Importance of Lean Muscle Maintenance to Improve Insulin Resistance by Body Weight Reduction in Female Patients with Obesity
Article information
Abstract
Background
It has recently been suggested that skeletal muscle has an important role in insulin resistance in obesity, in addition to exercise tolerance and the fat index. The aim of this study was to identify body composition factors that contribute to improvement of insulin resistance in female patients with obesity who reduce body weight.
Methods
We studied 92 female obese patients (age 40.9±10.4 years, body mass index 33.2±4.6 kg/m2) who reduced body weight by ≥5% after an intervention program including diet, exercise therapy, and cognitive behavioral therapy. Before and after the intervention, body composition was evaluated by dual-energy X-ray absorptiometry to examine changes in skeletal muscle mass. Homeostasis model assessment of insulin resistance (HOMA-IR) was measured as an index of insulin resistance. Cardiopulmonary exercise was also performed by all patients.
Results
There were significant improvements in body weight (–10.3%±4.5%), exercise tolerance (anaerobic threshold oxygen uptake 9.1%±18.4%, peak oxygen uptake 11.0%±14.2%), and HOMA-IR (–20.2%±38.3%). Regarding body composition, there were significant decreases in total body fat (–19.3%±9.6%), total fat-free mass (–2.7%±4.3%), and % body fat (–10.1%±7.5%), whereas % skeletal muscle significantly increased (8.9%±7.2%). In stepwise multiple linear regression analysis with change in HOMA-IR as the dependent variable, the change in % skeletal muscle was identified as an independent predictor (β=–0.280, R2=0.068, P<0.01).
Conclusion
Improvement of insulin resistance in female obese patients requires maintenance of skeletal muscle mass.
INTRODUCTION
The number of patients in Japan with obesity due to insufficient exercise and overeating has increased in recent years. Based on insulin resistance, obesity may cause metabolic syndrome, which is related to development of hypertension, abnormal lipid metabolism, and diabetes, and increases the risk of arteriosclerosis, ischemic cardiac disease, and cerebrovascular disease. Thus, obesity requires appropriate treatment and prevention [12]. Obese patients have accumulation of visceral fat and enlarged fat cells [3], and these cells can lead to insulin resistance and may cause various diseases due to vascular endothelial function disorder [456]. Therefore, the main goals of obesity treatment are to decrease fat and improve insulin resistance, mainly through intervention with aerobic exercise [7].
It has recently been suggested that skeletal muscle also has an important role in the cause of insulin resistance [24]. The exercise/metabolism function and oxygen consumption of skeletal muscles are dependent on muscle cells and mitochondria [89]. Furthermore, skeletal muscles are target tissues of insulin and have endocrine function to produce cytokines called myokines. Thus, a decrease in skeletal muscle mass may be a factor in insulin resistance [1011], and maintenance and increase of the skeletal muscle mass might be useful for improvement of insulin resistance. However, the details of this process are still unclear. Therefore, we examined the relationship between changes in skeletal muscle mass in obese patients who reduced body weight and insulin resistance measured before and after the decrease in body weight.
METHODS
Subjects and study protocol
Medical records were reviewed for 213 females who underwent our obesity program to reduce body weight. Of these patients, 52 aged over 60 years old were excluded. Of the remaining 161 patients, 111 and 50 (including 10 who subsequently dropped out) had reductions of body weight of >5% and <5%, respectively. After this evaluation, 19 patients with diabetes under treatment with insulin or oral diabetes drugs, extremely severe obesity (body mass index [BMI] ≥46 kg/m2), or significantly decreased skeletal muscle index (SMI <5.45 kg/m2) were excluded. A retrospective study was performed in 92 patients with obesity with BMI ≥30 kg/m2. There were some differences between patients with reductions of body weight of >5% and <5%. However, we want to emphasize the important factors for insulin resistance, and therefore we do not show data for subjects with body weight reduction <5%.
Based on the ethical code of Kansai Medical University, this study was performed after providing an explanation of the objectives, details and notes to all subjects, and obtaining written informed consent for participation in the study.
Body weight control program and monitoring
The body weight control program included exercise therapy, monthly nutritional guidance, and psychological counseling. A symptom-limited cardiopulmonary exercise (CPX) was performed before the program was used to determine the anaerobic threshold oxygen uptake (ATVO2) and peak oxygen uptake (peak VO2) in each subject. The exercise program lasted for 70 minutes, and consisted of 30 minutes of aerobic exercise, such as that on a bicycle or treadmill, and gravity level resistance exercise of stretching [12]. Over a period of 6 months, exercise therapy was supervised by a health exercise instructor at least once or twice a month in our health science center. The subjects were asked to perform similar exercise three times a week at home. For exercise at home, a pedometer was given to each subject to measure individual activity. The individual exercise amounts were determined from the pedometer at every visit to the center. The health exercise instructor also provided guidance to subjects at each visit. For nutritional guidance, education on eating behavior and dietary instruction was mainly provided. The dietitian mainly provided advice on eating behavior based on cognitive behavioral medicine. In psychological counseling, a clinical psychotherapist provided guidance, with a focus on self-monitoring and self-efficacy, based on cognitive behavioral therapy [1314].
Examinations
Physical findings (height and body weight), body composition, and blood tests in a fasting state were measured prior to and after the 6-month intervention. Blood was collected after overnight fasting. Serum insulin was measured by chemiluminescent immunoassay (Elecsys Insulin Assay; Roche Diagnostics Ltd., Tokyo, Japan) and determined using an automatic electrochemiluminescence immunoassay analyzer (Modular Analytics EE; Roche Diagnostics). The homeostasis model assessment of insulin resistance (HOMA-IR) was used to estimate insulin resistance, using fasting blood insulin in the early morning (IRI) and the fasting blood sugar (FBS) levels, based on the equation: HOMA-IR=(IRI×FBS)/405 [15]. This equation is most appropriate for evaluation of insulin resistance in patients without diabetes.
Cardiopulmonary exercise
A symptom-limited CPX was performed by all patients using a bicycle ergometer (232C-XL; Combi Co. Ltd., Tokyo, Japan). In analysis of expired gas, the measurement was performed with the breath-by-breath method using an expired gas analyzer (AE-300 system; Minato Medical Science Co. Ltd., Osaka, Japan). AT was determined using the V-slope method. peak VO2 was defined as the highest level under load.
Body composition and skeletal muscle mass indexes
Body fat and fat-free mass were measured using dual-energy X-ray absorptiometry (DXA; DPX-NT system; General Electric, Fairfield, CT, USA). After the 6-month intervention, these items were determined before other effects were evaluated. Using absolute values of fat-free mass (whole body, upper extremities, body trunk, and lower extremities) from DXA. The SMI was obtained by adjusting the skeletal muscle mass for height (skeletal muscle mass of extremities/height2 [kg/m2]), the appendicular skeletal muscle (ASM) index (/body weight) was obtained by adjusting the ASM mass for body weight (skeletal muscle mass of extremities/body weight [%]), and the % skeletal muscle was obtained by adjusting the total fat-free mass for body weight (total fat-free mass/body weight [%]).
Statistical analysis
All data are expressed as mean±standard deviation. Individual values obtained before and after the intervention were compared by paired t-test and Wilcoxon signed-rank test. For comparison between groups, an independent t-test and Mann-Whitney U test were used. Pearson correlation and Spearman rank-correlation coefficients were used to examine the relationship of changes in individual values. Stepwise multiple linear regression analysis was used for multivariate analysis. All calculations were performed using SPSS version 21 (IBM Co., Armonk, NY, USA). Differences at P<0.05 were considered significant in all analyses.
RESULTS
Characteristics
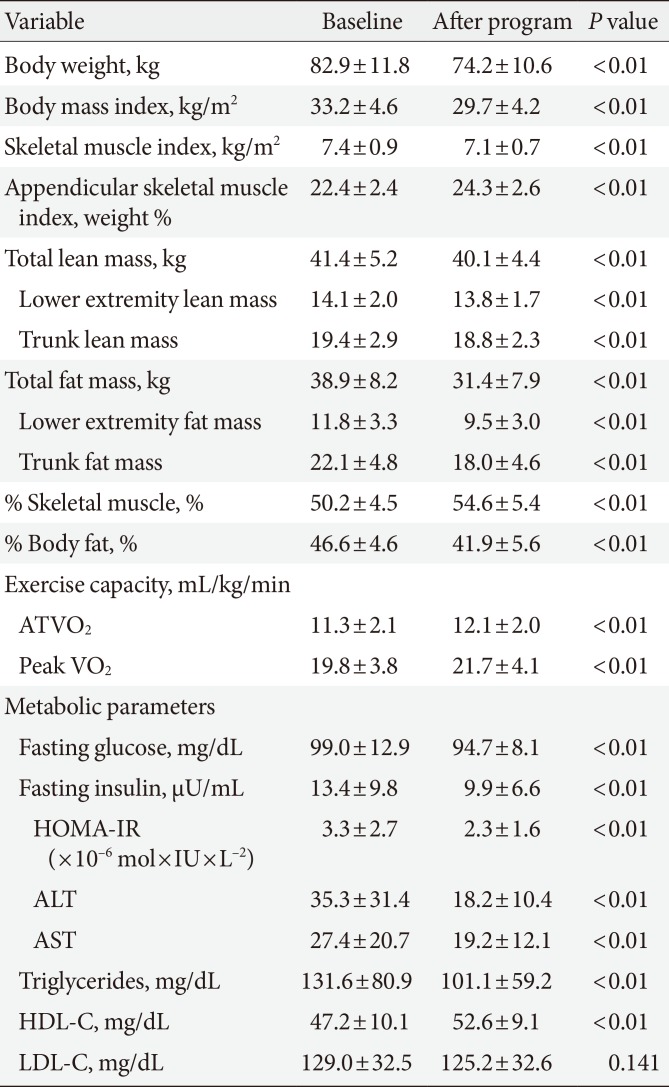
A retrospective study was performed in 92 patients with obesity with BMI ≥30 kg/m2. The average age, body weight and BMI were 40.9±10.4 years, 82.9±11.8 kg, and 33.2±4.6 kg/m2, respectively (Table 1).
Changes caused by the exercise intervention body weight control program
The background of the patients is shown in Table 1. After the 6-month body weight control program, all subjects had a body weight decrease of ≥5% and the amounts of fat and fat-free mass were also significantly reduced. The body weight change from before to after intervention was –10.3%±4.5% (82.9±11.8 to 74.2±10.6 kg, P<0.01), the decrease in total fat mass was –19.3%±9.6% (38.9±8.2 to 31.4±7.9 kg, P<0.01), the decrease in total fat-free mass was –2.7%±4.3% (41.4±5.2 to 40.1±4.4 kg, P<0.01), and the decrease in HOMA-IR was –20.2%±38.3% (3.3±2.7 to 2.3±1.6, P<0.01).
For skeletal muscle mass, SMI significantly decreased by 2.3%±1.6% (7.4±0.9 to 7.1±0.7 kg, P<0.01), ASM index (/body weight) significantly increased by 8.9%±8.0% (22.4±2.4 to 24.3±2.6 kg, P<0.01). % Body fat significantly decreased by –10.1%±7.5%, and % skeletal muscle significantly increased by 8.9%±7.2% (50.2±4.5 to 54.6±5.4 kg, P<0.01). Thus, all these parameters changed significantly from before to after the intervention.
In exercise tolerance, ATVO2 significantly increased by 9.1%±18.4% (11.3±2.1 to 12.1±2.0 mL/kg/min), and peak VO2 significantly increased by 11.0%±14.2% (19.8±3.8 to 21.7±4.1 mL/kg/min) (Table 2).
Relationship between the change in HOMA-IR and changes in body composition
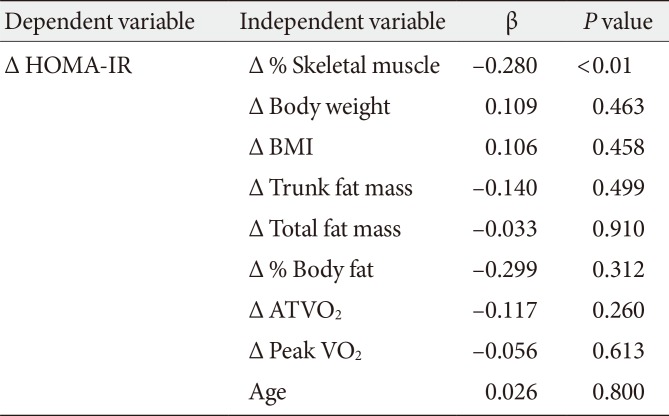
In the 92 subjects, the change in HOMA-IR was significantly positively correlated with changes in body weight (r=0.256, P<0.05), BMI (r=0.251, P<0.05), amount of fat (whole body: r=0.258, P<0.05; body trunk: r=0.210, P<0.05), and % body fat (r=0.228, P<0.05), and significantly negatively correlated with the change in % skeletal muscle mass (r=–0.280, P<0.01). In stepwise multiple linear regression analysis of these parameters and age as independent variables and the change in HOMA-IR as the dependent variable, a change in % skeletal muscle was identified as an independent predictor of HOMA-IR (β=–0.280, R2=0.068, P<0.01) (Table 3).
DISCUSSION
Female patients with obesity who completed a 6-month body weight control program and successfully reduced their body weight were selected as subjects of this study, to identify changes in body composition factors that are associated with improvement of insulin resistance. Comparison of data obtained before and after the intervention showed that % skeletal muscle was maintained, while body weight was significantly decreased by ≥5%, fat mass was reduced, and exercise tolerance and insulin resistance improved. The program mainly comprised aerobic exercise, and active resistance training for increase of skeletal muscle was not performed. Therefore, there were no cases in this study whose skeletal muscle mass was significantly decreased more than 5% instead of fat mass reduction.
A decrease in SMI occurred from before to after the intervention. Since sex hormones are highly correlated with maintenance of skeletal muscle [16], only female patients aged ≤60 years were included in the study. In obesity treatment, since total body weight is reduced in body weight control, a certain decrease in skeletal muscles is physiologically appropriate. In this study, fat-free mass and SMI significantly decreased after the intervention, while ASM index (/body weight) and % skeletal muscle significantly increased. These parameters reflect the amount of skeletal muscle adjusted for height, body weight, and total fat-free mass [17]. Nomura and Ouchi [7] found that body composition tends to vary more with aging, and suggested the importance of the amount of visceral fat and the skeletal muscle mass in obesity treatment. Therefore in this study, we propose that the % skeletal muscle can be used for appropriate evaluation of skeletal muscle in body composition after obese patients reduce their body weight.
Fat is thought to be the most important factor in insulin resistance, and obesity with a large amount of visceral fat has a particularly strong correlation with insulin resistance [181920]. We did not examine visceral fat in this study, but Yamanaka et al. [21] has shown relationships of insulin resistance with an increase in fat and the characteristics of the fat. In addition, enlarged fat cells have decreased production of adiponectin, which facilitates insulin sensitivity in skeletal muscles, and increased production of harmful adipocytokines. Such harmful cytokines include tumor necrosis factor α and resistin, and with free fatty acids these cytokines induce insulin resistance by inhibiting insulin signaling in skeletal muscle and the liver [456].
In contrast, in this study, the most important factor for insulin resistance in female patients with obesity was the % skeletal muscle, rather than exercise tolerance or the fat index. This finding is consistent with recent reports suggesting that tissue changes in skeletal muscles are strongly involved in insulin resistance. The motor/metabolism function and oxygen consumption of skeletal muscles are promoted by a close mitochondria network on the surface of the myocyte membrane or myofibril septum [89]. If the amount or function of such mitochondria is decreased, fatty acids in skeletal muscles are not metabolized and accumulate as diacylglycerol, long-chain acyl-coenzyme A, and ceramide, which may aggravate insulin resistance by inhibiting insulin signaling, including a decrease of glucose transporter type 4 (GLUT4) follicles [356]. Furthermore, skeletal muscles are target tissues for insulin and have endocrine function to produce myokines [2223]. A decrease in skeletal muscle mass induces a decrease of mitochondria, and thus fatty acids in skeletal muscle are not metabolized, resulting in insulin resistance [1011]. This suggests that a decrease of fat and maintenance or an increase in % skeletal muscle can improve insulin resistance and contribute to a favorable outcome of obesity treatment.
In stepwise multiple linear regression analysis in this study, the coefficient for the relationship of the change in % skeletal muscle with improvement of insulin resistance was only 0.068. This low value may be due to use of HOMA-IR as the index of insulin resistance, since this value mainly reflects insulin resistance in the liver [24] and might be largely influenced by visceral fat [23]. No relationships with other skeletal muscle mass indexes were found in this analysis. In 2012, Kim et al. [25] compared the validities of diagnoses using extremity SMIs adjusted by height and body weight in patients and found that sarcopenia and sarcopenia with obesity were under evaluated using SMI (adjusted for height). Moon et al. [10] used data adjusted for body weight to investigate the role of skeletal muscle in development of non-alcoholic fatty liver disease [2627]. When evaluating muscle-related conditions of obese subjects, their whole body size should be considered. The % skeletal muscle might be the most appropriate index for skeletal muscle in obese patients with large changes in body weight, since no other indexes were significant in this study. Our results also suggest that maintenance of skeletal muscle is more important than increased exercise tolerance in obese female patients who reduce their body weight.
There were several limitations in the study. First, we did not evaluate menopausal stage, which is a concern because sex hormones are correlated with body composition. However, there are a few studies of important factors, other than menopause, in women under 60 years [2829]. Lee et al. [30] also found that such subjects had significant negative correlations of HOMA-IR with ASM and ASM/height2, after adjustment for body weight. Therefore subjects over 60 years old were excluded and we consider that the effects of menopause were excluded to a certain extent in this study. Second, although the subjects were obese patients with BMI ≥30 kg/m2, adiponectin produced from fat tissues was not measured. We mainly examined body composition in regard to insulin resistance, but further studies on nutritional factors, such as proteins and amino acids, that may affect skeletal muscles are also required. In addition, male subjects and wider BMI and age groups should be included in future studies.
In conclusion, significant decreases in body weight and % body fat were found in female patients with obesity who completed a body weight control program. These patients also had increased exercise tolerance and % skeletal muscle. We suggest that % skeletal muscle is a useful index that is related to a change in HOMA-IR. This finding indicates that skeletal muscles contribute to improvement of HOMA-IR in female patients with obesity.
The present study indicated that maintaining skeletal muscle mass during reducing body weight in female patients with obesity may be the essential of success for a body weight management. Further, this results suggested that the independent factor of the % skeletal muscle was solely HOMA-IR. An adequate parameter for muscle-related conditions of obese patients may be the % skeletal muscle.
ACKNOWLEDGMENTS
We would like to thank the subjects who participated in the study. We express our gratitude for the assistance of staff members of the Health Science Center of Kansai Medical University. Parts of this study were reported at the 35th Annual Meeting of the Japan Society for the Study of Obesity (Miyazaki, October 2014).
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.