus Pneumonia Using Elastic Net Penalized Cox Proportional Hazard Algorithm with Enhanced Explainability
Yu-Chiang Wang, Wan-Ying Lin, Yi-Ju Tseng, Yiwen Fu, Weijia Li, Yu-Chen Huang, Hsin-Yao Wang
Journal of Clinical Medicine.2023; 12(13): 4489. CrossRef - Bee Honey Extract Attenuates Hyperglycemia in Induced Type 1 Diabetes: Impact of Antioxidant and Angiogenesis Activities on Diabetic Severity In Vivo
Ahmed H. Alghamdi, Ibrahim M. Shatla, Soliman Shreed, Atif H. Khirelsied, Mohamed F. El-Refaei
Applied Sciences.2023; 13(14): 8045. CrossRef - Increased Left Atrial Stiffness is Significantly Associated with Paroxysmal Atrial Fibrillation in Diabetic Patients
Diana-Aurora Arnautu, Sergiu-Florin Arnautu, Mirela-Cleopatra Tomescu, Silvia Luca, Constantin-Tudor Luca
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 2077. CrossRef - Cost-effectiveness analysis of implementing polygenic risk score in a workplace cardiovascular disease prevention program
Deo Mujwara, Jen Kintzle, Paolo Di Domenico, George B. Busby, Giordano Bottà
Frontiers in Public Health.2023;[Epub] CrossRef - Diabetes associates with mortality in critically ill patients with SARS-CoV-2 pneumonia: No diabetes paradox in COVID-19
Priscila Bellaver, Larissa Schneider, Ariell F. Schaeffer, Lilian Rodrigues Henrique, Joíza Lins Camargo, Fernando Gerchman, Cristiane B. Leitão, Tatiana H. Rech
Heliyon.2023; 9(8): e18554. CrossRef - New Insights into the Role of Oxidative Stress in the Development of Diabetes Mellitus and Its Complications
Julia Matzenbacher dos Santos, Qing Zhong, Sandra A. Benite-Ribeiro, Thiago Gomes Heck
Journal of Diabetes Research.2023; 2023: 1. CrossRef - A paradigm shift for cardiovascular outcome evaluation in diabetes: Major adverse cardiovascular events (MACE) to major adverse vascular events (MAVE)
Ashu Rastogi, Anand Sudhayakumar, Nicolaas C. Schaper, Edward B. Jude
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(11): 102875. CrossRef - Assessing Elevated Blood Glucose Levels Through Blood Glucose Evaluation and Monitoring Using Machine Learning and Wearable Photoplethysmography Sensors: Algorithm Development and Validation
Bohan Shi, Satvinder Singh Dhaliwal, Marcus Soo, Cheri Chan, Jocelin Wong, Natalie W C Lam, Entong Zhou, Vivien Paitimusa, Kum Yin Loke, Joel Chin, Mei Tuan Chua, Kathy Chiew Suan Liaw, Amos W H Lim, Fadil Fatin Insyirah, Shih-Cheng Yen, Arthur Tay, Seng
JMIR AI.2023; 2: e48340. CrossRef - Cost-Utility of an Exercise Referral Scheme Versus Doing Nothing in Flemish Adults: Exploring the Impact of Key Assumptions
Amber Werbrouck, Masja Schmidt, Koen Putman, Steven Simoens, Nick Verhaeghe, Lieven Annemans
Journal of Physical Activity and Health.2023; : 1. CrossRef - Community-based models of care for management of type 2 diabetes mellitus among non-pregnant adults in sub-Saharan Africa: A scoping review
Emmanuel Firima, Lucia Gonzalez, Fabiola Ursprung, Elena Robinson, Jacqueline Huber, Jennifer M. Belus, Fabian Raeber, Ravi Gupta, Gibrilla F. Deen, Alain Amstutz, Bailah Leigh, Maja Weisser, Niklaus Daniel Labhardt, Rehana Abdus Salam
PLOS ONE.2023; 18(11): e0278353. CrossRef - Omega-3 supplementation and diabetes: A systematic review and meta-analysis
Felipe Mendes Delpino, Lílian Munhoz Figueiredo, Bruna Gonçalves Cordeiro da Silva, Taiciane Gonçalves da Silva, Gicele Costa Mintem, Renata Moraes Bielemann, Denise Petrucci Gigante
Critical Reviews in Food Science and Nutrition.2022; 62(16): 4435. CrossRef - The effect of real‐time monitoring of physical activity intensity in diabetic patients
Rumi Tanaka, Kimie Fujita, Satoko Maeno, Kanako Yakushiji, Satomi Tanaka, Keizo Ohnaka, Kenji Ashida, Shohei Sakamoto, Masatoshi Nomura
Japan Journal of Nursing Science.2022;[Epub] CrossRef - Factors associated with influenza vaccine coverage among patients with diabetes: Korea National Health and Nutrition Examination Survey 2016–2018
A. Lum Han
International Journal of Diabetes in Developing Countries.2022; 42(2): 297. CrossRef - Interacción entre el estadio de la enfermedad renal crónica y la diabetes mellitus como factores asociados con mortalidad en pacientes con enfermedad renal crónica: un estudio de cohortes externas
Laura E. Villegas Sierra, Melisa Buriticá Agudelo, Carlos Enrique Yepes Delgado, Yanett Marcela Montoya Jaramillo, Fabián Jaimes Barragan
Nefrología.2022; 42(5): 540. CrossRef - Influence of glucose supplementation on biofilm formation of Candida albicans and Candida glabrata isolated from diabetic and non-diabetic individuals
Pedro Castania Amadio Domingues, Viviane de Cássia Oliveira, Felipe Lazarini Bim, Carolina Patrícia Aires, André Pereira dos Santos, Denise Tornavoi de Castro, Cláudia Helena Silva-Lovato, Denise de Andrade, Evandro Watanabe
Archives of Oral Biology.2022; 134: 105339. CrossRef - The influence of the disclosure of diabetes on the cognitive, physical ability and diabetes self‐management in diabetic employed adults in Saudi Arabia
Sitah Alshutwi, Eman Miligi, Lujain Alhumidan, Adel F. Almutairi
Nursing Open.2022; 9(2): 978. CrossRef - Effect of fermented cassava tuber on the gene expression of PI3K/Akt signaling and AMPK pathway in STZ-NA-induced diabetic rats
Rio Jati Kusuma, Desty Ervira Puspaningtyas, Puspita Mardika Sari
Nutrition & Food Science .2022; 52(2): 213. CrossRef - A fractional order control model for Diabetes and COVID-19 co-dynamics with Mittag-Leffler function
Andrew Omame, Ugochukwu K. Nwajeri, M. Abbas, Chibueze P. Onyenegecha
Alexandria Engineering Journal.2022; 61(10): 7619. CrossRef - Enhancing Choices Regarding the Administration of Insulin Among Patients With Diabetes Requiring Insulin Across Countries and Implications for Future Care
Ileana Mardare, Stephen M. Campbell, Johanna C. Meyer, Israel Abebrese Sefah, Amos Massele, Brian Godman
Frontiers in Pharmacology.2022;[Epub] CrossRef - Dyslipidemia Among Patients With Type 1 Diabetes and Its Associated Factors in Saudi Arabia: An Analytical Cross-Sectional Study
Abdullah A Alrasheed
Cureus.2022;[Epub] CrossRef - Prickly Pear Cacti (Opuntia spp.) Cladodes as a Functional Ingredient for Hyperglycemia Management: A Brief Narrative Review
Rao Raahim Kashif, Nathan M. D’Cunha, Duane D. Mellor, Natalie I. Alexopoulos, Domenico Sergi, Nenad Naumovski
Medicina.2022; 58(2): 300. CrossRef - Machine learning for predicting chronic diseases: a systematic review
F.M. Delpino, Â.K. Costa, S.R. Farias, A.D.P. Chiavegatto Filho, R.A. Arcêncio, B.P. Nunes
Public Health.2022; 205: 14. CrossRef - Effects of blueberry and cranberry on type 2 diabetes parameters in individuals with or without diabetes: A systematic review and meta-analysis of randomized clinical trials
Felipe Mendes Delpino, Lílian Munhoz Figueiredo, Taiciane Gonçalves da Silva, Thaynã Ramos Flores
Nutrition, Metabolism and Cardiovascular Diseases.2022; 32(5): 1093. CrossRef - Momordica charantia nanoparticles potentiate insulin release and modulate antioxidant gene expression in pancreas of diabetic rats
Olusola Olalekan Elekofehinti
Egyptian Journal of Medical Human Genetics.2022;[Epub] CrossRef - GLP-1 receptor agonists in diabetic kidney disease: current evidence and future directions
Ji Hee Yu, So Young Park, Da Young Lee, Nan Hee Kim, Ji A Seo
Kidney Research and Clinical Practice.2022; 41(2): 136. CrossRef - Pharmacological Study: Synergistic Antidiabetic Activity of Cinnamon Bark and Zingiber Extract in Streptozotocin-Induced Diabetic Rats
Eva Nurinda, Nurul Kusumawardani, Ari Susiana Wulandari , Annisa Fatmawati, E. Emelda, Husnatun Nisa, Nurjani A. Hasan, Wahyu Fajar Iriyanti, Mardiatun Rohmah, Puji Lestari, Veriani Aprilia
Open Access Macedonian Journal of Medical Sciences.2022; 10(T8): 1. CrossRef - Integrating a Polygenic Risk Score for Coronary Artery Disease as a Risk‐Enhancing Factor in the Pooled Cohort Equation: A Cost‐Effectiveness Analysis Study
Deo Mujwara, Geoffrey Henno, Stephen T. Vernon, Siyang Peng, Paolo Di Domenico, Brock Schroeder, George B. Busby, Gemma A Figtree, Giordano Bottà
Journal of the American Heart Association.2022;[Epub] CrossRef - Comparison of mechanistic pathways of bariatric surgery in patients with diabetes mellitus: A Bayesian network meta‐analysis
Chaoxing Lin, Trevor James Jun‐Ming Yeong, Wen Hui Lim, Cheng Han Ng, Chun En Yau, Yip Han Chin, Mark D. Muthiah, Poay Huan Loh, Roger S. Y. Foo, Shao Feng Mok, Asim Shabbir, Georgios K. Dimitriadis, Chin Meng Khoo, Nicholas W. S. Chew
Obesity.2022; 30(7): 1380. CrossRef - Diabetes and COVID-19: Biological profile of type 2 diabetic patients with COVID-19 in Pointe-Noire, Congo
Anicet Boumba Luc Magloire, Batchy Aladin Atandi, Elenga-Bongo Charley, Pouki Freddy Saturnin, Kibouilou Fredy, Balanda Christ Nkouanga, Dabo Tidiane Cheick Ahmed, Wahar Saar Abdoul, Mahouanga Didel Mampassi, Voumbi Ghislain Loubano, Moukassa Donatien
Global Journal of Clinical Virology.2022; 7(1): 001. CrossRef - Integrated Nutritional Supports for Diabetic Patients During COVID-19
Infection: A Comprehensive Review
A.K. Obidul Huq, Abu Naim Mohammad Bazlur Rahim, S.M. Golam Moktadir, Ielias Uddin, Mohammad Zahidul Manir, Muhammad Abu Bakr Siddique, Khaleda Islam, Md. Sirajul Islam
Current Diabetes Reviews.2022;[Epub] CrossRef - Mechanisms of COVID-19 pathogenesis in diabetes
Chandrakala Aluganti Narasimhulu, Dinender K. Singla
American Journal of Physiology-Heart and Circulatory Physiology.2022; 323(3): H403. CrossRef - Improvement in Age at Mortality and Changes in Causes of Death in the Population with Diabetes: An Analysis of Data from the Korean National Health Insurance and Statistical Information Service, 2006 to 2018
Eugene Han, Sun Ok Song, Hye Soon Kim, Kang Ju Son, Sun Ha Jee, Bong-Soo Cha, Byung-Wan Lee
Endocrinology and Metabolism.2022; 37(3): 466. CrossRef - Gender difference in association between diabetes mellitus and all-cause mortality in atrial fibrillation patients
Li Tian, Yan-min Yang, Jun Zhu, Han Zhang, Xing-hui Shao
Journal of Diabetes and its Complications.2022; 36(9): 108265. CrossRef - An Overview of Diabetes Mellitus in Egypt and the Significance of Integrating Preventive Cardiology in Diabetes Management
Mohamed R Abouzid, Karim Ali, Ibrahim Elkhawas, Shorouk M Elshafei
Cureus.2022;[Epub] CrossRef - The association between diabetes mellitus and functionality in knee osteoarthritis: a cross-sectional study
Serdar KAYMAZ, Sanem Aslıhan AYKAN
Journal of Health Sciences and Medicine.2022; 5(4): 1114. CrossRef - In-hospital hyperglycemia but not diabetes mellitus alone is associated with increased in-hospital mortality in community-acquired pneumonia (CAP): a systematic review and meta-analysis of observational studies prior to COVID-19
Rahul D Barmanray, Nathan Cheuk, Spiros Fourlanos, Peter B Greenberg, Peter G Colman, Leon J Worth
BMJ Open Diabetes Research & Care.2022; 10(4): e002880. CrossRef - The association between leukocyte telomere length and chronic obstructive pulmonary disease is partially mediated by inflammation: a meta-analysis and population-based mediation study
Tieshan Wang, Zhaoqi Jia, Sen Li, Yuxin Li, Tingting Yu, Tao Lu, Yuanyuan Shi
BMC Pulmonary Medicine.2022;[Epub] CrossRef - The Influence of Diabetic Peripheral Neuropathy on the Duration of Sciatic Nerve Block with 1.3% Liposomal Bupivacaine and 0.25% Bupivacaine Hydrochloride in a Mouse Model
Liljana Markova, Erika Cvetko, Chiedozie Kenneth Ugwoke, Simon Horvat, Nejc Umek, Tatjana Stopar Pintarič
Pharmaceutics.2022; 14(9): 1824. CrossRef - Free, Conjugated, and Bound Phenolics in Peel and Pulp from Four Wampee Varieties: Relationship between Phenolic Composition and Bio-Activities by Multivariate Analysis
Xue Lin, Yousheng Shi, Pan Wen, Xiaoping Hu, Lu Wang
Antioxidants.2022; 11(9): 1831. CrossRef - Protocol: Developing a framework to improve glycaemic control among patients with type 2 diabetes mellitus in Kinshasa, Democratic Republic of the Congo
Jean-Pierre Fina Lubaki, Olufemi Babatunde Omole, Joel Msafiri Francis, David Desseauve
PLOS ONE.2022; 17(9): e0268177. CrossRef - Triglyceride-glucose index is associated with poor sleep quality in apparently healthy subjects: A cross-sectional study
Daniela Carolina Avelino, Alessandra da Silva, Larissa Oliveira Chaves, Júlia Cristina Cardoso Carraro, Fernanda de Carvalho Vidigal, Josefina Bressan
Archives of Endocrinology and Metabolism.2022;[Epub] CrossRef - Molecular mechanisms interlinking biological clock and diabetes mellitus: Effective tools for better management
Chandrasekaran Sankaranarayanan, Perumal Subramanian
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2022; 16(11): 102639. CrossRef - Amyloid Fibrillation of Insulin: Amelioration Strategies and Implications for Translation
Megren H. A. Fagihi, Sourav Bhattacharjee
ACS Pharmacology & Translational Science.2022; 5(11): 1050. CrossRef - Potential Roles of Anti-Inflammatory Plant-Derived Bioactive Compounds Targeting Inflammation in Microvascular Complications of Diabetes
Yahia A. Kaabi
Molecules.2022; 27(21): 7352. CrossRef - Additive interaction of diabetes mellitus and chronic kidney disease in cancer patient mortality risk
Seohyun Kim, Gyuri Kim, Jae Hyeon Kim
Scientific Reports.2022;[Epub] CrossRef - Re-understanding and focusing on normoalbuminuric diabetic kidney disease
Na An, Bi-tao Wu, Yu-wei Yang, Zheng-hong Huang, Jia-fu Feng
Frontiers in Endocrinology.2022;[Epub] CrossRef - Coexistent Lichen Amyloidosis and Acquired Reactive Perforating Collagenosis in Type 2 Diabetes Mellitus and Post-Thyroidectomy Hypothyroidism Due to Papillary Thyroid Carcinoma: A Rare Case
Eva Krishna Sutedja, Muhamad Radyn Haryadi Widjaya, Hartati Purbo Dharmadji, Pati Aji Achdiat, Laila Tsaqilah
International Medical Case Reports Journal.2022; Volume 15: 745. CrossRef - Interaction between the stage of chronic kidney disease and diabetes mellitus as factors associated with mortality in chronic kidney disease patients: An external cohort study
Laura E. Villegas Sierra, Melisa Buriticá Agudelo, Carlos Enrique Yepes Delgado, Yanett Marcela Montoya Jaramillo, Fabián Jaimes Barragan
Nefrología (English Edition).2022; 42(5): 540. CrossRef - Effects of Pea Protein on Satiety, Postprandial Glucose Response and Appetite Hormones: A Literature Review
Amy Choi
Undergraduate Research in Natural and Clinical Science and Technology (URNCST) Journal.2022; 6(10): 1. CrossRef - Hemoglobin glycation index is associated with incident chronic kidney disease in subjects with impaired glucose metabolism: A 10-year longitudinal cohort study
Wonjin Kim, Taehwa Go, Dae Ryong Kang, Eun Jig Lee, Ji Hye Huh
Journal of Diabetes and its Complications.2021; 35(1): 107760. CrossRef - Coronavirus Disease 2019 (SARS-CoV-2) and polycystic ovarian disease: Is there a higher risk for these women?
Giuseppe Morgante, Libera Troìa, Vincenzo De Leo
The Journal of Steroid Biochemistry and Molecular Biology.2021; 205: 105770. CrossRef - Regional differences in diabetes across Europe – regression and causal forest analyses
Péter Elek, Anikó Bíró
Economics & Human Biology.2021; 40: 100948. CrossRef - Active ingredients and mechanisms of Phellinus linteus (grown on Rosa multiflora) for alleviation of Type 2 diabetes mellitus through network pharmacology
Ki Kwang Oh, Md. Adnan, Dong Ha Cho
Gene.2021; 768: 145320. CrossRef - Application of Telemedicine in Diabetes Care: The Time is Now
Felix Aberer, Daniel A. Hochfellner, Julia K. Mader
Diabetes Therapy.2021; 12(3): 629. CrossRef - Long-term outcomes after kidney transplant failure and variables related to risk of death and probability of retransplant: Results from a single-center cohort study in Brazil
Lúcio R. Requião-Moura, Cássio R. Moreira Albino, Paula Rebello Bicalho, Érika de Arruda Ferraz, Luciana Mello de Mello Barros Pires, Maurício Fregonesi Rodrigues da Silva, Alvaro Pacheco-Silva, Justyna Gołębiewska
PLOS ONE.2021; 16(1): e0245628. CrossRef - Estimating the disease burden of Korean type 2 diabetes mellitus patients considering its complications
Juyoung Kim, Seok-Jun Yoon, Min-Woo Jo, Brecht Devleesschauwer
PLOS ONE.2021; 16(2): e0246635. CrossRef - Susceptibility for Some Infectious Diseases in Patients With Diabetes: The Key Role of Glycemia
Jesús Chávez-Reyes, Carlos E. Escárcega-González, Erika Chavira-Suárez, Angel León-Buitimea, Priscila Vázquez-León, José R. Morones-Ramírez, Carlos M. Villalón, Andrés Quintanar-Stephano, Bruno A. Marichal-Cancino
Frontiers in Public Health.2021;[Epub] CrossRef - Prediabetes, Diabetes, and the Risk of All-Cause and Cause-Specific Mortality in a Japanese Working Population: Japan Epidemiology Collaboration on Occupational Health Study
Zobida Islam, Shamima Akter, Yosuke Inoue, Huan Hu, Keisuke Kuwahara, Tohru Nakagawa, Toru Honda, Shuichiro Yamamoto, Hiroko Okazaki, Toshiaki Miyamoto, Takayuki Ogasawara, Naoko Sasaki, Akihiko Uehara, Makoto Yamamoto, Takeshi Kochi, Masafumi Eguchi, Tai
Diabetes Care.2021; 44(3): 757. CrossRef - Proportion and mortality of Iranian diabetes mellitus, chronic kidney disease, hypertension and cardiovascular disease patients with COVID-19: a meta-analysis
Hamid Mirjalili, Seyed Alireza Dastgheib, Seyed Hossein Shaker, Reza Bahrami, Mahta Mazaheri, Seyed Mohamad Hossein Sadr-Bafghi, Jalal Sadeghizadeh-Yazdi, Hossein Neamatzadeh
Journal of Diabetes & Metabolic Disorders.2021; 20(1): 905. CrossRef - Prevalence and associated factors of diabetes mellitus among tuberculosis patients in Brunei Darussalam: A 6-year retrospective cohort study
Nurfakhrina Omar, Justin Wong, Kyaw Thu, Md Fathi Alikhan, Liling Chaw
International Journal of Infectious Diseases.2021; 105: 267. CrossRef - Diabetes and COVID-19
Zohair Jamil Gazzaz
Open Life Sciences.2021; 16(1): 297. CrossRef - Review of potential risk groups for coronavirus disease 2019 (COVID-19)
M. Naveed, M. Naeem, M. ur Rahman, M. Gul Hilal, M.A. Kakakhel, G. Ali, A. Hassan
New Microbes and New Infections.2021; 41: 100849. CrossRef - COVID-19 from the interdisciplinary standpoint. Round table
M. N. Mamedov, Yu. V. Rodionova, I. S. Yavelov, M. I. Smirnova, E. N. Dudinskaya, V. I. Potievskaya
Cardiovascular Therapy and Prevention.2021; 20(3): 2849. CrossRef - Diabetes is a major cause of influenza-associated mortality in Mexico
A. Gómez-Gómez, E.L. Sánchez-Ramos, D.E. Noyola
Revue d'Épidémiologie et de Santé Publique.2021; 69(4): 205. CrossRef - Ameliorative potential of Operculina turpethum against streptozotocin-induced diabetes in rats: biochemical and histopathological studies
Neeraj Choudhary, Gopal L. Khatik, Rekha Sharma, Navneet Khurana, Richard Lobo, Shvetank Bhatt, Devesh Tewari, Ashish Suttee
3 Biotech.2021;[Epub] CrossRef - Current utilization patterns for long-acting insulin analogues including biosimilars among selected Asian countries and the implications for the future
Brian Godman, Mainul Haque, Santosh Kumar, Salequl Islam, Jaykaran Charan, Farhana Akter, Amanj Kurdi, Eleonora Allocati, Muhammed Abu Bakar, Sagir Abdur Rahim, Nusrat Sultana, Farzana Deeba, M. A. Halim Khan, A. B. M Muksudul Alam, Iffat Jahan, Zubair Ma
Current Medical Research and Opinion.2021; 37(9): 1529. CrossRef - Association between cardiometabolic risk factors and COVID-19 susceptibility, severity and mortality: a review
Yasaman Sharifi, Moloud Payab, Erfan Mohammadi-Vajari, Seyed Morsal Mosallami Aghili, Farshad Sharifi, Neda Mehrdad, Elham Kashani, Zhaleh Shadman, Bagher Larijani, Mahbube Ebrahimpur
Journal of Diabetes & Metabolic Disorders.2021; 20(2): 1743. CrossRef - Association of ACE I/D and PAI-1 4G/5G polymorphisms with susceptibility to type 2 diabetes mellitus
Somaye Miri, Mohammad Hasan Sheikhha, Seyed Alireza Dastgheib, Seyed Amir Shaker, Hossein Neamatzadeh
Journal of Diabetes & Metabolic Disorders.2021; 20(2): 1191. CrossRef - Assessment and Management of Diabetic Patients During the COVID-19 Pandemic
Amit K Verma, Mirza Masroor Ali Beg, Deepti Bhatt, Kapil Dev, Mohammed A Alsahli, Arshad Husain Rahmani, Yamini Goyal
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 3131. CrossRef - Higher admission activated partial thromboplastin time, neutrophil-lymphocyte ratio, serum sodium, and anticoagulant use predict in-hospital COVID-19 mortality in people with Diabetes: Findings from Two University Hospitals in the U.K
Ahmed Iqbal, Marni Greig, Muhammad Fahad Arshad, Thomas H. Julian, Sher Ee Tan, Jackie Elliott
Diabetes Research and Clinical Practice.2021; 178: 108955. CrossRef - Risk factors for severe outcomes in people with diabetes hospitalised for COVID-19: a cross-sectional database study
Emilio Ortega, Rosa Corcoy, Mònica Gratacòs, Francesc Xavier Cos Claramunt, Manel Mata-Cases, Ramon Puig-Treserra, Jordi Real, Bogdan Vlacho, Esmeralda Castelblanco, Pere Domingo, Kamlesh Khunti, Josep Franch-Nadal, Didac Mauricio
BMJ Open.2021; 11(7): e051237. CrossRef - Diabetes increases the risk of COVID-19 in an altitude dependent manner: An analysis of 1,280,806 Mexican patients
Juan Alonso Leon-Abarca, Arianna Portmann-Baracco, Mayte Bryce-Alberti, Carlos Ruiz-Sánchez, Roberto Alfonso Accinelli, Jorge Soliz, Gustavo Francisco Gonzales, Chiara Lazzeri
PLOS ONE.2021; 16(8): e0255144. CrossRef - Botanical Interventions to Improve Glucose Control and Options for Diabetes Therapy
Peter Smoak, Susan J. Burke, J. Jason Collier
SN Comprehensive Clinical Medicine.2021; 3(12): 2465. CrossRef - Antidiabetic bioactive compounds from Tetrastigma angustifolia (Roxb.) Deb and Oxalis debilis Kunth.: Validation of ethnomedicinal claim by in vitro and in silico studies
Julfikar Ali Junejo, Kamaruz Zaman, Mithun Rudrapal, Ismail Celik, Emmanuel Ifeanyi Attah
South African Journal of Botany.2021; 143: 164. CrossRef - SGLT2 Inhibition for Cardiovascular Diseases, Chronic Kidney Disease, and NAFLD
Moein Ala
Endocrinology.2021;[Epub] CrossRef - Hospitalization costs with degludec versus glargine U100 for patients with type 2 diabetes at high cardiovascular risk: Canadian costs applied to SAEs from a randomized outcomes trial
Jean-Eric Tarride, Mansoor Husain, Andreas Andersen, Jens Gundgaard, Maria Luckevich, Thomas Mark, Lily Wagner, Thomas R. Pieber
Journal of Medical Economics.2021; 24(1): 1318. CrossRef - Traditional Uses, Pharmacological Effects, and Molecular Mechanisms of Licorice in Potential Therapy of COVID-19
Qian-hui Zhang, Hao-zhou Huang, Min Qiu, Zhen-feng Wu, Zhan-chang Xin, Xin-fu Cai, Qiang Shang, Jun-zhi Lin, Ding-kun Zhang, Li Han
Frontiers in Pharmacology.2021;[Epub] CrossRef - Recent Updates on Free Fatty Acid Receptor 1 (GPR-40) Agonists for the Treatment of Type 2 Diabetes Mellitus
Lata Rani, Ajmer Singh Grewal, Neelam Sharma, Sukhbir Singh
Mini-Reviews in Medicinal Chemistry.2021; 21(4): 426. CrossRef - Low Levels of Influenza Vaccine Uptake among the Diabetic Population in Spain: A Time Trend Study from 2011 to 2020
Jose J. Zamorano-Leon, Rodrigo Jimenez-Garcia, Ana Lopez-de-Andres, Javier de-Miguel-Diez, David Carabantes-Alarcon, Romana Albaladejo-Vicente, Rosa Villanueva-Orbaiz, Khaoula Zekri-Nechar, Sara Sanz-Rojo
Journal of Clinical Medicine.2021; 11(1): 68. CrossRef - Effects of Short-term Mobile Application Use on Weight Reduction for Patients with Type 2 Diabetes
Seung Eun Lee, Su-Kyung Park, Ye-Seul Park, Kyoung-Ah Kim, Han Seok Choi, Sang Woo Oh
Journal of Obesity & Metabolic Syndrome.2021; 30(4): 345. CrossRef - Diabetes mellitus in combination with COVID-19: modern views on therapy
V.I. Tsymbaliuk, M.D. Tronko, Y.G. Antypkin, S.V. Kushnirenko, V.V. Popova
REPRODUCTIVE ENDOCRINOLOGY.2021; (57): 8. CrossRef - Dietary aldose reductase inhibitors and prevention of diabetic complications
Ameena Anjum, Janamolla Sreeja, Yarlagadda Swapna, Rajesh Bolleddu, Sama Venkatesh
Indian Journal of Health Sciences and Biomedical Research (KLEU).2021; 14(2): 194. CrossRef - Cohort Profile: The Maule Cohort (MAUCO)
Catterina Ferreccio, Andrea Huidobro, Sandra Cortés, Claudia Bambs, Pablo Toro, Vanessa Van De Wyngard, Johanna Acevedo, Fabio Paredes, Pía Venegas, Hugo Verdejo, Ximena Oyarzún-González, Paz Cook, Pablo F Castro, Claudia Foerster, Claudio Vargas, Jill Ko
International Journal of Epidemiology.2020; 49(3): 760. CrossRef - Treating Dyslipidemias in the Primary Prevention of Atherosclerotic Cardiovascular Disease in Older Adults with Diabetes Mellitus
MengHee Tan, Mark Paul MacEachern
Clinics in Geriatric Medicine.2020; 36(3): 457. CrossRef - Increased Mortality Risk in People with Type 2 Diabetes Mellitus in Lithuania
Donata Linkeviciute-Ulinskiene, Auguste Kaceniene, Audrius Dulskas, Ausvydas Patasius, Lina Zabuliene, Giedre Smailyte
International Journal of Environmental Research and Public Health.2020; 17(18): 6870. CrossRef - Proteinuria Is Associated with Carotid Artery Atherosclerosis in Non-Albuminuric Type 2 Diabetes: A Cross-Sectional Study
Jaehyun Bae, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Journal of Clinical Medicine.2020; 9(1): 136. CrossRef - COVID-19 and diabetes: Knowledge in progress
Akhtar Hussain, Bishwajit Bhowmik, Nayla Cristina do Vale Moreira
Diabetes Research and Clinical Practice.2020; 162: 108142. CrossRef - Peripheral arterial endothelial dysfunction predicts future cardiovascular events in diabetic patients with albuminuria: a prospective cohort study
Bo Kyung Koo, Woo-Young Chung, Min Kyong Moon
Cardiovascular Diabetology.2020;[Epub] CrossRef - Risk Factors in and Long-Term Survival of Patients with Post-Transplantation Diabetes Mellitus: A Retrospective Cohort Study
Ching-Yao Cheng, Cheng-Hsu Chen, Ming-Fen Wu, Ming-Ju Wu, Jun-Peng Chen, Ying-Mei Liu, Yu-Chi Hou, Hue-Yu Wang
International Journal of Environmental Research and Public Health.2020; 17(12): 4581. CrossRef - Fasting Plasma Glucose Variability and Gastric Cancer Risk in Individuals Without Diabetes Mellitus: A Nationwide Population-Based Cohort Study
So-hyeon Hong, Eunjin Noh, Jinsil Kim, Soon Young Hwang, Jun A. Kim, You-Bin Lee, Eun Roh, Kyung Mook Choi, Sei Hyun Baik, Geum Joon Cho, Hye Jin Yoo
Clinical and Translational Gastroenterology.2020; 11(9): e00221. CrossRef - Trimetazidine Inhibits Renal Tubular Epithelial Cells to Mesenchymal Transition in Diabetic Rats via Upregulation of Sirt1
Yong Yang, Yong Wang, Zuowen He, Yunchang Liu, Chen Chen, Yan Wang, Dao Wen Wang, Hong Wang
Frontiers in Pharmacology.2020;[Epub] CrossRef - Beta Cell Imaging—From Pre-Clinical Validation to First in Man Testing
Stephane Demine, Michael L. Schulte, Paul R. Territo, Decio L. Eizirik
International Journal of Molecular Sciences.2020; 21(19): 7274. CrossRef - The role of dental insurance in mitigating mortality among working‐age U.S. adults with periodontitis
Naveed Sadiq, Janice C. Probst, Anwar T. Merchant, Amy B. Martin, Deepika Shrestha, M. Mahmud Khan
Journal of Clinical Periodontology.2020; 47(11): 1294. CrossRef - Antidiabetic Activity of Triterpenoids from Anisophyllea disticha
Miftahul Fikriyah, Muhammad Almurdani, Yum Eryanti, Hilwan Y. Teruna
Journal of Physics: Conference Series.2020; 1655(1): 012033. CrossRef - Therapeutic efficacy of umbilical cord-derived stem cells for diabetes mellitus: a meta-analysis study
Dina H. Kassem, Mohamed M. Kamal
Stem Cell Research & Therapy.2020;[Epub] CrossRef - Loss of Smell in COVID-19 Patients: Lessons and Opportunities
Dennis Mathew
Frontiers in Human Neuroscience.2020;[Epub] CrossRef - Diabetes mellitus and COVID-19: current issues of pathogenesis, clinic and therapy. Literature review
В. І. Цимбалюк, М. Д. Тронько, Ю. Г. Антипкін, В. В. Попова
REPRODUCTIVE ENDOCRINOLOGY.2020; (54): 8. CrossRef - Increased Age of Death and Change in Causes of Death Among Persons With Diabetes Mellitus From the Korean National Health Insurance and Statistical Information Service, 2006 to 2018
Eugene Han, Sun Ok Song, Hye Soon Kim, Kang Ju Son, Sun Ha Jee, Bong-Soo Cha, Byung-Wan Lee
SSRN Electronic Journal .2020;[Epub] CrossRef - Purva Rupeeyam of bhela indriya sthana-an explorative study
Kshama Gupta, Prasad Mamidi
International Journal of Complementary & Alternative Medicine.2020; 13(6): 228. CrossRef - The COVID-19 pandemic and diabetes mellitus
Athanasia Papazafiropoulou, Stavros Antonopoulos
Archives of Medical Science – Atherosclerotic Diseases.2020; 5(1): 200. CrossRef - Diabetes and Cancer: Cancer Should Be Screened in Routine Diabetes Assessment
Sunghwan Suh, Kwang-Won Kim
Diabetes & Metabolism Journal.2019; 43(6): 733. CrossRef - Diabetes Mellitus, Still Major Threat to Mortality from Various Causes
Nam Hoon Kim
Diabetes & Metabolism Journal.2019; 43(3): 273. CrossRef - The Need to Improve the Quality of Diabetes Care in Korea
Seung Jin Han, Dae Jung Kim
Journal of Korean Medical Science.2019;[Epub] CrossRef
- Figure
- Related articles
-
- Psychotic Disorders and the Risk of Type 2 Diabetes Mellitus, Atherosclerotic Cardiovascular Diseases, and All-Cause Mortality: A Population-Based Matched Cohort Study
- Gestational Diabetes Mellitus and Its Implications across the Life Span
- Metabolic Dysfunction-Associated Fatty Liver Disease and Mortality: A Population-Based Cohort Study

 KDA
KDA PubReader
PubReader Cite
Cite