- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 43(2); 2019 > Article
-
ReviewClinical Diabetes & Therapeutics Nonalcoholic Fatty Liver Disease and Diabetes: Part II: Treatment
-
Kyung-Soo Kim1, Byung-Wan Lee2
 , Yong Jin Kim3, Dae Ho Lee4, Bong-Soo Cha2, Cheol-Young Park5
, Yong Jin Kim3, Dae Ho Lee4, Bong-Soo Cha2, Cheol-Young Park5
-
Diabetes & Metabolism Journal 2019;43(2):127-143.
DOI: https://doi.org/10.4093/dmj.2019.0034
Published online: April 15, 2019
1Division of Endocrinology and Metabolism, Department of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea.
2Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
3Department of Surgery, Soonchunhyang University Seoul Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
4Division of Endocrinology and Metabolism, Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
5Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding author: Byung-Wan Lee. Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea. bwanlee@yuhs.ac
- Corresponding author: Cheol-Young Park. Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 03181, Korea. cydoctor@chol.com
Copyright © 2019 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Nonalcoholic fatty liver disease (NAFLD) and diabetes are common metabolic disorders that are often comorbid conditions. Among many proposed treatments, weight reduction is the only approved option for NAFLD to date. However, it is not easy to maintain weight loss by lifestyle modification alone; pharmacological treatments are helpful in this regard. Although many drugs have been investigated, pioglitazone could be a first-line therapy in patients with NAFLD and diabetes. Many more drugs are currently being developed and investigated, and it is likely that combination strategies will be used for future treatment of NAFLD and diabetes. Attention should be paid to the management of NAFLD and diabetes and efforts should be made to intervene early and individualize treatment of NAFLD in patients with diabetes.
- Epidemiological evidence suggests a strong bidirectional relationship between type 2 diabetes mellitus (T2DM), including its risks, and the severity of nonalcoholic fatty liver disease (NAFLD), progression to nonalcoholic steatohepatitis (NASH), and advanced fibrosis, independent of liver enzymes [12]. Furthermore, the coexistence of T2DM and NAFLD results in an unfavorable metabolic profile and increased cardiovascular risk [234], which makes lifestyle correction fundamental in all patients regardless of diabetes. Based on epidemiologic and clinical trial data, management of T2DM patients with NAFLD should aim to reduce risk factors associated with the high cardiovascular risk of these patients [3], decrease hepatic fat accumulation, and delay the progression of inflammation and fibrosis [56].
INTRODUCTION
- It is well established that weight loss in overweight or obese individuals with T2DM, either by lifestyle modification or bariatric surgery, results in significant improvement or resolution of T2DM and its comorbidities such as hypertension and hyperlipidemia [378]. In addition, weight reduction has been associated with a reduction in intrahepatic fat content and normalization of aminotransferase levels [9]. Even a relatively small amount of weight loss can reduce liver fat and improve hepatic insulin resistance. Petersen et al. [10] demonstrated that moderate weight loss (86 kg before weight loss, 78 kg after weight loss) in patients with poorly controlled T2DM could normalize fasting hyperglycemia by mobilizing the relatively small pool of intrahepatic lipids, which reversed hepatic insulin resistance and normalized rates of basal glucose production, independent of any changes in insulin-stimulated peripheral glucose metabolism. These results are applicable not only to the obese adult population, but also the non-obese adult population. In both nonobese and obese subjects with NAFLD [1112], lifestyle intervention involving the combination of diet, exercise, and behavior modification with a goal of 7% to 10% weight reduction results in more weight loss, more frequent resolution of NASH, and a borderline higher reduction in NAFLD activity score (NAS). However, in a 12-month uncontrolled study of subjects participating in a diet and exercise program, 15 subjects (out of 23 enrolled) who completed the study failed to lose weight and there was no change in their liver histology based on liver biopsies at the start and end of the study [13].
- A systematic review of 23 studies of adult populations with NAFLD to determine the effects of lifestyle interventions such as dietary modifications, physical activity, and/or exercise on hepatic indicators of steatosis, inflammation, and fibrosis, and glucose control/insulin sensitivity revealed that reductions in liver fat and/or liver aminotransferase concentrations were the most strongly correlated with weight loss [14]. Of the five studies that investigated changes in histopathology, all showed a trend towards reduction in inflammation, and this was statistically significant in two of the studies. The majority of studies also reported improvements in glucose control and insulin sensitivity following intervention. These studies concluded that diet-based lifestyle modifications leading to weight reduction and/or increased physical activity consistently reduced liver fat and improved glucose control/insulin sensitivity [14]. However, it should be borne in mind that sustainable maintenance of weight loss with lifestyle interventions for long-term periods was achieved in only 3% to 6% of subjects [915]. Furthermore, little is known about the effects of lifestyle intervention on liver histology after 1 year, or what the best strategy to maintain weight loss over time is.
- Another systematic review and meta-analysis of 15 studies (766 paired liver biopsies) that examined the effects of weight loss after bariatric surgical procedures on NAFLD reported a mean reduction in body mass index (BMI) after bariatric surgery ranging from 19.1% to 41.8%. The pooled proportion of patients with improvement or resolution of steatosis was 91.6%, followed by 81.3% for steatohepatitis and 65.5% for fibrosis. The proportion of patients with complete resolution of NASH was 69.5% [16].
- Magnitude of weight reduction
- In adult populations with NAFLD, a greater degree of weight loss, induced by either lifestyle modifications or bariatric surgery, is associated with greater improvement in histologic features. In this context, the amount of reduction in body weight is a determinant of histologic improvement of liver injury and fibrosis [9]. Among the few well-controlled studies with paired liver biopsies, an improvement in hepatic steatosis and necroinflammation was observed in patients with ≥7% weight reduction over 48 weeks based on a moderate-intensity hypocaloric diet and exercise program (200 min/wk) [11]. A similar paired biopsy study in 261 patients who underwent a 12-month intervention with a hypocaloric diet combined with walking 200 min/wk reported similar benefits, although this study was not controlled. In this study, a dose-response relationship was clear among weight loss and all NASH-related histologic parameters, with the greatest reduction observed in those with the greatest weight loss. The highest rates of NAS reduction, steatohepatitis resolution, and fibrosis regression occurred in patients with ≥10% weight loss [17]. In patients with poorly controlled T2DM, a relatively modest weight reduction in body weight (<10%) can lead to a marked reduction in intrahepatic lipid content, improved hepatic insulin sensitivity, and normalization of fasting plasma glucose concentration [710].
- Summarizing all current available reports, it appears that weight loss in the range of 5% to 7% clearly decreases steatosis and associated metabolic parameters, but 8% to 10% weight reduction is needed to reverse steatohepatitis [3710141618, 19]. Although weight loss ≥7% also improves NAS, fibrosis remains unchanged. The threshold of 7% weight loss is achieved by <50% of patients, even with intensive multidisciplinary lifestyle interventions [711].
WEIGHT REDUCTION
- Currently, weight loss is the treatment of choice to decrease hepatic fat accumulation and delay the progression of inflammation and fibrosis. Of the ways to achieve weight reduction, lifestyle modifications including a programmed diet and exercise are effective and sound treatment options for all patients with NAFLD and NASH [5192021]. In addition, lifestyle modifications can also improve hyperglycemia, atherogenic dyslipidemia, and blood pressure levels. In some studies, hepatic fat reductions in T2DM patients were achieved by lifestyle modification even with minimal or no weight loss, which implies that other factors beyond weight loss also play a role in NAFLD and NASH improvement [35621]. However, diets with different macronutrient compositions (e.g., low fat versus low carbohydrate) and types of exercise protocols (e.g., aerobic versus resistance training) have resulted in inconsistent findings among studies. Another challenge may be that different diets may be unacceptable because of cultural food differences and underlying metabolic abnormalities. For example, the Mediterranean diet has beneficial gluco-metabolic effects in those individuals with metabolic abnormalities such as T2DM, NAFLD, and obesity, but has not been evaluated in those of Asian ethnicity or with specific metabolic abnormalities.
- Of the lifestyle modifications, however, weight reduction by dietary program or intervention remains the cornerstone for all patients, including T2DM patients with NAFLD and NASH. Numerous challenges remain in the quest to find an effective and safe dietary intervention program, although several trials have now prioritized patients with NAFLD. It seems that caloric restriction has a greater role in reducing hepatic fat accumulation than dietary macronutrient composition; this reduction of hepatic fat accumulation delays the progression of inflammation and fibrosis in patients with NAFLD [7910111417]. In addition, it has been established that a hypocaloric diet attenuates the development and progression of T2DM and cardiovascular risk [71018]. Comparable effects have been observed with equally hypocaloric low-carbohydrate versus high-carbohydrate diets [22] and low-fat versus low-carbohydrate diets [23]. Haufe et al. [23] demonstrated that a prolonged hypocaloric diet low in carbohydrates and high in fat had the same beneficial effects on intrahepatic lipid accumulation as a traditional low-fat hypocaloric diet.
- Similar to dietary modification, the type of exercise that is most effective and the fitness level that is optimal for diabetes patients with NAFLD remain to be determined. Baseline characteristics of study subjects and exercise protocols are heterogeneous among studies, and the amount of weight loss or changes in surrogate biomarkers achieved after trial intervention can be inconsistent. Although it is still not clear if exercise improves weight loss and reduces hepatic fat reduction, exercise itself has been shown to improve liver enzymes and insulin resistance in all diabetes patients with NAFLD and NASH [321]. A systematic review of 12 studies compared the effects of regular aerobic exercise and/or progressive resistance training to no exercise on changes in liver fat and/or alanine aminotransferase (ALT) levels in adults. Exercise therapy had a beneficial effect on liver fat but not ALT level with minimal or no weight loss [24]. For adults with T2DM, a systematic review of 14 (11 randomized and three nonrandomized) controlled trials was performed to evaluate the effect of exercise interventions (duration ≥8 weeks) on glycosylated hemoglobin (HbA1c) and BMI. Exercise training reduced HbA1c compared to the non-exercising control group (7.65% in exercisers vs. 8.31% in non-exercisers, P<0.001), but there was no significant change in BMI between the two groups [25]. To determine the effect of exercise on NAFLD in T2DM subjects, a randomized controlled trial (RCT) compared the effects of 4 months of either aerobic training or resistance training, and showed that both exercise interventions resulted in similar weight reduction and were equally effective at reducing liver triglyceride content (by about 30%) in patients with T2DM and NAFLD [26]. Physical activity, either aerobic or resistance training, should be strongly promoted for the management of fatty liver in T2DM subjects, and the benefits of exercise are not exclusively contingent upon weight loss [27]. One study of 233,676 Koreans to determine the optimal amount of exercise to improve NAFLD demonstrated that moderate to vigorous exercise >5 times per week (lasting at least 10 minutes per exercise session) decreased the risk of development of new fatty liver or improved resolution of existing fatty liver as assessed by ultrasound (US) during 5 years of follow-up [28].
- Combined dietary and exercise interventions, especially those performed long-term, significantly improve NAFLD. However, a combined diet and exercise approach does not always have a synergistic effect relative to diet alone or exercise alone for reasons that are unclear. One possible reason for this is that hepatic fat reduction is correlated with the amount of weight reduction. Studies that have compared a hypocaloric diet versus a hypocaloric diet plus exercise have failed to show an improvement in liver fat content with a combined approach [212930]. A possible explanation is that body weight reduction was similar for both interventions. A meta-analysis by Keating et al. [24] showed that combined dietary and exercise intervention had no significant pooled effects size (ES) when compared to diet alone (ES, −0.05; 95% confidence interval [CI], −0.38 to 0.27; P=0.76). The long-term effects of diet-plus-exercise versus diet alone might, however, yield different results. A systematic review of 18 studies investigated whether a diet-plus-exercise intervention for at least 6 months was more effective at promoting weight loss than a diet-only intervention among obese or overweight adults. This review reported that a combined diet-plus-exercise intervention provided greater long-term weight loss than a diet-only intervention [31]. Furthermore, in studies lasting 2 years or longer, diet-plus-exercise interventions have been shown to result in significantly greater weight loss than diet-only interventions. However, both diet-only and diet-plus-exercise programs are associated with partial weight regain [31]. Pragmatic approaches combining dietary restriction and a progressive increase in aerobic exercise/resistance training are preferable and should be individually tailored [313233]. However, the long-term effects of these approaches, especially on weight regain, are currently unknown.
- Even though the effects of lifestyle modification in the absence of weight loss on NAFLD and NASH are unclear, it is clear that lifestyle changes comprising a hypocaloric diet, exercise, or both can reduce the risk of cardiovascular disease as well as the onset and progression of T2DM [3435]. Clearly, more studies are needed to completely understand the role of lifestyle intervention in the treatment of NASH and to establish the best treatment strategy for patients with T2DM and NASH [3].
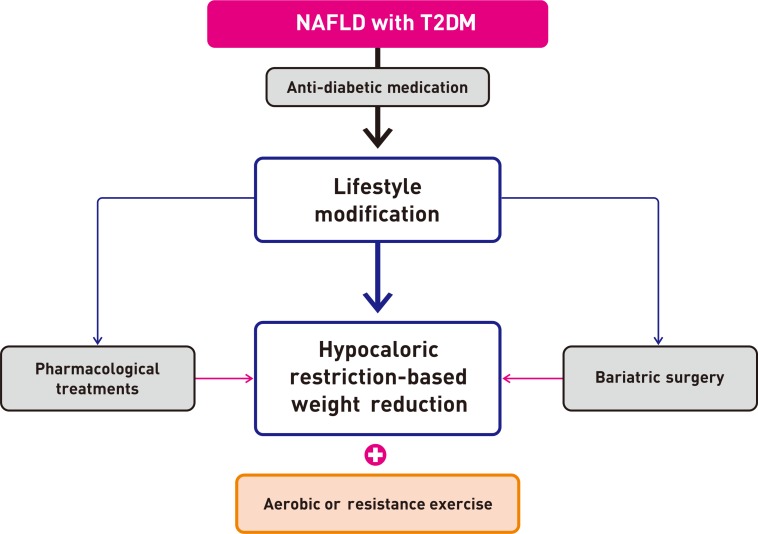
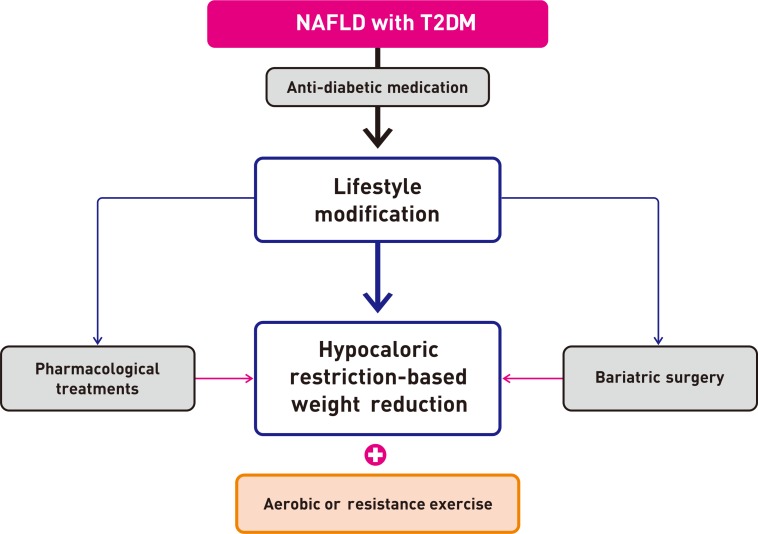
- For T2DM patients with NAFLD, however, pharmacological treatment is more powerful than lifestyle modification for glucose control. Moreover, lifestyle modification plus anti-diabetic drugs are likely to have a synergistic effect on reducing the risk factors associated with cardiovascular risk [3] and decreasing hepatic fat accumulation in these patients, thereby delaying the progression of inflammation and fibrosis. Therefore, all patients, especially those with T2DM, should be strongly encouraged to adopt both lifestyle changes and anti-diabetic medication (Fig. 1).
LIFESTYLE MODIFICATION FOR WEIGHT REDUCTION
- Patients with prediabetes or T2DM and NAFLD have the highest cardiometabolic disease risk [3637]. Because patients with NAFLD but without any fibrosis have an excellent prognosis, pharmacological treatment should be considered in biopsy-proven NASH in patients with T2DM. There are no definite pharmacological treatments for NASH in patients with T2DM, but many drugs have been evaluated and are under investigation (Table 1).
- Anti-diabetic agents
- Metformin is an insulin sensitizer and the first line agent for T2DM. It has been widely tested in NAFLD because insulin resistance is a major pathogenic feature of T2DM patients. Early open-label studies with metformin suggested a histologic benefit [3839], but recent RCTs have yielded negative results [2040]. Furthermore, two meta-analyses found that metformin did not improve liver histology in patients with NAFLD and NASH [4142]. Because there is scarce evidence of the efficacy of metformin in patients with NAFLD and T2DM, metformin is not recommended for treating NAFLD in patients with T2DM.
- Pioglitazone is a peroxisome proliferator-activated receptor (PPAR)-γ agonist with insulin-sensitizing effects and belongs to a class of drugs known as thiazolidinediones (TZDs). Pioglitazone has a lot of evidences for the treatment of NASH in patients with T2DM and has been shown to improve liver histology. In one RCT, 55 patients with impaired glucose tolerance or T2DM (mean HbA1c 6.2%) and liver biopsy-confirmed NASH were randomly assigned to 6 months of treatment with a hypocaloric diet plus pioglitazone (45 mg daily) or a hypocaloric diet and placebo. Pioglitazone improved insulin sensitivity and aminotransferase, steatosis (P=0.003), ballooning necrosis (P=0.02), and inflammation (P=0.008) [43]. Another RCT evaluated the efficacy and safety of long-term pioglitazone treatment in patients (n=101) with biopsy-proven NASH and prediabetes or T2DM (n=52). All patients were prescribed a hypocaloric diet (500 kcal/day deficit from weight-maintaining caloric intake) and were then randomly assigned to pioglitazone (45 mg/day) or placebo for 18 months, followed by an 18-month open-label phase of pioglitazone treatment. Among patients randomly assigned to pioglitazone, 58% achieved the primary outcome (a reduction of at least 2 points in NAS in two histologic categories without worsening of fibrosis) and 51% had resolution of NASH (P<0.001 for each). Pioglitazone treatment was also associated with an improvement in histologic score (P=0.039). All 18- month metabolic and histologic improvements persisted over 36 months of therapy [44]. In addition, pioglitazone reduced liver fibrosis and increased adipose tissue insulin sensitivity to a significantly greater extent in patients with T2DM than in patients with prediabetes [45]. In the case of advanced fibrosis (stage F3–F4), meta-analysis showed that pioglitazone use improved fibrosis in patients with NASH and T2DM, even in patients without diabetes [46]. Weight gain, fluid retention, and bone loss are common side effects of pioglitazone treatment, so risks and benefits should be considered before starting therapy. Pioglitazone could be a first line therapy for patients with NASH and T2DM, similar to metformin for the initial treatment of patients with T2DM. However, more data are required before pioglitazone is routinely used to treat NAFLD in patients with T2DM.
- Lobeglitazone is a novel TZD and is currently being prescribed for T2DM in Korea. Lobeglitazone improved hepatic steatosis in an animal model, similar to what has been observed for other TZDs [47]. In a multicenter, prospective, open-label, exploratory clinical trial, lobeglitazone treatment (0.5 mg daily) for 24 weeks improved liver enzymes and ameliorated hepatic fat content as assessed by transient liver elastography with controlled attenuation parameter (CAP) in 43 patients with T2DM and NAFLD [48].
- Glucagon-like peptide-1 receptor agonists (GLP-1 RA) are appealing candidates for the treatment of NAFLD and NASH because they can reduce weight and enhance insulin action. However, GLP-1 RA is not currently recommended for the treatment of NAFLD in patients with T2DM because of limited data.
- The Liraglutide Effect and Action in Diabetes (LEAD) program performed individual patient data meta-analysis using six 26-week, phase III, randomized controlled T2DM trials. Twenty-six weeks of treatment with liraglutide 1.8 mg reduced liver enzymes in patients with T2DM (liraglutide −8.20 IU/L vs. placebo −5.01 IU/L, P=0.003). This effect was likely mediated by liraglutide's weight loss and glycemic control effects [49]. In the Liraglutide Efficacy and Action in NASH (LEAN) study, which was a randomized, placebo-controlled trial consisting of 52 patients with biopsy-proven NASH, liraglutide (1.8 mg daily) for 48 weeks was associated with greater resolution of steatohepatitis and less progression of fibrosis. Among the LEAN study population, 32.7% of participants were patients with T2DM [50]. Larger studies with liraglutide are needed to confirm its effects on NASH in patients with T2DM.
- No study has investigated exenatide treatment of NASH in patients with T2DM, but one study investigated its effects on NAFLD. The investigators treated 60 newly diagnosed patients with obesity, NAFLD with elevated liver enzymes, and T2DM with exenatide plus insulin glargine or insulin aspart plus insulin glargine. Levels of ALT, aspartate aminotransferase (AST), and γ-glutamyl transpeptidase (γ-GTP) in the exenatide group were significantly lower than in the intensive insulin group (P<0.001), and the reversal rate of fatty liver was significantly higher in the exenatide group (93.3% vs. 66.7%, respectively; P<0.01) [51].
- The results of 12 RCTs on lixisenatide versus placebo and three RCTs with active comparators were analyzed by meta-analysis to evaluate the effects of lixisenatide on elevated liver enzyme levels in patients with T2DM. Lixisenatide increased the proportion of patients with normalization of ALT compared with placebo or active comparators [52].
- Dipeptidyl peptidase-4 (DPP-4) inhibitors are commonly used in patients with T2DM, but there are few studies of the efficacy of DPP-4 inhibitors in patients with T2DM and NAFLD. These inhibitors are not believed to have a beneficial effect on NAFLD.
- Results for sitagliptin have been mixed. Significant decreases in plasma glucose and serum HbA1c, AST, ALT, and γ-GTP levels were reported after 4 months of treatment of NAFLD patients with T2DM with sitagliptin [53]. A pilot clinical study demonstrated that sitagliptin could improve hepatocyte ballooning and liver enzymes in patients with T2DM and NASH after 1 year of treatment [54]. However, many other studies have failed to show an effect of sitagliptin treatment on liver fat content [5556], liver enzyme levels [57], or liver stiffness [56].
- One study has investigated the efficacy of vildagliptin in patients with T2DM and NAFLD. In patients with T2DM and hepatic steatosis, mean fasting liver triglyceride content decreased by 27% with vildagliptin, from 7.3%±1.0% (baseline) to 5.3%±0.9% (endpoint) during 6 months of therapy, and this was unrelated to changes in body weight. Additionally, ALT fell from 27.2 to 20.3 IU/L in the vildagliptin group (P=0.007) [58].
- Sodium-glucose co-transporter 2 (SGLT2) inhibitors are increasingly been used to treat T2DM and they promote weight loss, which is an attractive property for the treatment of patients with NAFLD. Although SGLT2 inhibitors are not yet generally recommended for the treatment of NAFLD in patients with T2DM, studies reporting beneficial effects are accumulating.
- In a retrospective study comparing dapagliflozin and DPP-4 inhibitors, dapagliflozin treatment resulted in liver enzyme improvement in patients with T2DM and NAFLD [59]. Many small studies have reported that dapagliflozin treatment had beneficial effects on levels of liver enzymes, liver fat content, and/or liver stiffness in patients with T2DM and NAFLD [606162]. In a randomized, active-controlled, open-label trial, dapagliflozin significantly decreased CAP (314 to 290 dB/m; P=0.0424) and tended to decrease liver stiffness measurements in 57 patients with T2DMM and NAFLD [60]. The Effects of Omega-3 Carboxylic Acids and Dapagliflozin on Liver Fat Content in Diabetic Patients (EFFECT-II) study, which was a randomized, placebo-controlled, double-blind, parallel-group study, showed that combined treatment with dapagliflozin and omega-3 carboxylic acids significantly reduced liver fat content as assessed by magnetic resonance imaging (MRI)-derived proton density fat fraction (PDFF). In addition, dapagliflozin monotherapy reduced all measured hepatocyte injury markers in patients with T2DM and NAFLD [61]. Another prospective, randomized, active-controlled, single center study in 55 Japanese T2DM patients showed that dapagliflozin treatment for 6 months significantly reduced liver fat accumulation as assessed by the liver-to-spleen (L/S) attenuation ratio using abdominal computed tomography (CT) compared with non-SGLT2 inhibitor treatment [62].
- Pooled data from four 26-week and two 52-week phase III clinical trials of canagliflozin showed significant reductions in ALT, AST, γ-GTP, and alkaline phosphatase compared with placebo in patients with T2DM [63]. These protective effects of canagliflozin were also found in a meta-analysis of data extracted from RCTs [64]. In a small (n=20), prospective, non-randomized, open-label single-arm study, canagliflozin (100 mg daily) treatment for 12 months significantly reduced hepatic fat fraction (17.6% to 12.1%; P<0.005) as measured by MRI in patients with T2DM and NAFLD [65]. Fibrosis-4 (FIB-4) index values decreased after 6 months of treatment with canagliflozin (100 mg daily) in 35 patients with T2DM and NAFLD [66].
- In the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus (EMPA-REG OUTCOME) trial, empagliflozin reduced aminotransferase levels (ALT>AST) in patients with T2DM [67]. The Effect of Empagliflozin on Liver Fat Content in Patients With Type 2 Diabetes (E-LIFT) trial, which was an investigator-initiated, prospective, open-label, randomized clinical study, showed that empagliflozin (10 mg daily) significantly reduced liver fat (16.2% to 11.3%; P<0.0001) as measured by MRI-PDFF in 50 patients with T2DM and NAFLD [68].
- Ipragliflozin treatment for 24 weeks (50 mg daily) reduced liver enzymes in 43 patients with T2DM and NAFLD and tended to decrease liver stiffness as measured by transient elastography [69]. Subgroup analysis of data from the Specified drug use resulTs survEy of lpragLifLozin treAtment in type 2 diabetes patients: LONG-TERM use (STELLA-LONG TERM), an ongoing 3-year post-marketing surveillance study on the long-term efficacy and safety of ipragliflozin, showed that fatty liver index (FLI) decreased (63.2677 to 56.7137; P<0.05) and liver function improved in patients with T2DM [70]. Interestingly, in this open-label, randomized, active-controlled trial, the efficacy of ipragliflozin was compared to that of pioglitazone in 66 patients with T2DM and NAFLD. At week 24, the mean L/S ratio on CT had increased by 0.22 in the ipragliflozin group and 0.21 in the pioglitazone group (P=0.90), and both improvements were significant. The FIB-4 index decreased significantly by 0.22 in the ipragliflozin group [71].
- In a single-center, prospective, randomized, open-label, controlled study, luseogliflozin treatment (2.5 mg daily) for 6 months reduced liver fat deposition (L/S ratio on CT 0.907 to 1.033; P=0.0008) in 32 patients with T2DM and NAFLD [72]. A prospective, single-arm trial showed that luseogliflozin treatment (2.5 mg daily) for 24 weeks reduced hepatic fat content as evaluated by MRI in patients with T2DM and NAFLD, but hepatic fibrosis markers did not change [73].
- Non-anti-diabetic agents
- Vitamin E is an antioxidant that prevents liver injury by blocking intrinsic apoptotic pathways and protecting against oxidative stress [974]. Vitamin E improves liver histology in nondiabetic adults with biopsy-proven NASH [74], but is not recommended in patients with diabetes because of lack of evidence [6]. In addition, there are some concerns that long-term use of vitamin E may be associated with increased all-cause mortality, increased incidence of hemorrhagic stroke, and increased risk of prostate cancer [67576].
- Although ezetimibe and omega-3 polyunsaturated fatty acids have been shown to have a beneficial effect on NASH in animal studies, lipid-lowering agents such as statins, ezetimibe, fibrates, niacin, omega-3 polyunsaturated fatty acids, and colesevelam do not improve hepatic steatosis in patients with NAFLD [67778798081]. There is also no evidence that these agents are effective treatment options in patients with T2DM and NAFLD.
- Ursodeoxycholic acid (UDCA), a naturally occurring bile acid, reduces oxidative stress and has antiapoptotic properties [8283]. Several studies have reported that UDCA improved liver enzymes and hepatic steatosis in patients with NAFLD [8485]. This bile acid was expected to be effective in treating NASH, but both conventional (13 to 15 mg/kg daily) and high (23 to 28/kg daily) doses of UDCA failed to result in histologic improvements in patients with NASH [8687].
- Pentoxifylline inhibited a number of pro-inflammatory cytokines including tumor necrosis factor-α, and had a beneficial effect on NASH in an animal study [88]. Pentoxifylline (400 mg three times a day) improved the histological features of NASH in 55 adults with biopsy-confirmed NASH, but only 9.1% of participants had T2DM [89]. Another study reported no histological improvement in 30 patients, so larger studies are needed to establish its potential utility in patients with NASH and T2DM [90].
- Obeticholic acid is a natural agonist of the farnesoid X receptor and has been shown to decrease insulin resistance and hepatic steatosis in an animal model [91]. In a double-blind, placebo-controlled, proof-of-concept study, obeticholic acid treatment for 6 weeks improved insulin resistance and reduced markers of liver inflammation and fibrosis in patients with T2DM and NAFLD [92]. The Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment (FLINT) trial, which was a multicenter, randomized, placebo-controlled trial, showed that obeticholic acid (25 mg daily) treatment for 72 weeks improved liver histology in patients with NASH. Among the FLINT trial participants, 52.7% had T2DM. Main concerns associated with obeticholic acid treatment are increased low density lipoprotein cholesterol and pruritus [3393]. Further studies are needed to clarify the long-term benefits and safety of obeticholic acid in patients with T2DM and NAFLD.
- Elafibranor is a dual agonist of PPAR-α and PPAR-δ and improves insulin resistance in liver and peripheral tissue [94]. In a phase IIb international, randomized, double-blind placebo-controlled trial, elafibranor treatment (120 mg daily) for 52 weeks tended to induce resolution of NASH without fibrosis worsening despite some methodological limitations. The predefined end point was not met in the intention to treat population and 39.1% of study participants had T2DM [95]. A phase III trial (The Efficacy and Safety of Elafibranor Versus Placebo in Patients with Nonalcoholic Steatohepatitis [RESOLVE-IT]) is currently underway to evaluate the ability of elafibranor to achieve resolution of NASH without worsening fibrosis.
- Cenicriviroc, a dual antagonist of C-C chemokine receptors type 2 and 5 (CCR2/CCR5), was shown to have potent anti-inflammatory and antifibrotic activity in an animal model [96]. In the Efficacy and Safety Study of Cenicriviroc for the treatment of Nonalcoholic Steatohepatitis in Adult Subjects with Liver Fibrosis (CENTAUR) study, which was a randomized, double-blind, multinational phase IIb study, cenicriviroc treatment (150 mg daily) for 1 year resulted in improvement in fibrosis and no worsening of steatohepatitis compared with placebo [97]. In that study, half of the participants had T2DM and two-thirds had metabolic syndrome.
- Carnitine is a modulator of mitochondrial free fatty acid transport and oxidation and has anti-oxidative activity in hepatocytes [98]. In patients with NASH, L-carnitine treatment (1 g daily) for 24 weeks improved liver enzymes and histological manifestations [99]. In the Carnitine-OROtate in NAFLD patients with diabetes (CORONA) trial, which was a randomized, controlled, double-blind trial, treatment with carnitine-orotate complex (824 mg three times daily) for 12 weeks improved serum ALT and appeared to improve hepatic steatosis as assessed by CT in 78 patients with T2DM and NAFLD [100].
- Saroglitazar is a dual PPAR-α/γ agonist that has been approved in India for the treatment of dyslipidemia in patients with diabetes. In a 16-week prospective, multicenter, randomized, double-blind, placebo-controlled, three-arm phase III study in subjects with hypertriglyceridemia (>200 and <500 mg/dL) and T2DM, saroglitazar reduced alkaline phosphatase as well as triglyceride levels [101]. No study has investigated the effect on saroglitazar in patients with NASH, although saroglitazar improved NASH in an animal model [102].
- Because weight reduction is a key component of the treatment of NAFLD, anti-obesity drugs are potential pharmacological candidates. However, few studies have investigated the efficacy of anti-obesity drugs on the treatment of NAFLD, with the exception of orlistat [103].
- Orlistat inhibits fat absorption and has been approved as an anti-obesity drug. In a randomized, double-blind, placebo-controlled study of 52 patients with NAFLD as diagnosed by US and confirmed by liver biopsy, orlistat (120 mg three times daily for 6 months) improved serum ALT level (48.0% vs. 26.4%) and steatosis on US in NAFLD patients beyond its effect on weight reduction. Among the study participants, 21% had T2DM [104]. Another study evaluated the effect of orlistat on NAFLD in patients who received a 1,400 kcal/day diet plus vitamin E (800 IU) daily. Orlistat (120 mg three times a day) did not enhance weight loss or improve liver enzymes and histopathology in 50 overweight subjects (10% of subjects had been T2DM). However, subjects who lost ≥5% of body weight over 9 months showed improved insulin resistance and steatosis, and those subjects who lost ≥9% also achieved improvement in liver histology and NAS [105]. In a meta-analysis, orlistat improved liver enzymes but not liver fibrosis score (P=0.71) in patients with NAFLD [106].
- Lorcaserin is a serotonin 2c receptor agonist that promotes weight loss while improving T2DM. One study has evaluated the effect of lorcaserin on cardiometabolic parameters. In a 6-month-long, randomized (1:1), placebo-controlled, double-blinded clinical trial, lorcaserin reduced FLI (P<0.001) in 48 obese subjects without T2DM [107]. Further studies are needed to determine if lorcaserin is effective for the treatment of NAFLD in patients with T2DM.
- The therapeutic landscape for NAFLD/NASH is evolving rapidly, with many drugs currently being assessed in clinical trials. Among them, some drugs have being evaluated in patients with NAFLD and T2DM (Table 2).
PHARMACOLOGICAL TREATMENTS
Metformin
Thiazolidinediones
Pioglitazone
Lobeglitazone
Glucagon-like peptide-1 receptor agonists
Liraglutide
Exenatide
Lixisenatide
Dipeptidyl peptidase-4 inhibitors
Sitagliptin
Vildagliptin
Sodium-glucose co-transporter 2 inhibitors
Dapagliflozin
Canagliflozin
Empagliflozin
Ipragliflozin
Luseogliflozin
Vitamin E
Lipid-lowering agents
Ursodeoxycholic acid
Pentoxifylline
Obeticholic acid
Elafibranor
Cenicriviroc
Carnitine
Saroglitazar
Anti-obesity drugs
Orlistat
Lorcaserin
Drugs under investigation
- In severely obese patients, bariatric surgery is accepted as the primary treatment modality for sustained weight loss and effective improvement or resolution of obesity-related metabolic complications. In a large cohort study, bariatric surgery improved survival and reduced the incidence of death from cardiovascular disease and malignancy, the two most common causes of death in patients with NAFLD.
- Mathurin et al. [108] prospectively correlated clinical and metabolic data with liver histology before surgery and at 1 and 5 years after surgery in 381 adult patients with severe obesity. Most histologic benefits were evident at 1 year, with no differences in liver histology between 1 and 5 years following bariatric surgery [108]. Lassailly et al. [109] prospectively examined 109 patients with NASH at the time of bariatric surgery and performed follow-up biopsies 1 year later. Eighty-five percent of patients had NASH resolution (95% CI, 75.8 to 92.2). Importantly, in contrast to past data, fibrosis improved at 1 year after surgery in 33% of patients. Among the study participants, 63.3% had T2DM [109]. A meta-analysis of available data in 2015 also showed that the majority of patients who underwent bariatric surgery had improvement or complete resolution of the histopathological features of steatosis, inflammation, and ballooning. Fibrosis also improved, as evidenced by a weighted mean decrease of 11.9% in the incidence of fibrosis [110]. A recent meta-analysis of 32 cohort studies including 3,093 biopsies reported consistent findings. Bariatric surgery resulted in biopsy-confirmed resolution of steatosis in 66% of patients (95% CI, 56% to 75%), inflammation in 50% (95% CI, 35% to 64%), ballooning degeneration in 76% (95% CI, 64% to 86%), and fibrosis in 40% (95% CI, 29% to 51%). Mean NAS also decreased significantly after bariatric surgery (mean difference, 2.39; 95% CI, 1.58 to 3.20; P<0.001) [111].
- With regard to procedure selection, there is no reliable data favoring sleeve gastrectomy over Roux-en-Y gastric bypass or vice versa. However, considering the safety of surgery itself, sleeve gastrectomy is recommended in patients with NASH cirrhosis [112113].
- Although no studies have reported the use of bariatric surgery to treat NAFLD/NASH in Korea, NAFLD/NASH is included in the clinical practice guidelines for performing bariatric surgery, which include BMI >35 kg/m2 or BMI >30 kg/m2 plus obesity-related comorbidities.
BARIATRIC SURGERY
- Despite many advances in the treatment of NAFLD, the only approved option for NAFLD in patients with T2DM is weight loss. However, it is not easy to maintain weight loss and T2DM itself confers high cardiovascular risk. Therefore, lifestyle intervention supported by pharmacological treatment is necessary in patients with T2DM and NAFLD. Although many drugs have been investigated, pioglitazone is currently the best first-line therapy in patients with T2DM and NAFLD. As new agents become available, combination strategies will likely be used to treat NAFLD, similar to how T2DM is currently treated. Much progress has been made in the understanding, diagnosis, and treatment of NAFLD over the past few decades. In the future, we expect early intervention and individualized treatment for NAFLD to become standard for all patients with T2DM.
CONCLUSIONS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
NOTES
- 1. Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM. Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012;56:943-951. ArticlePubMedPMC
- 2. Lomonaco R, Bril F, Portillo-Sanchez P, Ortiz-Lopez C, Orsak B, Biernacki D, Lo M, Suman A, Weber MH, Cusi K. Metabolic impact of nonalcoholic steatohepatitis in obese patients with type 2 diabetes. Diabetes Care 2016;39:632-638. ArticlePubMedPMCPDF
- 3. Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care 2017;40:419-430. ArticlePubMedPDF
- 4. Han E, Lee YH. Non-alcoholic fatty liver disease: the emerging burden in cardiometabolic and renal diseases. Diabetes Metab J 2017;41:430-437. ArticlePubMedPMCPDF
- 5. European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD). European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016;59:1121-1140. ArticlePubMedPDF
- 6. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328-357. ArticlePubMedPDF
- 7. Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, Wagenknecht LE, Pi-Sunyer FX, Kahn SE, Clark JM. Fatty Liver Subgroup of the Look AHEAD Research Group. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010;33:2156-2163. ArticlePubMedPMCPDF
- 8. Ikramuddin S, Korner J, Lee WJ, Connett JE, Inabnet WB, Billington CJ, Thomas AJ, Leslie DB, Chong K, Jeffery RW, Ahmed L, Vella A, Chuang LM, Bessler M, Sarr MG, Swain JM, Laqua P, Jensen MD, Bantle JP. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the diabetes surgery study randomized clinical trial. JAMA 2013;309:2240-2249. ArticlePubMedPMC
- 9. Younossi ZM, Loomba R, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, Corey KE, Friedman SL, Abdelmalek MF, Harrison SA, Sanyal AJ, Lavine JE, Mathurin P, Charlton MR, Chalasani NP, Anstee QM, Kowdley KV, George J, Goodman ZD, Lindor K. Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2018;68:361-371. ArticlePubMedPMCPDF
- 10. Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 2005;54:603-608. ArticlePubMedPMCPDF
- 11. Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51:121-129. ArticlePubMedPMC
- 12. Wong VW, Wong GL, Chan RS, Shu SS, Cheung BH, Li LS, Chim AM, Chan CK, Leung JK, Chu WC, Woo J, Chan HL. Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease. J Hepatol 2018;69:1349-1356. ArticlePubMed
- 13. Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, Emick D, Lok AS, Conjeevaram HS. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol 2005;100:1072-1081. ArticlePubMed
- 14. Thoma C, Day CP, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol 2012;56:255-266. ArticlePubMed
- 15. Kleiner DE, Makhlouf HR. Histology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in adults and children. Clin Liver Dis 2016;20:293-312. ArticlePubMed
- 16. Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2008;6:1396-1402. ArticlePubMed
- 17. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology 2015;14:367-378.Article
- 18. Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomized trials. Diabetologia 2012;55:885-904. ArticlePubMedPDF
- 19. Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263-2273. ArticlePubMed
- 20. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. American Gastroenterological Association. American Association for the Study of Liver Diseases. American College of Gastroenterology. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012;142:1592-1609. ArticlePubMed
- 21. Tamura Y, Tanaka Y, Sato F, Choi JB, Watada H, Niwa M, Kinoshita J, Ooka A, Kumashiro N, Igarashi Y, Kyogoku S, Maehara T, Kawasumi M, Hirose T, Kawamori R. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 2005;90:3191-3196. ArticlePubMed
- 22. Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009;136:1552-1560. ArticlePubMedPMC
- 23. Haufe S, Engeli S, Kast P, Bohnke J, Utz W, Haas V, Hermsdorf M, Mahler A, Wiesner S, Birkenfeld AL, Sell H, Otto C, Mehling H, Luft FC, Eckel J, Schulz-Menger J, Boschmann M, Jordan J. Randomized comparison of reduced fat and reduced carbohydrate hypocaloric diets on intrahepatic fat in overweight and obese human subjects. Hepatology 2011;53:1504-1514. ArticlePubMed
- 24. Keating SE, Hackett DA, George J, Johnson NA. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;57:157-166. ArticlePubMed
- 25. Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 2001;286:1218-1227. ArticlePubMed
- 26. Bacchi E, Negri C, Targher G, Faccioli N, Lanza M, Zoppini G, Zanolin E, Schena F, Bonora E, Moghetti P. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013;58:1287-1295. ArticlePubMed
- 27. Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 2009;50:1105-1112. ArticlePubMed
- 28. Sung KC, Ryu S, Lee JY, Kim JY, Wild SH, Byrne CD. Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J Hepatol 2016;65:791-797. ArticlePubMed
- 29. Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring) 2009;17:2162-2168. ArticlePubMedPMCPDF
- 30. Yoshimura E, Kumahara H, Tobina T, Matsuda T, Ayabe M, Kiyonaga A, Anzai K, Higaki Y, Tanaka H. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. J Obes 2014;2014:197216. ArticlePubMedPMCPDF
- 31. Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev 2009;10:313-323. ArticlePubMed
- 32. Rodriguez B, Torres DM, Harrison SA. Physical activity: an essential component of lifestyle modification in NAFLD. Nat Rev Gastroenterol Hepatol 2012;9:726-731. ArticlePubMedPDF
- 33. European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD). European Association for the Study of Obesity (EASO). EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-1402. PubMed
- 34. Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER trial. Circulation 2009;119:2026-2031. ArticlePubMedPMC
- 35. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393-403. ArticlePubMedPMC
- 36. Stefan N, Fritsche A, Schick F, Haring HU. Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol 2016;4:789-798. ArticlePubMed
- 37. Stefan N, Haring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol 2019;7:313-324. ArticlePubMed
- 38. Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, David E, Rizzetto M, Marchesini G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol 2005;100:1082-1090. ArticlePubMed
- 39. Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ, Hoofnagle JH. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2009;29:172-182. ArticlePubMedPMC
- 40. Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, Abrams SH, Scheimann AO, Sanyal AJ, Chalasani N, Tonascia J, Unalp A, Clark JM, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659-1668. ArticlePubMedPMC
- 41. Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep 2013;1:57-64. ArticlePubMed
- 42. Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology 2010;52:79-104. ArticlePubMed
- 43. Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J, Berria R, Ma JZ, Dwivedi S, Havranek R, Fincke C, DeFronzo R, Bannayan GA, Schenker S, Cusi K. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297-2307. ArticlePubMed
- 44. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, Tio F, Hardies J, Darland C, Musi N, Webb A, Portillo-Sanchez P. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med 2016;165:305-315. ArticlePubMed
- 45. Bril F, Kalavalapalli S, Clark VC, Lomonaco R, Soldevila-Pico C, Liu IC, Orsak B, Tio F, Cusi K. Response to pioglitazone in patients with nonalcoholic steatohepatitis with vs without type 2 diabetes. Clin Gastroenterol Hepatol 2018;16:558-566. ArticlePubMed
- 46. Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta-analysis. JAMA Intern Med 2017;177:633-640. ArticlePubMedPMC
- 47. Choung S, Joung KH, You BR, Park SK, Kim HJ, Ku BJ. Treatment with lobeglitazone attenuates hepatic steatosis in diet-induced obese mice. PPAR Res 2018;2018:4292509. ArticlePubMedPMCPDF
- 48. Lee YH, Kim JH, Kim SR, Jin HY, Rhee EJ, Cho YM, Lee BW. Lobeglitazone, a novel thiazolidinedione, improves non-alcoholic fatty liver disease in type 2 diabetes: its efficacy and predictive factors related to responsiveness. J Korean Med Sci 2017;32:60-69. ArticlePubMedPDF
- 49. Armstrong MJ, Houlihan DD, Rowe IA, Clausen WH, Elbrond B, Gough SC, Tomlinson JW, Newsome PN. Safety and efficacy of liraglutide in patients with type 2 diabetes and elevated liver enzymes: individual patient data meta-analysis of the LEAD program. Aliment Pharmacol Ther 2013;37:234-242. ArticlePubMed
- 50. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hubscher SG, Newsome PN. LEAN trial team. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387:679-690. ArticlePubMed
- 51. Shao N, Kuang HY, Hao M, Gao XY, Lin WJ, Zou W. Benefits of exenatide on obesity and non-alcoholic fatty liver disease with elevated liver enzymes in patients with type 2 diabetes. Diabetes Metab Res Rev 2014;30:521-529. ArticlePubMed
- 52. Gluud LL, Knop FK, Vilsboll T. Effects of lixisenatide on elevated liver transaminases: systematic review with individual patient data meta-analysis of randomised controlled trials on patients with type 2 diabetes. BMJ Open 2014;4:e005325.ArticlePubMedPMC
- 53. Iwasaki T, Yoneda M, Inamori M, Shirakawa J, Higurashi T, Maeda S, Terauchi Y, Nakajima A. Sitagliptin as a novel treatment agent for non-alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology 2011;58:2103-2105. ArticlePubMed
- 54. Yilmaz Y, Yonal O, Deyneli O, Celikel CA, Kalayci C, Duman DG. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg 2012;75:240-244. PubMed
- 55. Kato H, Nagai Y, Ohta A, Tenjin A, Nakamura Y, Tsukiyama H, Sasaki Y, Fukuda H, Ohshige T, Terashima Y, Sada Y, Kondo A, Sasaoka T, Tanaka Y. Effect of sitagliptin on intrahepatic lipid content and body fat in patients with type 2 diabetes. Diabetes Res Clin Pract 2015;109:199-205. ArticlePubMed
- 56. Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, Salotti J, Bhatt A, Hooker J, Haufe W, Hooker C, Brenner DA, Sirlin CB, Loomba R. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol 2016;65:369-376. ArticlePubMedPMC
- 57. Fukuhara T, Hyogo H, Ochi H, Fujino H, Kan H, Naeshiro N, Honda Y, Miyaki D, Kawaoka T, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Aikata H, Chayama K. Efficacy and safety of sitagliptin for the treatment of nonalcoholic fatty liver disease with type 2 diabetes mellitus. Hepatogastroenterology 2014;61:323-328. PubMed
- 58. Macauley M, Hollingsworth KG, Smith FE, Thelwall PE, Al-Mrabeh A, Schweizer A, Foley JE, Taylor R. Effect of vildagliptin on hepatic steatosis. J Clin Endocrinol Metab 2015;100:1578-1585. ArticlePubMedPMC
- 59. Choi DH, Jung CH, Mok JO, Kim CH, Kang SK, Kim BY. Effect of dapagliflozin on alanine aminotransferase improvement in type 2 diabetes mellitus with non-alcoholic fatty liver disease. Endocrinol Metab (Seoul) 2018;33:387-394. ArticlePubMedPMCPDF
- 60. Shimizu M, Suzuki K, Kato K, Jojima T, Iijima T, Murohisa T, Iijima M, Takekawa H, Usui I, Hiraishi H, Aso Y. Evaluation of the effects of dapagliflozin, a sodium-glucose co-transporter-2 inhibitor, on hepatic steatosis and fibrosis using transient elastography in patients with type 2 diabetes and non-alcoholic fatty liver disease. Diabetes Obes Metab 2019;21:285-292. ArticlePubMedPDF
- 61. Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnstrom M, Moris L, Miliotis T, Forsberg GB, Riserus U, Lind L, Oscarsson J. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia 2018;61:1923-1934. ArticlePubMedPMCPDF
- 62. Kurinami N, Sugiyama S, Yoshida A, Hieshima K, Miyamoto F, Kajiwara K, Jinnouch K, Jinnouchi T, Jinnouchi H. Dapagliflozin significantly reduced liver fat accumulation associated with a decrease in abdominal subcutaneous fat in patients with inadequately controlled type 2 diabetes mellitus. Diabetes Res Clin Pract 2018;142:254-263. ArticlePubMed
- 63. Leiter LA, Forst T, Polidori D, Balis DA, Xie J, Sha S. Effect of canagliflozin on liver function tests in patients with type 2 diabetes. Diabetes Metab 2016;42:25-32. ArticlePubMed
- 64. Li B, Wang Y, Ye Z, Yang H, Cui X, Wang Z, Liu L. Effects of canagliflozin on fatty liver indexes in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. J Pharm Pharm Sci 2018;21:222-235. ArticlePubMedPDF
- 65. Inoue M, Hayashi A, Taguchi T, Arai R, Sasaki S, Takano K, Inoue Y, Shichiri M. Effects of canagliflozin on body composition and hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease. J Diabetes Investig 2018 11 21 [Epub].ArticlePDF
- 66. Itani T, Ishihara T. Efficacy of canagliflozin against nonalcoholic fatty liver disease: a prospective cohort study. Obes Sci Pract 2018;4:477-482. ArticlePubMedPMCPDF
- 67. Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME trial. Diabetologia 2018;61:2155-2163. ArticlePubMedPMCPDF
- 68. Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care 2018;41:1801-1808. ArticlePubMedPDF
- 69. Miyake T, Yoshida S, Furukawa S, Sakai T, Tada F, Senba H, Yamamoto S, Koizumi Y, Yoshida O, Hirooka M, Kumagi T, Niiya T, Miyaoka H, Masanori A, Matsuura B, Hiasa Y. Ipragliflozin ameliorates liver damage in non-alcoholic fatty liver disease. Open Med (Wars) 2018;13:402-409. ArticlePubMedPMC
- 70. Tabuchi H, Maegawa H, Tobe K, Nakamura I, Uno S. Effect of ipragliflozin on liver function in Japanese type 2 diabetes mellitus patients: a subgroup analysis of the STELLA-LONG TERM study (3-month interim results). Endocr J 2019;66:31-41. ArticlePubMed
- 71. Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, Akiyama Y, Morimoto Y, Noda M, Shimada A. Comparison of ipragliflozin and pioglitazone effects on nonalcoholic fatty liver disease in patients with type 2 diabetes: a randomized, 24-week, open-label, active-controlled trial. Diabetes Care 2017;40:1364-1372. ArticlePubMedPDF
- 72. Shibuya T, Fushimi N, Kawai M, Yoshida Y, Hachiya H, Ito S, Kawai H, Ohashi N, Mori A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective randomized controlled pilot study. Diabetes Obes Metab 2018;20:438-442. ArticlePubMedPDF
- 73. Sumida Y, Murotani K, Saito M, Tamasawa A, Osonoi Y, Yoneda M, Osonoi T. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: a prospective, single-arm trial (LEAD trial). Hepatol Res 2019;49:64-71. ArticlePubMedPDF
- 74. Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675-1685. ArticlePubMedPMC
- 75. Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med 2005;142:37-46. ArticlePubMed
- 76. Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306:1549-1556. ArticlePubMedPMC
- 77. Cusi K. Treatment of patients with type 2 diabetes and non-alcoholic fatty liver disease: current approaches and future directions. Diabetologia 2016;59:1112-1120. ArticlePubMedPMCPDF
- 78. Nelson A, Torres DM, Morgan AE, Fincke C, Harrison SA. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: a randomized placebo-controlled trial. J Clin Gastroenterol 2009;43:990-994. PubMed
- 79. Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, Soaft L, Hooker J, Kono Y, Bhatt A, Hernandez L, Nguyen P, Noureddin M, Haufe W, Hooker C, Yin M, Ehman R, Lin GY, Valasek MA, Brenner DA, Richards L. San Diego Integrated NAFLD Research Consortium (SINC). Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology 2015;61:1239-1250. ArticlePubMedPMCPDF
- 80. Fabbrini E, Mohammed BS, Korenblat KM, Magkos F, McCrea J, Patterson BW, Klein S. Effect of fenofibrate and niacin on intrahepatic triglyceride content, very low-density lipoprotein kinetics, and insulin action in obese subjects with nonalcoholic fatty liver disease. J Clin Endocrinol Metab 2010;95:2727-2735. ArticlePubMedPMC
- 81. Dasarathy S, Dasarathy J, Khiyami A, Yerian L, Hawkins C, Sargent R, McCullough AJ. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol 2015;49:137-144. ArticlePubMedPMC
- 82. Neuman M, Angulo P, Malkiewicz I, Jorgensen R, Shear N, Dickson ER, Haber J, Katz G, Lindor K. Tumor necrosis factor-alpha and transforming growth factor-beta reflect severity of liver damage in primary biliary cirrhosis. J Gastroenterol Hepatol 2002;17:196-202. PubMed
- 83. Bellentani S. Immunomodulating and anti-apoptotic action of ursodeoxycholic acid: where are we and where should we go? Eur J Gastroenterol Hepatol 2005;17:137-140. ArticlePubMed
- 84. Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, Rakela J, McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology 1996;23:1464-1467. ArticlePubMed
- 85. Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, Zala JF, Helbling B, Steuerwald M, Zimmermann A. Swiss Association for the Study of the Liver. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2006;4:1537-1543. ArticlePubMed
- 86. Lindor KD, Kowdley KV, Heathcote EJ, Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L, Colin P. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology 2004;39:770-778. ArticlePubMed
- 87. Leuschner UF, Lindenthal B, Herrmann G, Arnold JC, Rossle M, Cordes HJ, Zeuzem S, Hein J, Berg T. NASH Study Group. High-dose ursodeoxycholic acid therapy for nonalcoholic steatohepatitis: a double-blind, randomized, placebo-controlled trial. Hepatology 2010;52:472-479. ArticlePubMed
- 88. Koppe SW, Sahai A, Malladi P, Whitington PF, Green RM. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol 2004;41:592-598. ArticlePubMed
- 89. Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, McCullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology 2011;54:1610-1619. ArticlePubMedPMC
- 90. Van Wagner LB, Koppe SW, Brunt EM, Gottstein J, Gardikiotes K, Green RM, Rinella ME. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: a randomized controlled trial. Ann Hepatol 2011;10:277-286. ArticlePubMed
- 91. Townsend SA, Newsome PN. Review article: new treatments in non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2017;46:494-507. ArticlePubMedPDF
- 92. Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E, Dillon P, Pruzanski M, Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013;145:574-582. ArticlePubMed
- 93. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B, Kowdley KV, McCullough A, Terrault N, Clark JM, Tonascia J, Brunt EM, Kleiner DE, Doo E. NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956-965. ArticlePubMed
- 94. Cariou B, Hanf R, Lambert-Porcheron S, Zair Y, Sauvinet V, Noel B, Flet L, Vidal H, Staels B, Laville M. Dual peroxisome proliferator-activated receptor α/δ agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care 2013;36:2923-2930. ArticlePubMedPMCPDF
- 95. Ratziu V, Harrison SA, Francque S, Bedossa P, Lehert P, Serfaty L, Romero-Gomez M, Boursier J, Abdelmalek M, Caldwell S, Drenth J, Anstee QM, Hum D, Hanf R, Roudot A, Megnien S, Staels B, Sanyal A. GOLDEN-505 Investigator Study Group. Elafibranor, an agonist of the peroxisome proliferator-activated receptor-α and -δ, induces resolution of nonalcoholic steatohepatitis without fibrosis worsening. Gastroenterology 2016;150:1147-1159. ArticlePubMed
- 96. Lefebvre E, Moyle G, Reshef R, Richman LP, Thompson M, Hong F, Chou HL, Hashiguchi T, Plato C, Poulin D, Richards T, Yoneyama H, Jenkins H, Wolfgang G, Friedman SL. Antifibrotic effects of the dual CCR2/CCR5 antagonist cenicriviroc in animal models of liver and kidney fibrosis. PLoS One 2016;11:e0158156. ArticlePubMedPMC
- 97. Friedman SL, Ratziu V, Harrison SA, Abdelmalek MF, Aithal GP, Caballeria J, Francque S, Farrell G, Kowdley KV, Craxi A, Simon K, Fischer L, Melchor-Khan L, Vest J, Wiens BL, Vig P, Seyedkazemi S, Goodman Z, Wong VW, Loomba R, Tacke F, Sanyal A, Lefebvre E. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67:1754-1767. ArticlePubMedPMCPDF
- 98. Li JL, Wang QY, Luan HY, Kang ZC, Wang CB. Effects of L-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha. J Biomed Sci 2012;19:32. ArticlePubMedPMC
- 99. Malaguarnera M, Gargante MP, Russo C, Antic T, Vacante M, Malaguarnera M, Avitabile T, Li Volti G, Galvano F. L-carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis. A randomized and controlled clinical trial. Am J Gastroenterol 2010;105:1338-1345. ArticlePubMedPDF
- 100. Bae JC, Lee WY, Yoon KH, Park JY, Son HS, Han KA, Lee KW, Woo JT, Ju YC, Lee WJ, Cho YY, Lee MK. Improvement of nonalcoholic fatty liver disease with carnitine-orotate complex in type 2 diabetes (CORONA): a randomized controlled trial. Diabetes Care 2015;38:1245-1252. ArticlePubMedPDF
- 101. Jani RH, Pai V, Jha P, Jariwala G, Mukhopadhyay S, Bhansali A, Joshi S. A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 mg compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI). Diabetes Technol Ther 2014;16:63-71. ArticlePubMedPMC
- 102. Jain MR, Giri SR, Bhoi B, Trivedi C, Rath A, Rathod R, Ranvir R, Kadam S, Patel H, Swain P, Roy SS, Das N, Karmakar E, Wahli W, Patel PR. Dual PPARα/γ agonist saroglitazar improves liver histopathology and biochemistry in experimental NASH models. Liver Int 2018;38:1084-1094. PubMed
- 103. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism 2019;92:82-97. ArticlePubMed
- 104. Zelber-Sagi S, Kessler A, Brazowsky E, Webb M, Lurie Y, Santo M, Leshno M, Blendis L, Halpern Z, Oren R. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2006;4:639-644. ArticlePubMed
- 105. Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: a randomized, prospective trial. Hepatology 2009;49:80-86. ArticlePubMed
- 106. Wang H, Wang L, Cheng Y, Xia Z, Liao Y, Cao J. Efficacy of orlistat in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep 2018;9:90-96. ArticlePubMedPMC
- 107. Tuccinardi D, Farr OM, Upadhyay J, Oussaada SM, Mathew H, Paschou SA, Perakakis N, Koniaris A, Kelesidis T, Mantzoros CS. Lorcaserin treatment decreases body weight and reduces cardiometabolic risk factors in obese adults: a six-month, randomized, placebo-controlled, double-blind clinical trial. Diabetes Obes Metab 2019 2 06 [Epub].ArticlePDF
- 108. Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, Pigeyre M, Verkindt H, Dharancy S, Louvet A, Romon M, Pattou F. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology 2009;137:532-540. ArticlePubMed
- 109. Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, Raverdy V, Leteurtre E, Dharancy S, Louvet A, Romon M, Duhamel A, Pattou F, Mathurin P. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology 2015;149:379-388. ArticlePubMed
- 110. Bower G, Toma T, Harling L, Jiao LR, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. Bariatric surgery and non-alcoholic fatty liver disease: a systematic review of liver biochemistry and histology. Obes Surg 2015;25:2280-2289. ArticlePubMedPDF
- 111. Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, Anvari M, Hong D. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018 10 13 [Epub].Article
- 112. Mosko JD, Nguyen GC. Increased perioperative mortality following bariatric surgery among patients with cirrhosis. Clin Gastroenterol Hepatol 2011;9:897-901. ArticlePubMed
- 113. Jan A, Narwaria M, Mahawar KK. A systematic review of bariatric surgery in patients with liver cirrhosis. Obes Surg 2015;25:1518-1526. ArticlePubMedPDF
REFERENCES
Suggested algorithm for the management of patients with nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM).
Effects of treatment in patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease
NAS, nonalcoholic fatty liver disease activity score; RCT, randomized controlled trial; MA, meta-analysis; SR, systematic review; NA, no data available; X, none; GLP-1 RA, glucagon-like peptide-1 receptor agonist; DPP-4, dipeptidyl peptidase-4; SGLT2, sodium-glucose co-transporter 2. aIt is a weak level of evidence because not all study participants have been diabetes, bMixed results, cEvaluated by non-invasive hepatic fibrosis markers (nonalcoholic fatty liver disease fibrosis score, fibrosis-4 [FIB-4] index).
Pharmacological agents currently under investigation for the treatment of nonalcoholic fatty liver disease in patients with diabetes
Figure & Data
References
Citations

- Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study
Kyung-Soo Kim, Sangmo Hong, Kyungdo Han, Cheol-Young Park
BMJ.2024; : e076388. CrossRef - Research Progress of Traditional Chinese Medicine and Western Medicine on Non-Alcoholic Fatty Liver Disease
强江 郭
Advances in Clinical Medicine.2024; 14(03): 561. CrossRef - Liver and cardiovascular disease outcomes in metabolic syndrome and diabetic populations: Bi-directional opportunities to multiply preventive strategies
Alhussain Yasin, Madison Nguyen, Angad Sidhu, Priyanka Majety, Jared Spitz, Amon Asgharpour, Mohammad S. Siddiqui, Laurence S. Sperling, Arshed A. Quyyumi, Anurag Mehta
Diabetes Research and Clinical Practice.2024; 211: 111650. CrossRef - Effect of aerobic training with silymarin consumption on glycemic indices and liver enzymes in men with type 2 diabetes
Keyvan Ghalandari, Mojtaba Shabani, Ali Khajehlandi, Amin Mohammadi
Archives of Physiology and Biochemistry.2023; 129(1): 76. CrossRef - Metabolic Dysfunction-Associated Fatty Liver Disease and Mortality: A Population-Based Cohort Study
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes & Metabolism Journal.2023; 47(2): 220. CrossRef - Comparative antihypertensive efficacy of combinations of azilsartan medoxomil or olmesartan medoxomil with amlodipine in patients with arterial hypertension, type 2 diabetes mellitus and non-alcoholic fatty liver disease
I. А. Lukonin, V. V. Skibitsky, A. V. Fendrikova, A. V. Skibitsky, I. A. Antipov
South Russian Journal of Therapeutic Practice.2023; 4(1): 68. CrossRef - An Ethyl Acetate Extract of Eryngium carlinae Inflorescences Attenuates Oxidative Stress and Inflammation in the Liver of Streptozotocin-Induced Diabetic Rats
Cristian M. Trejo-Hurtado, Cinthia I. Landa-Moreno, Jenaro Lemus-de la Cruz, Donovan J. Peña-Montes, Rocío Montoya-Pérez, Rafael Salgado-Garciglia, Salvador Manzo-Avalos, Christian Cortés-Rojo, Juan Luis Monribot-Villanueva, José Antonio Guerrero-Analco,
Antioxidants.2023; 12(6): 1235. CrossRef - Pharmacogenetics of glucagon-like peptide-1 agonists in the treatment of type 2 diabetes mellitus
Iu.G. Samoilova, A.E. Stankova, M.V. Matveeva, O.E. Vaizova, D.V. Podchinenova, D.A. Kudlay, T.A. Filippova, I.R. Grishkevich
Profilakticheskaya meditsina.2023; 26(12): 95. CrossRef - Obesity is an important determinant of severity in newly defined metabolic dysfunction-associated fatty liver disease
Ji Hye Huh, Kwang Joon Kim, Seung Up Kim, Bong-Soo Cha, Byung-Wan Lee
Hepatobiliary & Pancreatic Diseases International.2022; 21(3): 241. CrossRef - Triglyceride and glucose index is a simple and easy‐to‐calculate marker associated with nonalcoholic fatty liver disease
Kyung‐Soo Kim, Sangmo Hong, Hong‐Yup Ahn, Cheol‐Young Park
Obesity.2022; 30(6): 1279. CrossRef - Evaluating Triglyceride and Glucose Index as a Simple and Easy-to-Calculate Marker for All-Cause and Cardiovascular Mortality
Kyung-Soo Kim, Sangmo Hong, You-Cheol Hwang, Hong-Yup Ahn, Cheol-Young Park
Journal of General Internal Medicine.2022; 37(16): 4153. CrossRef - Pharmacological Treatment of Nonalcoholic Fatty Liver Disease: Antidiabetic Agents
Kyung-Soo Kim
The Journal of Korean Diabetes.2022; 23(2): 83. CrossRef - Efficacy and mechanism of Jiedu Tongluo Tiaogan Formula in treating type 2 diabetes mellitus combined with non-alcoholic fatty liver disease: Study protocol for a parallel-armed, randomized controlled trial
Jinghan Xu, Chunli Piao, Yue Qu, Tianjiao Liu, Yuting Peng, Qi Li, Xiaohua Zhao, Pei Li, Xuemin Wu, Yawen Fan, Binqin Chen, Jie Yang
Frontiers in Pharmacology.2022;[Epub] CrossRef - Intestinal microbiota in the treatment of metabolically associated fatty liver disease
Ji-Shuai Wang, Jin-Chun Liu
World Journal of Clinical Cases.2022; 10(31): 11240. CrossRef - Efficiency of combined antihypertensive pharmacotherapy in patients with arterial hypertension, combined with type 2 diabetes mellitus and non-alcoholic fatty liver disease
I. A. Lukonin, V. V. Skibitsky, A. V. Fendrikova, I. I. Pavlyuchenko, K. Yu. Lazarev, F. A. Kovalenko
Systemic Hypertension.2022; 19(1): 31. CrossRef - Diosgenin Ameliorated Type II Diabetes-Associated Nonalcoholic Fatty Liver Disease through Inhibiting De Novo Lipogenesis and Improving Fatty Acid Oxidation and Mitochondrial Function in Rats
Yujie Zhong, Zhiman Li, Ruyi Jin, Yanpeng Yao, Silan He, Min Lei, Xin Wang, Chao Shi, Li Gao, Xiaoli Peng
Nutrients.2022; 14(23): 4994. CrossRef - Pluchea indica Leaf Extract Alleviates Dyslipidemia and Hepatic Steatosis by Modifying the Expression of Lipid Metabolism-Related Genes in Rats Fed a High Fat-High Fructose Diet
Patcharin Singdam, Jarinyaporn Naowaboot, Laddawan Senggunprai, Kampeebhorn Boonloh, Patchareewan Pannangpetch
Preventive Nutrition and Food Science.2022; 27(4): 384. CrossRef - NAFLDin type 2 diabetes mellitus: Still many challenging questions
Simona Cernea, Itamar Raz
Diabetes/Metabolism Research and Reviews.2021;[Epub] CrossRef - Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell
Kyung-Soo Kim, Yeon Kyung Choi, Mi Jin Kim, Jung Wook Hwang, Kyunghoon Min, Sang Youn Jung, Soo-Kyung Kim, Yong-Soo Choi, Yong-Wook Cho
Diabetes & Metabolism Journal.2021; 45(2): 260. CrossRef - Comparative Efficacy of Lobeglitazone Versus Pioglitazone on Albuminuria in Patients with Type 2 Diabetes Mellitus
Kyung-Soo Kim, Sangmo Hong, Hong-Yup Ahn, Cheol-Young Park
Diabetes Therapy.2021; 12(1): 171. CrossRef - Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: Diagnosis and Treatment
Sook Jung Lee, Byung-Wan Lee
The Journal of Korean Diabetes.2021; 22(1): 38. CrossRef - Patient Management in Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus
A. E. Bagriy, A. D. Zubov, M. V. Khomenko, E. S. Mikhailichenko, E. A. Pylaeva, N. A. Khaustova, E. V. Bryukhovetskaya
Russian Journal of Gastroenterology, Hepatology, Coloproctology.2021; 31(2): 14. CrossRef - NAFLD and its link with diabetes: Why we should be worried
Louise Cremonesini, Emma Harkin
Independent Nurse.2021; 2021(8): 20. CrossRef - Albuminuria Is Associated with Steatosis Burden in Patients with Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease
Eugene Han, Mi Kyung Kim, Byoung Kuk Jang, Hye Soon Kim
Diabetes & Metabolism Journal.2021; 45(5): 698. CrossRef - Fatty liver index and development of cardiovascular disease in Koreans without pre-existing myocardial infarction and ischemic stroke: a large population-based study
Jun Hyung Kim, Jin Sil Moon, Seok Joon Byun, Jun Hyeok Lee, Dae Ryong Kang, Ki Chul Sung, Jang Young Kim, Ji Hye Huh
Cardiovascular Diabetology.2020;[Epub] CrossRef - Novel antisense inhibition of diacylglycerol O-acyltransferase 2 for treatment of non-alcoholic fatty liver disease: a multicentre, double-blind, randomised, placebo-controlled phase 2 trial
Rohit Loomba, Erin Morgan, Lynnetta Watts, Shuting Xia, Lisa A Hannan, Richard S Geary, Brenda F Baker, Sanjay Bhanot
The Lancet Gastroenterology & Hepatology.2020; 5(9): 829. CrossRef - Hepatic fibrosis is associated with total proteinuria in Korean patients with type 2 diabetes
Eugene Han, Yongin Cho, Kyung-won Kim, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-wan Lee
Medicine.2020; 99(33): e21038. CrossRef - Metabolic liver disease in diabetes – From mechanisms to clinical trials
Bedair Dewidar, Sabine Kahl, Kalliopi Pafili, Michael Roden
Metabolism.2020; 111: 154299. CrossRef - Managing NAFLD in Type 2 Diabetes: The Effect of Lifestyle Interventions, a Narrative Review
Siôn A. Parry, Leanne Hodson
Advances in Therapy.2020; 37(4): 1381. CrossRef -
Diabetes and Metabolism Journal in 2020: Good to Great
In-Kyung Jeong
Diabetes & Metabolism Journal.2020; 44(1): 1. CrossRef - Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Position Statement of the Fatty Liver Research Group of the Korean Diabetes Association
Byung-Wan Lee, Yong-ho Lee, Cheol-Young Park, Eun-Jung Rhee, Won-Young Lee, Nan-Hee Kim, Kyung Mook Choi, Keun-Gyu Park, Yeon-Kyung Choi, Bong-Soo Cha, Dae Ho Lee
Diabetes & Metabolism Journal.2020; 44(3): 382. CrossRef - Molecular mechanisms of hepatic insulin resistance in nonalcoholic fatty liver disease and potential treatment strategies
Chang-hua Zhang, Bu-gao Zhou, Jun-qing Sheng, Yang Chen, Ying-qian Cao, Chen Chen
Pharmacological Research.2020; 159: 104984. CrossRef - Beneficial effect of anti-diabetic drugs for nonalcoholic fatty liver disease
Kyung-Soo Kim, Byung-Wan Lee
Clinical and Molecular Hepatology.2020; 26(4): 430. CrossRef - Effects of sodium–glucose cotransporter 2 inhibitors on non‐alcoholic fatty liver disease in patients with type 2 diabetes: A meta‐analysis of randomized controlled trials
Baodi Xing, Yuhang Zhao, Bingzi Dong, Yue Zhou, Wenshan Lv, Wenjuan Zhao
Journal of Diabetes Investigation.2020; 11(5): 1238. CrossRef - Empaglifozin mitigates NAFLD in high-fat-fed mice by alleviating insulin resistance, lipogenesis and ER stress
Tamiris Ingrid Petito-da-Silva, Vanessa Souza-Mello, Sandra Barbosa-da-Silva
Molecular and Cellular Endocrinology.2019; 498: 110539. CrossRef

 KDA
KDA PubReader
PubReader Cite
Cite