- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 43(2); 2019 > Article
-
Original ArticleClinical Diabetes & Therapeutics Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors
-
Ji-Yeon Lee1, Yongin Cho1, Minyoung Lee1, You Jin Kim1,2, Yong-ho Lee1,3, Byung-Wan Lee1,3, Bong-Soo Cha1,3, Eun Seok Kang1,3

-
Diabetes & Metabolism Journal 2019;43(2):158-173.
DOI: https://doi.org/10.4093/dmj.2018.0057
Published online: January 25, 2019
1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
2Brain Korea 21 Plus Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
3Institute of Endocrine Research, Yonsei University College of Medicine, Seoul, Korea.
- Corresponding author: Eun Seok Kang. Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea. edgo@yuhs.ac
Copyright © 2019 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- We investigated the predictive markers for the therapeutic efficacy and the best combination of sodium-glucose co-transporter 2 (SGLT2) inhibitors (empagliflozin, dapagliflozin, and ipragliflozin) therapy in patients with type 2 diabetes mellitus (T2DM).
-
Methods
- A total of 804 patients with T2DM who had taken SGLT2 inhibitor as monotherapy or an add-on therapy were analyzed. Multivariate regression analyses were performed to identify the predictors of SGLT2 inhibitor response including the classes of baseline anti-diabetic medications.
-
Results
- After adjusting for age, sex, baseline body mass index (BMI), diabetes duration, duration of SGLT2 inhibitor use, initial glycosylated hemoglobin (HbA1c) level, estimated glomerular filtration rate (eGFR), and other anti-diabetic agent usage, multivariate analysis revealed that shorter diabetes duration, higher initial HbA1c and eGFR were associated with better glycemic response. However, baseline BMI was inversely correlated with glycemic status; lean subjects with well-controlled diabetes and obese subjects with inadequately controlled diabetes received more benefit from SGLT2 inhibitor treatment. In addition, dipeptidyl peptidase 4 (DPP4) inhibitor use was related to a greater reduction in HbA1c in patients with higher baseline HbA1c ≥7%. Sulfonylurea users experienced a larger change from baseline HbA1c but the significance was lost after adjustment for covariates and metformin and thiazolidinedione use did not affect the glycemic outcome.
-
Conclusion
- A better response to SGLT2 inhibitors is expected in Korean T2DM patients who have higher baseline HbA1c and eGFR with a shorter diabetes duration. Moreover, the add-on of an SGLT2 inhibitor to a DPP4 inhibitor is likely to show the greatest glycemic response.
- The prevalence of type 2 diabetes mellitus (T2DM) has increased along with increased obesity and sedentary lifestyles changes [1]. Most T2DM patients have metabolic disturbances with insulin resistance, such as hypertension, hyperlipidemia, and obesity, which increase the cardiovascular risk by two-fold compared to the risk in non-diabetic subjects [2]. Large randomized trials have demonstrated that optimal glycemic control is associated with the prevention of microvascular complications and long-term reduction of the risk of cardiovascular diseases [345]; however, only about 50% of patients achieve the glycosylated hemoglobin (HbA1c) levels <7.0% [6].
- The development of new classes of anti-diabetes drugs has allowed a wider range of therapeutic options for glycemic control [7]. Among them, sodium-glucose co-transporter 2 (SGLT2) inhibitors are a new class of drugs that were first approved by the U.S. Food and Drug Administration in March 2013 [8]. Their mechanism involves the inhibition of glucose reabsorption in the renal proximal tubule, leading to increased urinary glucose excretion [9]. This exerts diuretic and natriuretic effects, which also contribute to the reduction of body weight and blood pressure [1011]. In addition, the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) and Canagliflozin Cardiovascular Assessment Study (CANVAS) studies have shown that T2DM patients treated with either empagliflozin or canagliflozin had significantly lower rates of the primary outcome, a composite of death from cardiovascular causes, nonfatal myocardial infarction, and nonfatal stroke [1213]. These extraglycemic beneficial effects, as well as the glycemic effects, make SGLT2 inhibitors a promising anti-diabetic agent.
- Previously, we demonstrated that dapagliflozin treatment was more effective in patients with higher baseline HbA1c and estimated glomerular filtration rate (eGFR) [14]. The present study extended the scope of these findings to investigate the effectiveness of three widely used SGLT2 inhibitors (empagliflozin, dapagliflozin, and ipragliflozin) as monotherapy or add-on therapy and to analyze which baseline parameters or preexisting anti-diabetic drugs were associated with better response to SGLT2 inhibitor treatment in Korean T2DM patients.
INTRODUCTION
- Study populations
- We screened patients with T2DM who were treated at the outpatient diabetes clinic of Severance Hospital, Yonsei University College of Medicine, Republic of Korea, between April 2014 and August 2017. Patients aged 18 to 75 years who had taken an SGLT2 inhibitor (empagliflozin, 10 mg once daily; dapagliflozin, 10 mg once daily; or ipragliflozin, 50 mg once daily) as monotherapy or an add-on therapy for at least 90 consecutive days were included. We excluded patients with type 1 diabetes mellitus, gestational diabetes, insulin use, or significant renal impairment (eGFR <45 mL/min/1.73 m2). Patients who changed the class of SGLT2 inhibitors and those with missing data regarding body weight, HbA1c level, or eGFR were also excluded. Finally, a total of 804 subjects (128 empagliflozin users, 500 dapagliflozin users, and 176 ipragliflozin users) were enrolled and followed for a median of 192 days (interquartile range [IQR], 168 to 323 days). This study was approved by the Institutional Review Board of the Yonsei University Health System, Severance Hospital (No. 4-2018-0175). Informed consent was waived due to the retrospective nature of the study.
- Study design
- Demographic information including age, gender, time since diabetes diagnosis, other anti-diabetic drug use, and comorbidities (hypertension, hyperlipidemia, coronary artery occlusive disease, peripheral artery occlusive disease, stroke, and transient ischemic attack) was collected through an examination of electronic medical records. The subjects regularly visited the outpatient clinic every 3 to 6 months according to the degree of glycemic control. At each visit, the body weight and body mass index (BMI) were calculated as the body weight divided by the height squared (kg/m2). Blood samples were collected after an overnight fasting. Fasting plasma glucose (FPG) levels were assessed by enzymatic colorimetric assay using a Cobas c 702 (Roche, Basel, Switzerland) and Hitachi 7600-200-DDP (Hitachi, Tokyo, Japan). HbA1c levels were determined by high-performance liquid chromatography on a Cobas Integra 800 device (Roche). Lipid panels (total cholesterol, triglyceride, and high density lipoprotein cholesterol [HDL-C]), serum aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, and creatinine levels were measured using a Hitachi 7600 chemistry analyzer (Hitachi). We calculated low density lipoprotein cholesterol (LDL-C) levels using the following formula: LDL-C=(total cholesterol−HDL-C)−(triglyceride/5). The eGFR was determined using the 4-variable modification of diet in renal disease formula [15].
- We assessed the efficacy of SGLT2 inhibitor according to baseline anti-diabetic drug use: baseline metformin, sulfonylurea (SU), dipeptidyl peptidase 4 (DPP4) inhibitor, or thiazolidinedione (TZD) users and non-users. Glycemic response was evaluated using the change from baseline HbA1c (ΔHbA1c) and the percentage of subjects with baseline HbA1c ≥7% who achieved HbA1c <7% after follow-up. Body weight changes (Δweight loss) were adjusted by the baseline body weight as follows: [(baseline weight−follow-up weight)/baseline weight] ×100.
- Statistical analysis
- Normally distributed data are expressed as mean±standard deviations and non-normally distributed data are expressed as median (IQR). Independent t-test or Mann-Whitney U test for continuous variables and chi-square test for categorical variables were used to assess the differences in baseline characteristics. Changes in clinic-laboratory values between baseline and follow-up were analyzed by paired t-test or Wilcoxon signed-rank test. To compare the response according to the baseline anti-diabetic agent use, Mann-Whitney U test were used. Subgroups based on initial HbA1c and BMI categories were compared by Kruskal-Wallis test. We used linear regression analyses to determine the factors responsible for the changes in HbA1c. Multivariate model was adjusted for age, sex, initial BMI, diabetes duration, duration of SGLT2 inhibitor use, baseline HbA1c and eGFR levels, and anti-diabetic agent use (metformin, SU, DPP4 inhibitor, and TZD). IBM SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analyses and P<0.05 was considered significant.
METHODS
- Baseline characteristics of the study population
- The baseline characteristics of the study participants are described in Table 1. The median age was 57 years (IQR, 49 to 64 years), 54.4% were male, and the median diabetes duration was 7.2 years. The baseline median BMI was 27.3 kg/m2 and the initial HbA1c and FPG were 7.7% and 152 mg/dL, respectively. Of the 804 subjects, 767 (95.4%) were baseline metformin users, 364 (45.1%) were SU users, 217 (26.9%) were DPP4 inhibitor users, and 52 (6.5%) were TZD users.
- When classified by concurrently prescribed anti-diabetic drugs, the DPP4 inhibitor users showed a similar age and sex compared to non-users, but the diabetes duration was longer (Table 1). Fasting and postprandial plasma glucose levels were higher but there was no difference in initial median HbA1c levels (7.7% vs. 7.8%). When classified by metformin use, most of the subjects were on metformin therapy at baseline and the characteristics were balanced except for diabetes duration (Supplementary Table 1). The SU users were older, had a longer diabetes duration, and had the least controlled diabetes compared to non-users (Supplementary Table 2). A few patients were on TZD treatment and there was no difference in baseline glycemic status between users and non-users (Supplementary Table 3).
- Effect of SGLT2 inhibitors on anthropometric and biochemical parameters
- After administration of SGLT2 inhibitors (empagliflozin, dapagliflozin, or ipragliflozin) for a median of 192 days, the median HbA1c level had decreased by 0.7%, from 7.7% (IQR, 7.0% to 8.6%) to 7.0% (IQR, 6.5 to 7.8%; P<0.001) and fasting and postprandial glucose levels were significantly reduced (Table 2). The weight loss was about 3 kg, from 75 kg (IQR, 67 to 85 kg) to 72 kg (IQR, 63 to 82 kg; P<0.001). Serum triglyceride, HDL-C, and liver function test also improved and modest increases in serum hemoglobin and hematocrit levels were also observed. There were no significant changes in total cholesterol, LDL-C, and serum creatinine levels or eGFR. In addition, when patients were classified according to the kind of SGLT2 inhibitors, there were no significant differences in the changes in HbA1c and body weight (P=0.629 and P=0.125, respectively) (Supplementary Table 4).
- Efficacy of SGLT2 inhibitor according to baseline characteristics
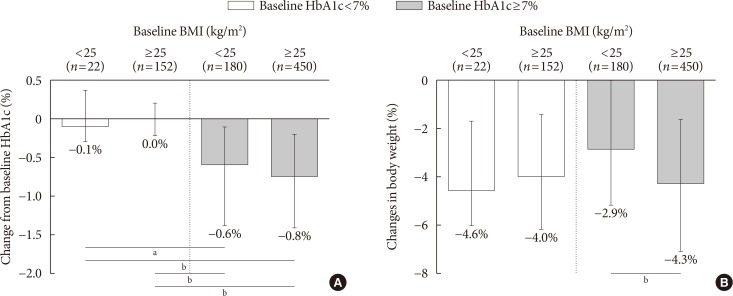
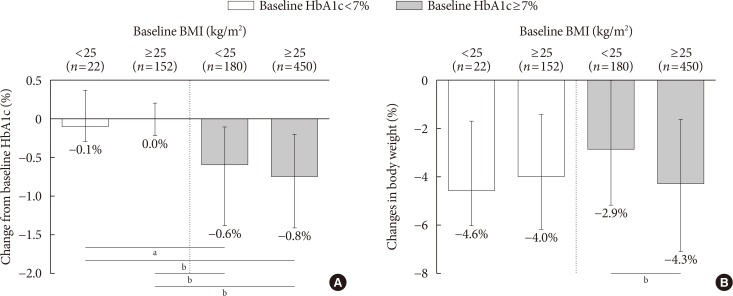
- The effects of SGLT2 inhibitors on the HbA1c level and body weight were compared according to baseline HbA1c (cut-off, 7%) and BMI (cut-off, 25 kg/m2). In well-controlled diabetes (left panel, Fig. 1A), the change from baseline HbA1c was minimal. In patients with inadequately controlled diabetes (right panel, Fig. 1A), the degree of HbA1c reduction was significantly larger and obese patients tended to have a better response (ΔHbA1c, −0.6% vs. −0.8%). In Fig. 1B, the adjusted weight loss did not differ in well-controlled diabetes (left panel, −4.6% vs. −4.0%), whereas significant weight reduction was observed in obese subjects with inadequately controlled diabetes (right panel, −2.9% vs. −4.3%).
- Glycemic responses according to other baseline characteristics are shown in Supplementary Fig. 1. There was no difference in glycemic response to SGLT2 inhibitors according to age and gender. However, a significantly larger HbA1c reduction following SGLT2 inhibitor therapy was shown in patients with shorter diabetes duration (<5 years), preserved eGFR (≥90 mL/min/1.73 m2), and poorly controlled diabetes (HbA1c ≥9%).
- Efficacy of SGLT2 inhibitor according to baseline anti-diabetes use
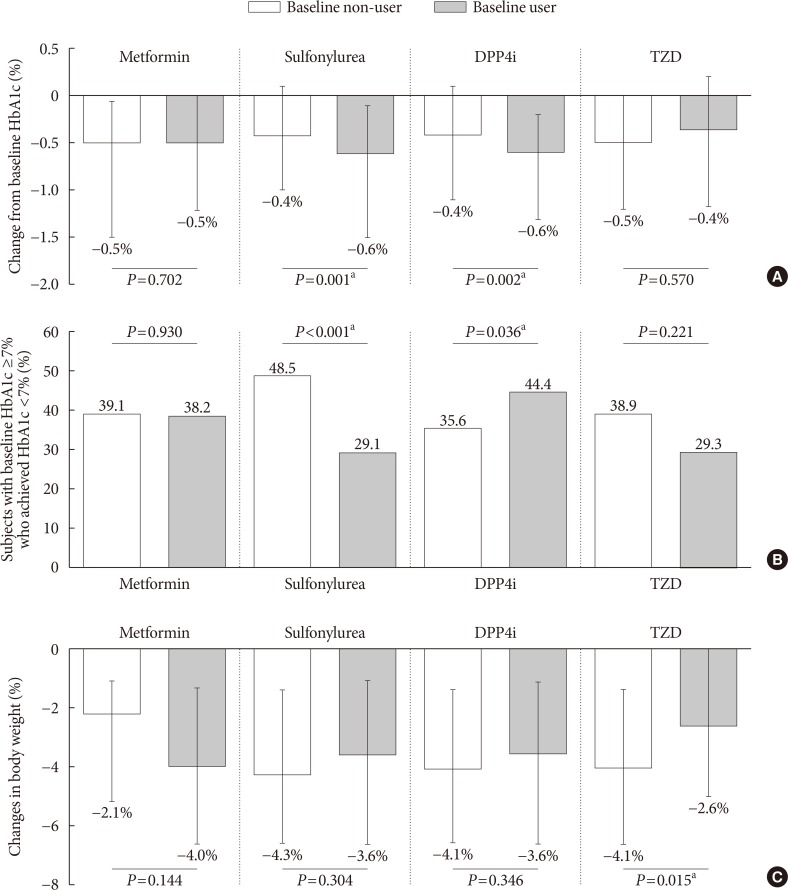
- Next, the effect of SGLT2 inhibitors was observed according to concomitant anti-diabetic medications. Baseline metformin or TZD use with SGLT2 inhibitor treatment did not impact the change from baseline HbA1c or the proportions of subjects achieving HbA1c <7% (Fig. 2A and B). SU users experienced a greater reduction in HbA1c after the addition of SGLT2 inhibitor versus non-users (−0.6% vs. −0.4%, P=0.001) (Fig. 2A), but the percentage of patients who reached the glycemic target was higher in SU non-users (29.1% vs. 48.5%, P<0.001) (Fig. 2B). As the SU users had inadequately controlled diabetes at baseline (Supplementary Table 2), it might be easier to lower the baseline high glucose, but it was not enough to reach the HbA1c <7%. On the other hand, the effect of SGLT2 inhibitor on prevalent DPP4 inhibitor users compared to non-users was significant both in the change from baseline HbA1c (−0.6% vs. −0.4%, P=0.002) and in the proportions of subjects who achieved HbA1c <7% (44.4% vs. 35.6%, P=0.036). The adjusted weight loss was about 4% and was not impacted by baseline metformin, SU, or DPP4 inhibitor use, whereas the combination therapy with TZD offset the weight loss effects of the SGLT2 inhibitor (P=0.015) (Fig. 2C).
- When subjects were divided according to respective drug combinations, 18 (2.2%) started SGLT2 inhibitor as monotherapy; 305 (37.9%) were on dual therapy; 353 (43.9%) were on triple combination therapy; and 128 (15.9%) were on quadruple combination therapy with addition of SGLT2 inhibitor to preexisting metformin, SU, TZD, or DPP4 inhibitor therapy. The detailed combinations of anti-diabetic drugs and the reduction of HbA1c are described in Supplementary Table 5. Because the combinations of anti-diabetic drugs were so diverse and those except for the four major combinations were difficult to analyze due to the small number of subjects, we analyzed subjects by baseline user or non-user through the manuscript.
- Analysis of predictive markers for the response to SGLT2 inhibitors
- To identify the predictive markers of SGLT2 inhibitors on HbA1c levels, linear regression analyses were conducted (Table 3). After adjusted for age, sex, initial BMI, diabetes duration, duration of SGLT2 inhibitor use, baseline HbA1c, eGFR, and other anti-diabetic agent use (metformin, SU, DPP4 inhibitor, and TZD), shorter diabetes duration, higher baseline HbA1c, and eGFR were the common predictors for a better response following SGLT2 inhibitor treatment in all subjects (all P<0.05). However, the baseline BMI had an inverse effect according to glycemic status. Lower BMI in well-controlled diabetes patients (baseline HbA1c <7%) and higher BMI in inadequately controlled diabetes patients (baseline HbA1c ≥7%) were associated with better response. Preexisting anti-diabetics use did not have an impact on subjects with lower baseline HbA1c <7%, but baseline SU and DPP4 inhibitor use were potential moderators of the SGLT2 inhibitor effects in subjects with baseline HbA1c ≥7%. SU use was associated with a lower response after adjusting for covariates whereas DPP4 inhibitor use was related to a significantly better response after the addition of SGLT2 inhibitor.
RESULTS
- In this study, we analyzed 804 patients who were administered three widely used SGLT2 inhibitors (empagliflozin, dapagliflozin, and ipragliflozin). After treatment for a median 192 days, the HbA1c level decreased by 0.7% (baseline 7.7%) and the weight loss was about 3.0 kg. Evaluation of the clinical factors affecting SGLT2 inhibitor response revealed that shorter diabetes duration, higher baseline HbA1c level and eGFR were associated with a greater reduction in HbA1c levels. The baseline BMI showed an opposite effect according to glycemic status and lean, tightly controlled subjects and obese, inadequately controlled subjects showed better responses. The type of anti-diabetic agents used before the addition of an SGLT2 inhibitor was also an important determinant. Baseline metformin and TZD use did not have an impact, but baseline DPP4 inhibitor users received the greatest benefit from SGLT2 inhibitor therapy. SU use was associated with a significantly lower response after adjusting for covariates.
- As the pathophysiology of T2DM is complex, the use of combination therapy with complementary mechanisms of action may offer additive or synergistic effects in glucose control [16]. DPP4 inhibitors prevent the degradation of incretin hormones such as glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide, which stimulate insulin secretion and inhibit glucagon release [17]. SGLT2 inhibitors improve glycemic control in an insulin-independent manner by promoting urinary glucose excretion [9]. Thus, the combination of DPP4 inhibitor and an SGLT2 inhibitor is an attractive approach. Furthermore, recent studies have shown that glucosuria produced by SGLT2 inhibitors is accompanied by increased endogenous glucose production (EGP), which may offset the glucose-lowering effect [18]. As DPP4 inhibitors suppress glucagon secretion from pancreatic α-cells and reduce EGP [17], combining DPP4 inhibitor and SGLT2 inhibitor may exert more beneficial effects [19].
- This issue includes several studies on the effect of combination therapy of DPP4 inhibitor and SGLT2 inhibitor. Rosenstock et al. [20] have assessed the efficacy and safety of the dual add-on of saxagliptin/dapagliflozin compared with those of saxagliptin or dapagliflozin added alone to metformin. Triple combination therapy showed a significantly greater HbA1c reduction than dual therapy with saxagliptin or dapagliflozin, with a mean change from baseline HbA1c of −1.5% versus −0.9% or −1.2%. Patients were well tolerated and hypoglycemia was rare, with no events of major hypoglycemia. DeFronzo et al. [21] reported similar findings after examining the effect of the combination of empagliflozin /linagliptin added to metformin versus each agent alone. As most of our study patients (95.4%) were already prescribed metformin, our results are in line with those of previous studies showing the greatest response in the combined therapy of metformin plus DPP4 inhibitor plus SGLT2 inhibitor. However, the degree of HbA1c reduction was slightly differed from the above studies. This discrepancy may probably due to the differences in drug compliance, which is much higher in randomized clinical trial, and the difference of baseline phenotype (ethnicity, initial HbA1c, and BMI) in patients.
- On the other hand, the decrease from baseline HbA1c level was larger in baseline SU users as absolute values, but the significance was reduced after adjusting for various factors in multiple regression analysis. Compared to other anti-diabetic agents, there were significant differences between SU users and non-users; the SU users were older, and had a longer diabetes duration, and were not well-controlled (Supplementary Table 2). Thus, it appears that these characteristics of baseline SU users are also related to the poor response to the addition of an SGLT2 inhibitor.
- The different effect of BMI on glucose control according to baseline HbA1c is a novel finding. In previous studies evaluating the clinical characteristics of glycemic response to SGLT2 inhibitors, baseline BMI was not associated with the glucose-lowering effect of SGLT2 inhibitor treatment [142223]. Higher baseline HbA1c, FPG, and eGFR are known independent predictors influencing the HbA1c reduction [142223]. However, as the baseline HbA1c may influence the response in different BMI groups, we compared the therapeutic efficacy of SGLT2 inhibitor among different HbA1c and BMI categories, demonstrating that obese T2DM patients with inadequate sugar control are more responsive to SGLT2 inhibitors. The increase in renal glucose excretion in proportion to the plasma glucose level [24] and the greater weight loss in obese subjects may have synergistic effects, though the precise mechanisms are unknown. However, considering of small sample number (n=22) in the lean and well-controlled diabetes patients, attention should be paid in interpreting this phenomenon.
- Our study has several limitations. First, we could not assess diet, exercise, or drug compliance during SGLT2 inhibitor treatment. Second, as this study design was retrospective, the type or dosage of other prescribing anti-diabetic agents was not standardized. Third, the degree of urinary glucose excretion was not measured. However, despite these limitations, we analyzed a relatively large number of patients who used SGLT2 inhibitors in a real-world setting, revealed the clinical factors, and identified the best SGLT2 inhibitor combination for optimal glucose-lowering efficacy.
- In summary, this retrospective observational study suggests that the addition of SGLT2 inhibitor use can provide greater glycemic benefit in inadequately controlled T2DM patients with a preserved renal function, short diabetes duration, and in baseline DPP4 inhibitor users. In association with BMI and HbA1c levels, lower BMI in well-controlled subjects and higher BMI in poorly controlled subjects were associated with better response. Further prospective studies are warranted to obtain more information on the therapeutic applications of SGLT2 inhibitors.
DISCUSSION
-
Acknowledgements
- We would like to thank Jiyu Sun (Statistician, Department of Preventive Medicine, Yonsei University College of Medicine) for the help with statistical consultation.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
NOTES
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Supplementary Table 2
Supplementary Table 3
Supplementary Table 4
Supplementary Table 5
Supplementary Fig. 1
- 1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4-14. ArticlePubMed
- 2. Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215-2222. ArticlePubMedPMC
- 3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-853. ArticlePubMed
- 4. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-1589. ArticlePubMed
- 5. Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, McCarren M, Duckworth WC, Emanuele NV. VADT Investigators. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197-2206. ArticlePubMed
- 6. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med 2013;368:1613-1624. ArticlePubMed
- 7. Montvida O, Shaw J, Atherton JJ, Stringer F, Paul SK. Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care 2018;41:69-78. ArticlePubMedPDF
- 8. Mullard A. 2013 FDA drug approvals. Nat Rev Drug Discov 2014;13:85-89. ArticlePubMedPDF
- 9. Chao EC, Henry RR. SGLT2 inhibition: a novel strategy for diabetes treatment. Nat Rev Drug Discov 2010;9:551-559. ArticlePubMedPDF
- 10. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care 2010;33:2217-2224. PubMedPMC
- 11. Jung CH, Jang JE, Park JY. A novel therapeutic agent for type 2 diabetes mellitus: SGLT2 inhibitor. Diabetes Metab J 2014;38:261-273. ArticlePubMedPMC
- 12. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117-2128. ArticlePubMed
- 13. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644-657. ArticlePubMed
- 14. Lee JY, Kim G, Kim SR, Lee YH, Lee BW, Cha BS, Kang ES. Clinical parameters affecting dapagliflozin response in patients with type 2 diabetes. Diabetes Metab 2017;43:191-194. ArticlePubMed
- 15. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-470. ArticlePubMed
- 16. Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773-795. PubMedPMC
- 17. Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004;89:2078-2084. ArticlePubMed
- 18. Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, Xiong J, Perez Z, Norton L, Abdul-Ghani MA, DeFronzo RA. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509-514. ArticlePubMedPMC
- 19. Abdul-Ghani M. Where does combination therapy with an SGLT2 inhibitor plus a DPP-4 inhibitor fit in the management of type 2 diabetes? Diabetes Care 2015;38:373-375. ArticlePubMedPDF
- 20. Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, Iqbal N. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care 2015;38:376-383. ArticlePubMedPDF
- 21. DeFronzo RA, Lewin A, Patel S, Liu D, Kaste R, Woerle HJ, Broedl UC. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 2015;38:384-393. ArticlePubMedPDF
- 22. Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab 2011;13:928-938. ArticlePubMed
- 23. Bujac S, Del Parigi A, Sugg J, Grandy S, Liptrot T, Karpefors M, Chamberlain C, Boothman AM. Patient characteristics are not associated with clinically important differential response to dapagliflozin: a staged analysis of phase 3 data. Diabetes Ther 2014;5:471-482. ArticlePubMedPMCPDF
- 24. Ferrannini E, Veltkamp SA, Smulders RA, Kadokura T. Renal glucose handling: impact of chronic kidney disease and sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care 2013;36:1260-1265. PubMedPMC
REFERENCES
Efficacy parameters according to baseline glycosylated hemoglobin (HbA1c) and body mass index (BMI). (A) Change from baseline HbA1c, (B) changes in body weight according to baseline HbA1c and BMI categories. Data are expressed as median (interquartile range). aP<0.01, bP<0.001.
Efficacy parameters according to baseline anti-diabetic drug use. (A) Change from baseline glycosylated hemoglobin (HbA1c), (B) subjects with baseline HbA1c ≥7% who achieved HbA1c <7%, (C) changes in body weight. Data are expressed as median (interquartile range) or number (%). DPP4i, dipeptidyl peptidase 4 inhibitor; TZD, thiazolidinedione. aP<0.05.
Baseline characteristics of patients
Values are presented as median (interquartile range), number (%), or mean±standard deviation.
DPP4, dipeptidyl peptidase 4; DM, diabetes mellitus; SGLT2, sodium-glucose co-transporter 2; HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; PP2, post-prandial 2-hour glucose; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CAOD/PAOD, coronary artery/peripheral artery occlusive disease; TIA, transient ischemic attack.
Changes in anthropometric and biochemical parameters in patients
Values are presented as median (interquartile range) or mean±standard deviation.
HbA1c, glycosylated hemoglobin; FPG, fasting plasma glucose; PP2, post-prandial 2-hour glucose; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Linear regression analysis for better glycemic response to SGLT2 inhibitors
SGLT2, sodium-glucose co-transporter 2; HbA1c, glycosylated hemoglobin; BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; SU, sulfonylurea; DPP4, dipeptidyl peptidase 4; TZD, thiazolidinedione.
aAdjusted for age, sex, initial BMI, diabetes duration, duration of SGLT2 inhibitor use, baseline HbA1c and eGFR levels, and anti-diabetic agent use (metformin, SU, DPP4 inhibitor, and TZD).
Figure & Data
References
Citations

- Predictors of efficacy of Sodium‐GLucose Transporter‐2 inhibitors and Glucagon‐Like Peptide 1 receptor agonists: A retrospective cohort study
Daniele Scoccimarro, Giacomo Cipani, Ilaria Dicembrini, Edoardo Mannucci
Diabetes/Metabolism Research and Reviews.2024;[Epub] CrossRef - Short-term effectiveness of dapagliflozin versus DPP-4 inhibitors in elderly patients with type 2 diabetes: a multicentre retrospective study
M. L. Morieri, I. Raz, A. Consoli, M. Rigato, A. Lapolla, F. Broglio, E. Bonora, A. Avogaro, G. P. Fadini, Federica Ginestra, Gloria Formoso, Agostino Consoli, Francesco Andreozzi, Giorgio Sesti, Salvatore Turco, Luigi Lucibelli, Adriano Gatti, Raffaella
Journal of Endocrinological Investigation.2023; 46(7): 1429. CrossRef - Treatment effect heterogeneity following type 2 diabetes treatment with GLP1-receptor agonists and SGLT2-inhibitors: a systematic review
Katherine G. Young, Eram Haider McInnes, Robert J. Massey, Anna R. Kahkoska, Scott J. Pilla, Sridharan Raghavan, Maggie A. Stanislawski, Deirdre K. Tobias, Andrew P. McGovern, Adem Y. Dawed, Angus G. Jones, Ewan R. Pearson, John M. Dennis, Deirdre K. Tobi
Communications Medicine.2023;[Epub] CrossRef - Predictors of HbA1c treatment response to add-on medication following metformin monotherapy: a population-based cohort study
Wei Ying Tan, Wynne Hsu, Mong Li Lee, Ngiap Chuan Tan
Scientific Reports.2023;[Epub] CrossRef - Efficacy and Safety of Evogliptin Add-on Therapy to Dapagliflozin/Metformin Combinations in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A 24-Week Multicenter Randomized Placebo-Controlled Parallel-Design Phase-3 Trial with a 28-Week Extensio
Jun Sung Moon, Il Rae Park, Hae Jin Kim, Choon Hee Chung, Kyu Chang Won, Kyung Ah Han, Cheol-Young Park, Jong Chul Won, Dong Jun Kim, Gwan Pyo Koh, Eun Sook Kim, Jae Myung Yu, Eun-Gyoung Hong, Chang Beom Lee, Kun-Ho Yoon
Diabetes & Metabolism Journal.2023; 47(6): 808. CrossRef - Effect of Dapagliflozin as an Add-on Therapy to Insulin on the Glycemic Variability in Subjects with Type 2 Diabetes Mellitus (DIVE): A Multicenter, Placebo-Controlled, Double-Blind, Randomized Study
Seung-Hwan Lee, Kyung-Wan Min, Byung-Wan Lee, In-Kyung Jeong, Soon-Jib Yoo, Hyuk-Sang Kwon, Yoon-Hee Choi, Kun-Ho Yoon
Diabetes & Metabolism Journal.2021; 45(3): 339. CrossRef - Angiotensin II up-regulates sodium-glucose co-transporter 2 expression and SGLT2 inhibitor attenuates Ang II-induced hypertensive renal injury in mice
Kana N. Miyata, Chao-Sheng Lo, Shuiling Zhao, Min-Chun Liao, Yuchao Pang, Shiao-Ying Chang, Junzheng Peng, Matthias Kretzler, Janos G. Filep, Julie R. Ingelfinger, Shao-Ling Zhang, John S.D. Chan
Clinical Science.2021; 135(7): 943. CrossRef - Sodium-Glucose Cotransporter-2 Inhibitor for Renal Function Preservation in Patients with Type 2 Diabetes Mellitus: A Korean Diabetes Association and Korean Society of Nephrology Consensus Statement
Tae Jung Oh, Ju-Young Moon, Kyu Yeon Hur, Seung Hyun Ko, Hyun Jung Kim, Taehee Kim, Dong Won Lee, Min Kyong Moon
Diabetes & Metabolism Journal.2020; 44(4): 489. CrossRef - Differential indication for SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with established atherosclerotic heart disease or at risk for congestive heart failure
Francesco Giorgino, Irene Caruso, Julia Moellmann, Michael Lehrke
Metabolism.2020; 104: 154045. CrossRef - Clinical Predictors of the Hypoglycemic Effect of Sodium–Glucose Co-transporter-2 Inhibitors in Hyperuricemic Patients: A Retrospective Descriptive Observational Study
Toshinori Hirai, Yuya Kawagoe, Motoki Kei, Ryuichi Ogawa, Toshimasa Itoh
Biological and Pharmaceutical Bulletin.2020; 43(5): 782. CrossRef - Sodium-glucose cotransporter-2 inhibitor for renal function preservation in patients with type 2 diabetes mellitus: A Korean Diabetes Association and Korean Society of Nephrology consensus statement
Tae Jung Oh, Ju-Young Moon, Kyu Yeon Hur, Seung Hyun Ko, Hyun Jung Kim, Taehee Kim, Dong Won Lee, Min Kyong Moon
Kidney Research and Clinical Practice.2020; 39(3): 269. CrossRef - Efficacy of Once-Weekly Semaglutide vs Empagliflozin Added to Metformin in Type 2 Diabetes: Patient-Level Meta-analysis
Ildiko Lingvay, Matthew S Capehorn, Andrei-Mircea Catarig, Pierre Johansen, Jack Lawson, Anna Sandberg, Robert Shaw, Abby Paine
The Journal of Clinical Endocrinology & Metabolism.2020; 105(12): e4593. CrossRef - Letter: Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors (Diabetes Metab J 2019;43:158–73)
Kyung-Soo Kim
Diabetes & Metabolism Journal.2019; 43(3): 377. CrossRef - Response: Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors (Diabetes Metab J 2019;43:158–73)
Ji-Yeon Lee, Eun Seok Kang
Diabetes & Metabolism Journal.2019; 43(3): 379. CrossRef - An Age of Sodium-Glucose Cotransporter-2 Inhibitor Priority: Are We Ready?
Ji A Seo
Diabetes & Metabolism Journal.2019; 43(5): 578. CrossRef

 KDA
KDA PubReader
PubReader Cite
Cite