Serum 25-Hydroxyvitamin D Concentration Is Independently Inversely Associated with Insulin Resistance in the Healthy, Non-Obese Korean Population
Article information
Abstract
Background
We evaluated the associations between 25-hydroxyvitamin D (25(OH)D) concentrations in serum and insulin resistance in the healthy Korean population.
Methods
We conducted this cross-sectional analysis in 1,807 healthy Korean people (628 men and 1,179 women) aged 30 to 64 years in the Cardiovascular and Metabolic Disease Etiologic Research Center study. All participants were assessed for 25(OH)D, fasting glucose, and insulin levels, and completed a health examination and lifestyle questionnaire according to standard procedures. Insulin resistance was defined as the homeostasis model assessment insulin resistance higher than the 75 percentile.
Results
Compared to those in the highest tertile (≥14.3 ng/mL), the odds ratio (OR) for insulin resistance was 1.37 (95% confidence interval [CI], 1.01 to 1.86) for the 1st tertile (<9.7 ng/mL) and 1.19 (95% CI, 0.08 to 1.62) for the 2nd tertile (9.7 to 14.3 ng/mL) after adjusting for age, gender, waist circumference, alcohol consumption, smoking status, physical exercise, season, and cohort. After stratification of the subjects by adiposity, these associations remained only in non-obese subjects (lowest tertile vs. highest tertile, multivariable OR, 1.64; 95% CI, 1.05 to 2.56).
Conclusion
Serum 25(OH)D has an independent inverse association with insulin resistance in the healthy, non-obese Korean population, even among people with vitamin D insufficiency.
INTRODUCTION
Vitamin D is not only a regulator of calcium-phosphorus metabolism, but it also influences the onset of diseases, including diabetes, cardiovascular disease, and cancer [12]. The association between vitamin D and glucose metabolism is particularly important. Some studies have reported that vitamin D insufficiency is more common in people with diabetes risk than people without such risk [345]. Other studies have linked lower 25-hydroxyvitamin D (25(OH)D) concentrations, a generally accepted indicator of vitamin D status, with insulin resistance and β-cell dysfunction in Western populations [467].
However, there are differences in vitamin D metabolism and nutritional status between ethnic groups [38910]. Asian countries, including Korea, have a relatively high prevalence of vitamin D insufficiency compared to other countries [111213]. However, few studies have examined the relationship between 25(OH)D concentration and insulin resistance in a healthy Asian population. In addition, previous studies suggested that this association may differ by obesity status [1415].
In this cross-sectional study, we investigated the associations between 25(OH)D levels in serum and insulin resistance (represented by the homeostasis model assessment insulin resistance [HOMA-IR]) in the healthy Korean population.
METHODS
Study design and participants
This study was performed as part of the Cardiovascular and Metabolic Disease Etiologic Research Center (CMERC) study, which was undertaken beginning in 2013 to evaluate traditional risk factors, explore new biomarkers, clarify etiology, and suggest prevention strategies for cardiovascular and metabolic diseases.
The CMERC population is a multicenter prospective cohort, which consists of two community-based cohorts and one hospital-based cohort of people at high risk for cardiovascular diseases. The community-based cohorts included residents of urban and rural areas. Community-based cohort 1 was based in the Seoul, Goyang-si, Gimpo-si, and Ganghwa-gun; community-based cohort 2 was based in the Suwon-si, Yongin-si, and Hwaseong-si. We excluded persons who had been diagnosed with a malignancy within 2 years, were currently being treated, or had a history of myocardial infarction, stroke, or heart failure.
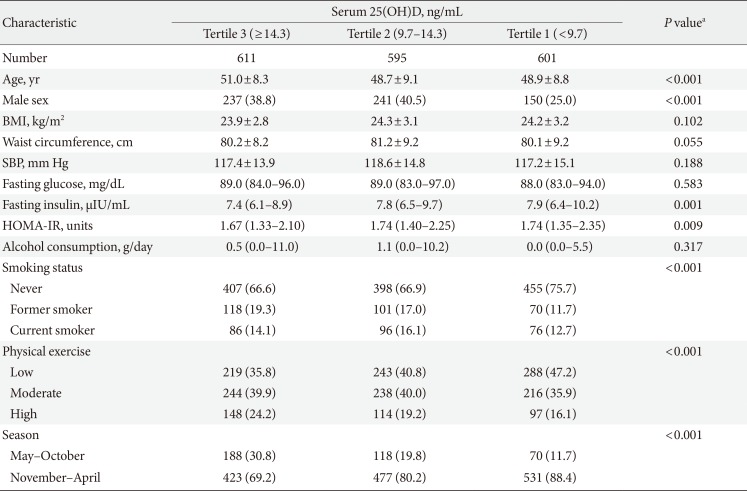
Anthropometric measurements and serum biochemistry profiles were made for 1,807 subjects, all of whom were included in this study. The level of serum 25(OH)D was 12.5 ng/mL (interquartile range [IQR], 9.8 to 16.1) in men and 11.1 ng/mL (IQR, 8.3 to 16.0) in women. No statistical difference was present (P=0.481), so we analyzed them together. The participants were divided into three categories based on 25(OH)D concentration: tertile 1 (<9.7 ng/mL), tertile 2 (9.7 to 14.3 ng/mL), and tertile 3 (≥14.3 ng/mL). The prevalence of high insulin resistance was compared among these three groups. In addition, participants were divided into normal and obese according to body mass index (BMI) and waist circumference. General obesity was defined as a BMI of 25 kg/m2 or greater [16]. Abdominal obesity was defined as a waist circumference of 90 cm or greater for male and 85 cm or greater for female [17]. The odds ratios (ORs) of insulin resistance according to 25(OH)D concentration were also compared among the groups.
This study was approved by the Institutional Review Boards of Severance Hospital at Yonsei University College of Medicine (IRB No. 4-2013-0661) and Ajou University Hospital (AJIRB-BMR-SUR-13-272), and informed consent was obtained from each participant.
Measurements
Information on subjects, including demographics, physical activities, and lifestyles were collected by face-to-face interviews. Trained researchers gathered all information using standardized questionnaires and protocols. All measurements were taken at the health-screening centers in Seoul and Suwon, South Korea.
Body weights and heights were measured using standardized techniques and equipment with participants who were wearing light indoor clothing without shoes (cohort 1, DS-102 Jenix, Seoul, Korea for height; BB-150 CAS, Seongnam, Korea for weight; cohort 2, BSM 330, Biospace, Seoul, Korea). BMI was calculated as weight divided by height squared (kg/m2). Waist circumference was measured over the midpoint between the lower border of the ribs and the iliac crest in a standing position using a plastic tapeline. Blood pressure was measured three times in the right arm using an electronic manometer (HEM-7080IC; Omron Healthcare Co. Ltd., Kyoto, Japan) after 5 minutes of rest in the sitting position, and the average of the final two measurements was used for the analysis.
Blood samples were obtained after a fasting period of at least 8 hours, and all laboratory tests were performed in a central laboratory (Seoul Clinical Laboratories, Seoul, Korea). Serum fasting glucose was measured using the colorimetric method using an autoanalyzer (ADVIA 1800 Auto Analyzer; Siemens Medical Solutions, Malvern, PA, USA). The insulin level in serum was measured using a radioimmunoassay (SR 300; STRATEC, Birkenfeld, Germany) and serum 25(OH)D concentration was measured using chemiluminescence immunoassay (CLIA) method (Liaison; DiaSorin, Dietzenbach, Germany). Insulin resistance was measured using the HOMA-IR: fasting insulin (µIU/mL)×fasting glucose (mg/dL)/405. In both men and women, high insulin resistance was defined as an HOMA-IR score higher than the 75th percentile [18].
Demographic characteristics including age, gender, smoking status, amount of alcohol consumption, physical exercise, and season of sampling (May to October, November to April) were collected by trained interviewers. Smoking status was divided into three categories: never smokers (if the subjects had smoked <100 cigarettes), former smokers (≥100 cigarettes in one's lifetime but not currently a smoker), and current smokers (≥100 cigarettes and reported "currently smoking" in the questionnaire). Alcohol consumption (in g/day) was assessed by inquiring about the frequency and average amount drunk during the most recent year; this was calculated by multiplying the daily intake of each alcoholic beverage by its ethanol content. Physical exercise was measured using the International Physical Activity Questionnaire (IPAQ) and classified into three categories based on standard scoring criteria (http://www.ipaq.ki.se): low, moderate, and high. Season was defined using the date participants completed blood collection and was classified as follows: May to October, November to April.
Statistical analysis
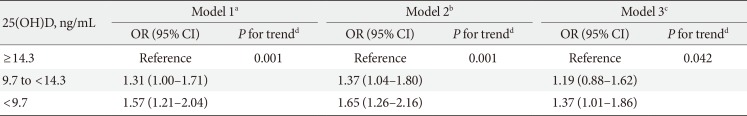
Differences between continuous variables according to 25(OH)D concentration were evaluated by analysis of variance and Tukey post hoc analyses. Categorical variables were compared using chi-square tests. All data are presented as mean±standard deviation or medians with IQRs. Multivariable logistic regression was used to assess the independent association of 25(OH)D concentrations and high insulin resistance. We used three models with progressive degrees of adjustment. Model 1 was the unadjusted analysis. Model 2 adjusted for age and gender. Model 3 additionally adjusted for waist circumference, alcohol consumption, smoking status, physical exercise, season, and cohort (cohort 1 or cohort 2). The results are expressed as ORs and 95% confidence intervals (CIs). Furthermore, stratified analysis between the normal and obese groups was performed. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA). P values <0.05 were considered statistically significant.
RESULTS
Participant characteristics
This study comprised a total of 1,807 subjects with a mean age of 49.6±8.8 years. Among them, 1,179 (65.2%) were women, and 628 (34.8%) were men. The median concentration of 25(OH)D was 11.7 ng/mL (IQR, 8.8 to 16.0) and HOMA-IR was 1.7 (IQR, 1.4 to 2.2).
Table 1 indicates the characteristics of each group according to serum 25(OH)D. The BMI, waist circumference, fasting glucose, systolic blood pressure, and amount of alcohol consumption did not differ significantly among groups (Table 1). However, the lowest tertile, with the lowest 25(OH)D levels (<9.7 ng/mL), represented a higher proportion of younger people and women, high fasting insulin level, and less frequent physical exercise compared to the other tertiles.
Associations between 25(OH)D concentration and insulin resistance
Insulin resistance was inversely associated with 25(OH)D concentrations (Table 2). Compared to those in the highest tertile, the ORs of those with high insulin resistance was 1.57 (95% CI, 1.21 to 2.04) in the lowest tertile and 1.31 (95% CI, 1.00 to 1.71) in the 2nd tertile (P for trend=0.001) in the unadjusted analysis (model 1). This pattern remained after adjustments. After adjusting for age, gender, waist circumference, alcohol intake, smoking status, physical exercise, season, and cohort, model 3 indicated higher ORs for high insulin resistance in the lowest tertile (OR, 1.37; 95% CI, 1.01 to 1.86) and middle tertile (OR, 1.19; 95% CI, 0.88 to 1.62) compared with the highest tertile (P for trend=0.042).
The results of the stratified analysis with general obesity by BMI and abdominal obesity by waist circumference are given in Table 3, Supplementary Table 1, Fig. 1. The results are based on the common reference group with serum 25(OH)D in the highest tertile and non-obese. Compared to the reference group, the risk of insulin resistance among subjects with general obesity (BMI ≥25 kg/m2) and serum 25(OH)D in the lowest tertile was 1.95 times higher, and subjects with normal (BMI <25 kg/m2) and serum 5(OH)D in the lowest tertile had about a 1.41-fold increase risk of insulin resistance. However, there was no difference in the results according to abdominal obesity (Fig. 1).

OR of high insulin resistance (>75 percentile) according to serum 25(OH)D levels stratified by adiposity
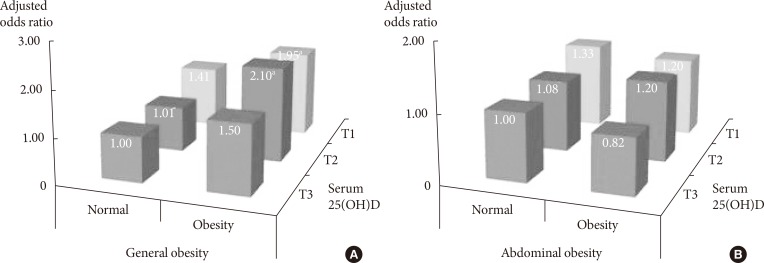
Adjusted odds ratio of high insulin resistance (>75 percentile) by tertiles (T) of serum 25-hydroxyvitamin D (25(OH)D) and adiposity status. (A) General obesity was defined as a body mass index of 25 kg/m2 or greater. (B) Abdominal obesity was defined as a waist circumference of 90 cm or greater for men and 85 cm or greater for women. Serum 25(OH)D was categorized into T. T1, first tertile; T2, second tertile; T3, third tertile. Model adjusted for age, gender, waist circumference, alcohol consumption, smoking status, physical exercise, season, and cohort. aP<0.05.
Table 3 shows the association between insulin resistance and 25(OH)D concentrations that remained in the non-general obesity group; however, there was no significant association in the general obesity group (lowest tertile vs. highest tertile; normal obesity [OR, 1.64; 95% CI, 1.05 to 2.56]; general obesity [OR, 0.97; 95% CI, 0.63 to 1.48]). After stratification by abdominal obesity, there was no significant association for each of the groups (lowest tertile vs. highest tertile; normal obesity [OR, 1.33; 95% CI, 0.92 to 1.93]; abdominal obesity [OR, 1.38; 95% CI, 0.81 to 2.37]).
DISCUSSION
We found that lower serum 25(OH)D concentrations are independently associated with high insulin resistance even after adjustments. This inverse relationship is consistent with some previous epidemiologic studies [8910]. A large cross-sectional study from the Third National Health and Nutrition Examination Survey revealed an inverse association between vitamin D status and insulin resistance in non-Hispanic white people and Mexican-Americans [10]. Similarly, studies on Asian populations have indicated that low circulating 25(OH)D levels are correlated with high fasting insulin levels and high HOMA-IR scores [89].
With regard to the Korean population, the Korean Genome Epidemiology Study (KoGES)-Kangwha study reported an inverse relationship between 25(OH)D concentrations and HOMA-IR score, but the association was no longer present after adjusting for sex, age, BMI, smoking status, alcohol intake, and regular exercise [19]. In contrast, in our study, the inverse associations remained after adjustments. However, the KoGES-Kangwha study population consisted of 807 rural subjects aged 40 to 89 years old, and the authors found that 25(OH)D concentrations were much higher than those in both our study and another study based on the Korea National Health and Nutrition Examination Survey (KNHANES IV) [13]. Therefore, the differences in results between the KoGES-Kangwha study and our study might be attributable to differences in 25(OH)D levels.
No significant difference of serum 25(OH)D levels based on gender was present in this study. Although several studies showed serum 25(OH)D levels were higher in males than in females [1320], other studies showed no difference [21] and even higher serum 25(OH)D levels in females [22]. Statistical adjustment for gender was made as well, with the result that younger people showed much lower serum 25(OH)D levels, which is consistent with other studies [2123]. This might be the result of their higher indoor activity, lower physical activity, and lower vitamin D supplement intake.
Most previous studies on vitamin D levels and insulin resistance have focused on the categories of vitamin D deficiency (<20 ng/mL), insufficiency (20 to 30 ng/mL), and sufficiency (≥30 ng/mL). However, the KNHANES IV reported that the prevalence of vitamin D insufficiency was very high, impacting more than 60% of Korean adults [13]. Therefore, additional categories of vitamin D deficiency or insufficiency are needed in Korea. We divided subjects based on 25(OH)D concentration, using cutoff points of 9.7 and 14.3 ng/mL, both lower than the level currently defined as vitamin D deficiency (20 ng/mL or 50 nmol/L). To the best of our knowledge, this is the first study to have evaluated the association between subdivisions of vitamin D deficiency and insulin resistance. Similar negative associations were observed between vitamin D levels and insulin resistance in the vitamin D deficiency subdivisions.
Stratified analyses indicated that the association between 25(OH)D levels and insulin resistance is not amplified by abdominal obesity, although it is amplified by general obesity. This result is not consistent with previous studies, which found that abdominal obesity is more strongly associated with insulin resistance than general obesity [24]. Kang et al. [25] reported results that were similar to our study, finding a stronger association between 25(OH)D and insulin resistance when stratified by BMI than when stratified by abdominal obesity. They insisted that BMI is more reflective of subcutaneous fat than waist circumference. Also, previous studies have shown that 25(OH)D and obesity may have a synergistic effect on insulin resistance risk [14]. However, our study showed that the association of 25(OH)D levels with insulin resistance was only present in the normal obesity group. Although the storage of 25(OH)D in fat tissues can cause lower bioavailable vitamin D, obesity may be a greater effect than 25(OH)D because obesity is an established risk factor of insulin resistance [26]. So, the relation of 25(OH)D levels with insulin resistance was only significant in the normal obesity group, not in the obesity group.
Vitamin D is a multifunctional hormone that is essential to many important functions, ranging from calcium and phosphorus homeostasis to glucose regulation [12]. Although previous studies have reported that vitamin D deficiency is associated with insulin resistance, the mechanism of this association has not fully been explained. One potential mechanism is that vitamin D may have a direct effect on insulin sensitivity through stimulation of insulin receptor expression, together with improved insulin-dependent glucose transport [2728]. Another potential mechanism is that vitamin D suppresses chronic inflammation, which is associated with obesity and insulin resistance [28]. Vitamin D has anti-inflammatory actions. It dose-dependently suppresses the release of tumor necrosis factor-α and interleukin 6 (IL-6) [2930], while upregulating the synthesis of the anti-inflammatory cytokine IL-10, thus potentially partly counteracting the inflammatory consequences of increased adiposity [28].
Strengths of this study were the large sample size and the coverage of both rural and urban areas. Thus, our study population may be representative of the Korean general population. In addition, we included many potential confounders (age, gender, BMI, waist circumference, smoking, alcohol consumption, physical activity, and season) that could affect glucose and vitamin D metabolism, and the final results were obtained after adjusting for these confounding factors. However, this study also had some limitations. First, the blood samples were not collected during the same season. So, we adjusted for season (May to October, November to April) to obtain more precise results. Second, data on sunscreen use, sun exposure time, diet pattern, and calcium or vitamin D supplement use were not collected. Third, this study cannot establish causality between 25(OH)D concentrations and insulin resistance due to the cross-sectional design. Finally, there is a possibility that unmeasured confounding factors, such as calcium and parathyroid hormone levels, might have influenced the results.
In conclusion, our study shows that 25(OH)D concentrations in serum have a substantial inverse association with insulin resistance. These results suggest that vitamin D is independently associated with insulin resistance in the healthy, non-obese Korean population.
ACKNOWLEDGMENTS
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI13C0715).
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
SUPPLEMENTARY MATERIAL
Supplementary Table 1
Numbers of participants with high insulin resistance (>75 percentile) by tertiles