- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 39(6); 2015 > Article
-
Original ArticleOthers Metformin Promotes Apoptosis but Suppresses Autophagy in Glucose-Deprived H4IIE Hepatocellular Carcinoma Cells
- Deok-Bae Park
-
Diabetes & Metabolism Journal 2015;39(6):518-527.
DOI: https://doi.org/10.4093/dmj.2015.39.6.518
Published online: December 11, 2015
Department of Histology and Institute of Medical Science, Jeju National University School of Medicine, Jeju, Korea.
- Corresponding author: Deok-Bae Park. Department of Histology and Institute of Medical Science, Jeju National University School of Medicine, 102 Jejudaehang-ro, Jeju 54987, Korea. parkdb@jejunu.ac.kr
• Received: November 17, 2014 • Accepted: February 26, 2015
Copyright © 2015 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Metformin, a well-known anti-diabetic drug, has gained interest due to its association with the reduction of the prevalence of cancer in patients with type 2 diabetes and the anti-proliferative effect of metformin in several cancer cells. Here, we investigated the anti-proliferative effect of metformin with respect to apoptosis and autophagy in H4IIE hepatocellular carcinoma cells.
-
Methods
- H4IIE rat cells were treated with metformin in glucose-free medium for 24 hours and were then subjected to experiments examining the onset of apoptosis and/or autophagy as well as the related signaling pathways.
-
Results
- When H4IIE cells were incubated in glucose-free media for 24 hours, metformin and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) reduced the viability of cells. Inhibition of AMP-activated protein kinase (AMPK) by compound C significantly blocked cell death induced by metformin or AICAR. Pro-apoptotic events (nuclear condensation, hydrolysis of intact poly ADP ribose polymerase and caspase-3) were stimulated by metformin and then suppressed by compound C. Interestingly, the formation of acidic intracellular vesicles, a marker of autophagy, was stimulated by compound C. Although the deprivation of amino acids in culture media also induced apoptosis, neither metformin nor compound C affected cell viability. The expression levels of all of the autophagy-related proteins examined decreased with metformin, and two proteins (light chain 3 and beclin-1) were sensitive to compound C. Among the tested inhibitors against MAP kinases and phosphatidylinositol-3-kinase/mammalian target of rapamycin, SB202190 (against p38MAP kinase) significantly interrupted the effects of metformin.
-
Conclusion
- Our data suggest that metformin induces apoptosis, but suppresses autophagy, in hepatocellular carcinoma cells via signaling pathways, including AMPK and p38 mitogen-activated protein kinase.
- Metformin, a well-established, safe, effective anti-diabetic drug, has gained interest due to its association with the reduced risk of several cancers in type 2 diabetes patients [12]. A number of epidemiological and preclinical studies have suggested various mechanisms underlying the anticancer activity of metformin in different cancer types [3]. Previous studies have found diverse anticancer effects of metformin in human lung cancer cells [4], gastric cancer cells [5], endometrial cancer cells [6], breast cancer cells [7], and other types of cancer cells. These studies suggest that metformin inhibits cell proliferation by unique mechanisms in different types of cancer cells. As an example, metformin decreases the growth of cells by regulating lipogenesis, independent of AMP-activated protein kinase (AMPK) in hepatocellular carcinoma [8], whereas the anticancer effects of metformin in many other cancer cells are dependent on AMPK, a target molecule of metformin [9].
- AMPK is a cellular energy sensor that regulates metabolism [9]. It diminishes hepatic gluconeogenesis and enhances muscular glucose uptake. Studies have also indicated that AMPK is involved in the suppression of cancer cell proliferation [10]. However, it is not yet understood whether AMPK plays a key role in mediating metformin's anticancer activity because metformin also induces cell damage by directly interrupting the mitochondrial complex independent of AMPK [11].
- Macroautophagy (hereafter referred to as autophagy) is a recycling process that is used to maintain cellular nutrient balance and the function of intracellular organelles [12]. Autophagy can remove cells that have undergone apoptosis. Alternatively, autophagy may result in a form of non-apoptotic cell death [13]. Thus, autophagy can either promote or suppress cell death under different conditions.
- Recently, previous studies have shown that metformin induces apoptosis [14] or inhibits proliferation in hepatocellular carcinoma Huh-7 cells [15]. However, there is little evidence of autophagy when hepatocellular carcinoma cells are exposed to metformin. Here, we show that metformin induces apoptosis and suppresses autophagy in H4IIE hepatocellular carcinoma cells in glucose-deprived culture conditions. The effect of metformin is sensitive to the inhibition of AMPK and p38 mitogen-activated protein kinase (p38MAPK) signaling pathways.
INTRODUCTION
- Cells
- H4IIE rat hepatocellular carcinoma cells were obtained from the Korean Cell Line Bank (Seoul, Korea) and maintained in Dulbecco's minimal essential medium (DMEM, 1 g/L glucose) with 10% fetal bovine serum (FBS). At the beginning of the experiments, H4IIE cells were incubated in serum-free DMEM overnight. Cells were washed twice with Dulbecco's phosphate-buffered saline (D-PBS) and again incubated in serum- and glucose-free DMEM (GFM) supplemented with 2 mM pyruvate and 20 mM lactate for 30 minutes before treatment with reagents.
- Materials
- DMEM, Hank's-balanced salt solution (HBSS), D-PBS, trypsin-ethylenediaminetetraacetic acid solution, metformin, compound C, 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT), 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), SB202190, SP600125, wortmannin, rapamycin, H33342, and acridine orange (AO) were purchased from Sigma Chemical Corp. (St. Louis, MO, USA). FBS was obtained from Life Technologies, Inc. (Rockville, MD, USA). Polyclonal antibodies against poly ADP ribose polymerase (PARP), cleaved caspase-3, autophagy-related (3, 5, 7), β-actin, and monoclonal antibodies against beclin-1 and microtubule-associated protein 1 light chain 3B (LC3B) were purchased from Cell Signaling Technology (Danvers, MA, USA). Polyclonal antibodies against phospho-AMPK were obtained from Millipore (Billerica, MA, USA). Polyclonal anti-PARP and anti-rabbit goat immunoglobulin G-horseradish peroxidase (HRP) secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Electrophoresis reagents (including Bis-Tris gels, running buffer, and polyvinylidene difluoride [PVDF] membranes) were obtained from Invitrogen (Carlsbad, CA, USA).
- MTT assay
- Cell viability was analyzed using the MTT assay as previously described [16]. Briefly, H4IIE cells in 24-well plates were pre-incubated in serum-free DMEM overnight, washed twice with D-PBS and then further incubated for 24 hours after pretreatment and treatment with reagents in GFM. After the treatments, the same amount of MTT reagent (1 g/L in D-PBS) was added to each well. After a 30 minute incubation at 37℃, cells were washed with D-PBS, and the blue-colored formazan product was subsequently solubilized in 0.5 mL of 2-propanol for 20 minutes. The absorbance of the converted dye was measured at a wavelength of 570 nm.
- Western blotting analysis
- H4IIE cells in 6-well plates were pre-incubated in serum-free DMEM overnight, washed twice with D-PBS, and then further incubated in GFM-containing reagents. After treatment, the cells were lysed in an ice-cold lysis buffer (50 mM Tris-HCl, 1% nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM sodium orthovanadate, 1 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, 1 mM leupeptin, and 1 mM pepstatin A). Equal amounts (10 to 20 µg) of protein were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 4% to 12% polyacrylamide gels and transferred onto PVDF membranes. The membranes were incubated in blocking buffer (5% nonfat dry milk in Tris-buffered saline [TBS]-0.1% Tween-20 [TBS-T]) for 1 hour at room temperature, after which the membranes were probed with different primary antibodies (at dilutions of 1:1,000 to 1:2,000). After a series of washes, the membranes were further incubated with the respective HRP-conjugated secondary antibodies at a dilution of 1:10,000. The signal was detected using the enhanced chemiluminescence detection system (Intron, Seongnam, Korea).
- Acridine orange/H33342 staining
- Acidic intracellular vesicles, a marker of the onset of autophagy were visualized using AO staining. After treatment, the cells were washed twice with D-PBS and stained in 2% acetone in D-PBS containing AO (1 µg/mL) for 15 minutes at room temperature. To observe the degree of nuclear condensation (a marker of apoptosis), H33342 (1 µg/mL) was added alone or in combination with AO. After removal of the AO/H33342 staining solution, the cells were immediately observed under a fluorescent microscope (IX70; Olympus, Tokyo, Japan) and imaged using a digital camera (DP-70; Olympus).
- Statistics
- The experimental results are presented as the mean±standard error. The significance of the differences among groups was determined using Student t-test. P<0.05 was considered statistically significant.
METHODS
- Metformin inhibits cell viability and induces apoptosis in H4IIE cells
- Our preliminary results showed that the viability of H4IIE cells was not significantly affected within the tested ranges of the glucose concentration (0 to 5.5 mM) after 24 hours (data not shown). Thus, we regarded the cells incubated in GFM as the control group in the experiments. The culture conditions mimic glucose-deprived environments for proliferating cancer cells with respect to the induction of apoptosis and/or autophagy.
- To test whether metformin affects the viability of hepatocellular carcinoma cells, H4IIE cells were treated with metformin in GFM. To remove the residual proliferative effects of serum, cells were pre-incubated in serum-free DMEM for 24 hours and then treated with metformin and AICAR, typical activators of AMPK, in fresh GFM. Stimulation of AMPK was detected using Western blotting analyses for phospho-AMPK after 4 hours of treatment (Fig. 1A). While lower doses of metformin (0.2 mM) or AICAR (0.1 mM) did not change the levels of phospho-AMPK, 1 mM AICAR weakly increased the levels of p-AMPK (approximately 1.3-fold over the non-treated control) and 2 mM metformin strongly increased the levels of p-AMPK (2.2-fold over the control). Pretreatment with compound C (10 µM), an inhibitor of AMPK, blocked the stimulation of AMPK by metformin or AICAR. Cell viability was measured using the MTT assay after 24 hours of treatment with metformin and AICAR. AICAR (1 mM) decreased MTT reactivity, but was significantly restored by pretreatment with compound C (50.44%±3.53% vs. 72.56%±0.88% of the non-treated control, respectively) (Fig. 1B). Metformin (2 mM) markedly decreased MTT reactivity, and such a decrease was also significantly restored by pretreatment with compound C (10.79%±1.13% vs. 57.8%±1.73% of the non-treated control, respectively) (Fig. 1C). These results indicated that pharmacological stimulation of AMPK by AICAR or metformin exerts cytotoxicity in H4IIE hepatocellular carcinoma cells. After determining that stimulation of AMPK and reduction of MTT reactivity were stronger with metformin (2 mM) than AICAR (1 mM) treatment, we next examined whether the decrease in MTT reactivity caused by metformin reflected an induction of apoptosis via the stimulation of AMPK. Induction of apoptosis was detected using fluorescent staining of condensed nuclear chromatin (apoptotic bodies) and Western blotting analyses for cleaved caspase-3 and PARP. In the control group (cells pre-incubated in serum-free DMEM for 24 hours and further incubated in GFM for an additional 24 hours), little change was observed in the degree of apoptotic body formation and the cleavage of caspase-3 and PARP (Fig. 1D and E). The addition of 2 mM metformin increased the number of apoptotic bodies (Fig. 1D) and the amount of cleaved caspase-3 and PARP (Fig. 1E). Compound C also suppressed the effects of metformin.
- Inhibition of AMPK induces autophagy
- Recent studies have suggested that metformin induces autophagy in various cells [171819]. However, it is unknown if metformin (or AMPK) modulates autophagy with respect to its anti-proliferative activity in hepatocellular carcinoma. Because autophagy is initiated by the depletion of energy sources, including glucose [2021] or amino acids [22], we tested whether deprivation of glucose or amino acids induced autophagy in H4IIE cells. In addition, the effect of metformin on the induction of autophagy was examined. As shown in Fig. 2A, deprivation of glucose did not enhance the formation of acidic intracellular vesicles or apoptotic bodies per se. However, compound C stimulated the formation of acid vesicles (yellow fluorescence within cells) in most of the treated cells. The number of apoptotic cells increased with metformin, while pretreatment with compound C completely reversed the effect of metformin. In an enlarged fluorescent image, acidic vesicles were observed only in healthy, non-apoptotic cells, but were not observed in apoptotic cells (arrows in Fig. 2B) when co-treated with metformin and compound C in GFM. Compared to the results observed with glucose treatment, deprivation of amino acids (incubation in amino acid-free HBSS containing 5.5 mM glucose) markedly increased the number of apoptotic cells (Fig. 2C). In amino acid-deprived cells, metformin did not affect the viability of cells compared to the control, but AICAR accelerated cell death (Fig. 2D). However, in amino acid-fed cells (together with 5.5 mM glucose), metformin decreased cell viability and the cells that were sensitive to compound C (Fig. 2D). Although compound C enhanced the formation of acidic vesicles within cells, it failed to rescue cells from apoptosis in all of the treatments evaluated (Fig. 2C and D). These results indicated that metformin induced apoptotic cell death and inhibited autophagy in glucose-deprived environments, but not in amino acid-deprived conditions in H4IIE cells. Moreover, compound C suppressed the pro-apoptotic activity of metformin, indicating a role for AMPK in the control of apoptosis/autophagy in H4IIE cells.
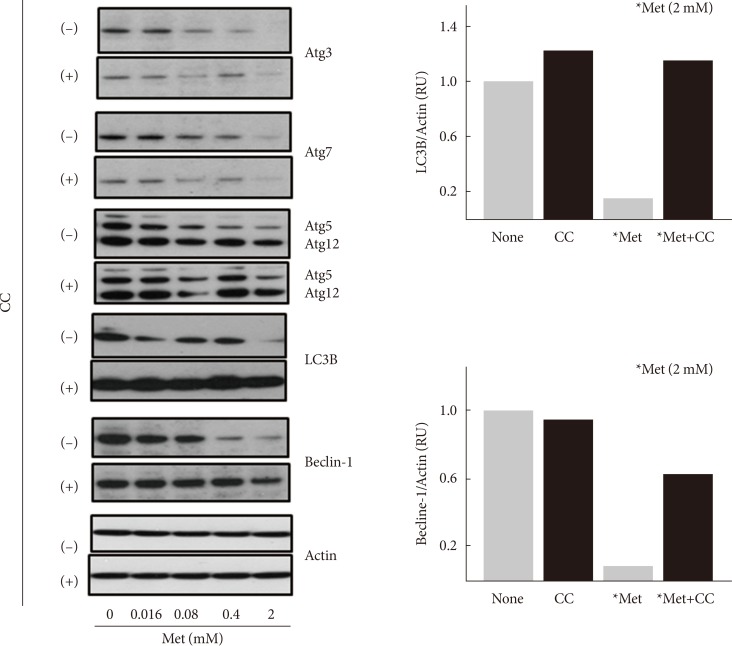
- Metformin decreases the expression levels of autophagy-related proteins
- Although compound C stimulated the formation of acidic intracellular vesicles, it is unknown whether metformin inhibits autophagy in an AMPK-dependent manner. Thus, the effect of metformin on the expression of various autophagy-related proteins (Atg3, Atg5, Atg7, Atg12, LC3B, and beclin-1) was examined. Serum-starved (for 24 hours) H4IIE cells were treated with different concentrations of metformin (0.016 to 2 mM) for 24 hours in GFM. Western blotting analyses revealed that metformin decreased the expression levels of all six proteins tested in a dose-dependent manner (Fig. 3). Pretreatment with compound C blocked the metformin-induced decrease of LC3B and beclin-1 proteins. Because these two proteins are essential components in the formation of the autophagosome compared to the other four proteins, these results suggested that metformin inhibits autophagy in an AMPK-dependent manner.
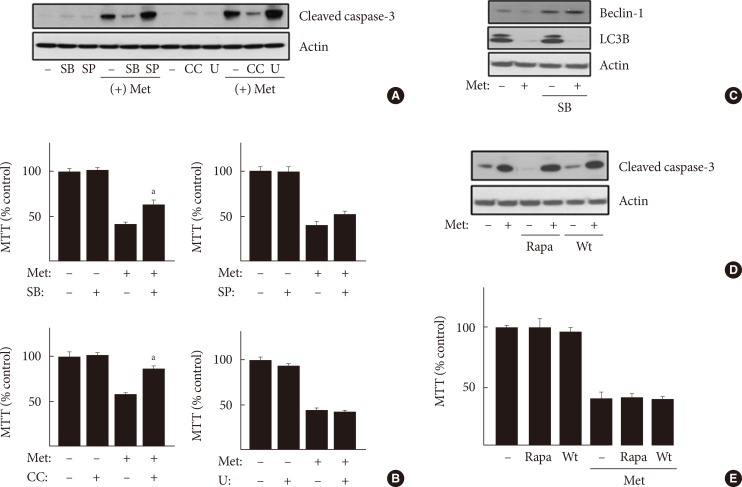
- Inhibition of p38MAPK as well as AMPK suppresses apoptosis induced by metformin
- Our results showed that metformin induces apoptosis and inhibits autophagy in an AMPK-dependent manner in H4IIE cells under glucose-deprived conditions. However, little evidence is available regarding the involvement of signaling pathways other than AMPK for the pro-apoptotic and anti-autophagic activities of metformin. We tested the involvement of two major signaling pathways with regard to metformin activity. Members of the MAPK and phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) families play diverse roles in controlling the viability of normal and cancer cells. First, inhibitors against MAPKs were pre-incubated for 30 minutes prior to metformin treatment for 24 hours. Only the inhibition of p38MAPK by 50 µM SB202190 significantly suppressed metformin's pro-apoptotic activity (Fig. 4A, cleavage of caspase-3; Fig. 4B, MTT reactivity), whereas inhibition of ERKs (extracellular signal-regulated kinases) by 10 µM U0126 or inhibition of JNKs (c-Jun N-terminal kinases) did not suppress the pro-apoptotic activity of metformin. SB202190 also restored the reduced expression of beclin-1 by metformin (Fig. 4C). However, the reduced expression of LC3B by metformin was not restored by SB202190, whereas it was restored by compound C (Fig. 3). Taken together, these results suggest that a member of the MAPK family, p38MAPK, combined with AMPK, also mediate metformin's pro-apoptotic and anti-autophagic activity in H4IIE cells. In addition, inhibition of PI3K and mTOR by wortmannin (100 nM) and rapamycin (100 nM) failed to change metformin's pro-apoptotic activity (Fig. 4D and E). On the basis of these results, p38MAPK combined with AMPK is likely a key player contributing to metformin's pro-apoptotic and anti-autophagic activities.
RESULTS
- Many studies have suggested a link between type 2 diabetes and an increased risk of cancer [12]. Several metabolic disorders, such as hyperglycemia, hyperinsulinemia, and dyslipidemia in type 2 diabetes are also likely potential risk factors for cancer; however, the correlation between these two diseases has not yet been elucidated [23]. Although a number of reports have indicated the association between cancer risk and hypoglycemic drugs, this link is still controversial [24]. In particular, metformin has gained attention for its anticancer potential [2526]. Metformin, a synthetic biguanide, is the most widely prescribed drug in patients with type 2 diabetes [27]. However, the precise nature of metformin action is not yet known. Metformin is a positively charged molecule. It accumulates within the mitochondrial matrix and inhibits mitochondrial electron transport, ultimately resulting in a reduction in nicotinamide adenine dinucleotide oxidation and ATP synthesis [28]. The reduction of ATP synthesis activates AMPK, and activated AMPK inhibits hepatic gluconeogenesis while stimulating muscular glucose uptake [29]. Many studies have found that metformin inhibits cell proliferation via unique mechanisms in different types of cancer cells [34567]. However, the association between metformin (or AMPK) and hepatocellular carcinoma has not yet been elucidated. Thus, we investigated the role of metformin in the control of cell viability as well as the precise nature of metformin activity in H4IIE hepatocellular carcinoma cells.
- We found that metformin severely reduced the viability of H4IIE cells and stimulated apoptosis in an AMPK-dependent manner. Similar results were shown in a recent study using HepG2 [30], a human hepatocellular carcinoma cell line. The study also showed that metformin results in cell cycle arrest at G0/G1 and decreased BrdU incorporation in an AMPK-dependent manner. However, this study did not provide evidence for metformin's induction of apoptosis. Thus, metformin may exert its anticancer activity via the suppression of DNA synthesis and induction of cell cycle arrest and apoptosis. In particular, the anticancer effect of metformin is completely dependent on AMPK because metformin does not inhibit the growth of AMPK-deficient cells, such as Hela [31], or differentiated normal cells, including L6 myocytes [32]. This hypothesis is further supported by a new report that shows that metformin inhibits the growth of esophageal squamous carcinoma cells (ESCC), but fails to inhibit the growth of normal esophageal epithelial cell lines [33]. However, in ESCC, metformin promotes autophagy as well as apoptosis by down-regulating Stat3 signaling [33]. There are a number of studies on the role of AMPK regarding the control of autophagy. AMPK (or metformin) promotes autophagy in pancreatic β-cells [17], the cerebral artery [18], breast cancer cells [34], colorectal cancer cells [35], and endometrial cancer cells [36]. These findings indicated that the anticancer or anti-proliferative effect of AMPK (or metformin) is partially or completely dependent on autophagy. To date, there are no data showing that metformin inhibits autophagy.
- Our study clearly demonstrates that metformin inhibits autophagy. Under glucose-deprived conditions, metformin decreased the expression levels of six different autophagy-related proteins, including LC3B and beclin-1, in an AMPK-dependent manner. Moreover, inhibition of AMPK by compound C stimulated the formation of acidic intracellular vesicles, a morphological marker of autophagy. To the best of our knowledge, our study is the first report demonstrating that metformin inhibits autophagy but induces apoptosis in hepatocellular carcinoma cells.
- Both autophagy and apoptosis are essential mechanisms for the control of cell death or survival. However, the upstream regulator(s) between autophagy and apoptosis are unknown. Ben et al. [37] hypothesized that the inhibition of autophagy promotes apoptosis in cancer cells with intact apoptotic signaling pathways. Our results correlated with this hypothesis because metformin inhibited autophagy and subsequently decreased cell survival and stimulated various pro-apoptotic markers. However, the precise mechanisms of metformin-regulated autophagy and/or apoptosis in various cancer types or different metabolic environments remains to be further investigated.
- The direct effects of metformin on apoptosis or autophagy are thought to be primarily mediated by AMPK. However, different signaling pathways also play roles in regulating cell death and survival. Members of the MAPK and PI3K/mTOR family play diverse roles in controlling the viability of a number of normal and cancerous cells. Our data showed that the inhibition of autophagy and induction of apoptosis by metformin were primarily dependent on AMPK and were sensitive to compound C. In addition, inhibition of p38MAPK also restored metformin-inhibited cell viability, whereas other inhibitors of the MAPK and PI3K/mTOR signaling pathways did not. In MCF-7 breast cancer cells, metformin activated p38MAPK as well as AMPK and induced apoptosis and cell cycle arrest [38]. Recent studies also suggested the pro-apoptotic activity of p38MAPK in hepatocellular carcinoma cells [39]. Thus, we proposed that p38MAPK also mediates pro-apoptotic activity together with AMPK in H4IIE cells.
- In summary, we report that metformin promotes apoptosis, but inhibits autophagy, in an AMPK-dependent manner under glucose-deprived conditions. Furthermore, p38MAPK mediates metformin's proapoptotic activity; however, the precise mechanism of the autophagy-to-apoptosis association of metformin has not been elucidated and requires further study.
DISCUSSION
-
Acknowledgements
- This work was supported by the National Research Foundation of Korea (NRFK) grant (2010-0022036).
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
NOTES
- 1. Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 2012;2:778-790. ArticlePubMedPDF
- 2. Park HK. Metformin and cancer in type 2 diabetes. Diabetes Metab J 2013;37:113-116. ArticlePubMedPMC
- 3. Rizos CV, Elisaf MS. Metformin and cancer. Eur J Pharmacol 2013;705:96-108. ArticlePubMed
- 4. Ashinuma H, Takiguchi Y, Kitazono S, Kitazono-Saitoh M, Kitamura A, Chiba T, Tada Y, Kurosu K, Sakaida E, Sekine I, Tanabe N, Iwama A, Yokosuka O, Tatsumi K. Antiproliferative action of metformin in human lung cancer cell lines. Oncol Rep 2012;28:8-14. PubMed
- 5. Kato K, Gong J, Iwama H, Kitanaka A, Tani J, Miyoshi H, Nomura K, Mimura S, Kobayashi M, Aritomo Y, Kobara H, Mori H, Himoto T, Okano K, Suzuki Y, Murao K, Masaki T. The antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivo. Mol Cancer Ther 2012;11:549-560. ArticlePubMedPDF
- 6. Cantrell LA, Zhou C, Mendivil A, Malloy KM, Gehrig PA, Bae-Jump VL. Metformin is a potent inhibitor of endometrial cancer cell proliferation: implications for a novel treatment strategy. Gynecol Oncol 2010;116:92-98. ArticlePubMed
- 7. Du Y, Zheng H, Wang J, Ren Y, Li M, Gong C, Xu F, Yang C. Metformin inhibits histone H2B monoubiquitination and downstream gene transcription in human breast cancer cells. Oncol Lett 2014;8:809-812. ArticlePubMedPMC
- 8. Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG, Wong KK, Saxena NK, Biswal S, Girnun GD. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila) 2012;5:544-552. ArticlePubMedPMCPDF
- 9. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 2012;13:251-262. ArticlePubMedPMCPDF
- 10. Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. Mol Cells 2013;36:279-287. ArticlePubMedPMCPDF
- 11. Emami Riedmaier A, Fisel P, Nies AT, Schaeffeler E, Schwab M. Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharmacol Sci 2013;34:126-135. ArticlePubMed
- 12. Quan W, Lee MS. Role of autophagy in the control of body metabolism. Endocrinol Metab (Seoul) 2013;28:6-11. ArticlePubMedPMC
- 13. Gonzalez CD, Lee MS, Marchetti P, Pietropaolo M, Towns R, Vaccaro MI, Watada H, Wiley JW. The emerging role of autophagy in the pathophysiology of diabetes mellitus. Autophagy 2011;7:2-11. ArticlePubMedPMC
- 14. Lin F, Yan W, Wen T, Wu GY. Metformin induces apoptosis in hepatocellular carcinoma Huh-7 cells in vitro and its mechanism. Zhonghua Zhong Liu Za Zhi 2013;35:742-746. PubMed
- 15. Miyoshi H, Kato K, Iwama H, Maeda E, Sakamoto T, Fujita K, Toyota Y, Tani J, Nomura T, Mimura S, Kobayashi M, Morishita A, Kobara H, Mori H, Yoneyama H, Deguchi A, Himoto T, Kurokohchi K, Okano K, Suzuki Y, Murao K, Masaki T. Effect of the anti-diabetic drug metformin in hepatocellular carcinoma in vitro and in vivo. Int J Oncol 2014;45:322-332. ArticlePubMed
- 16. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63. ArticlePubMed
- 17. Jiang Y, Huang W, Wang J, Xu Z, He J, Lin X, Zhou Z, Zhang J. Metformin plays a dual role in MIN6 pancreatic beta cell function through AMPK-dependent autophagy. Int J Biol Sci 2014;10:268-277. ArticlePubMedPMC
- 18. Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD, Tan L. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 2014;171:3146-3157. ArticlePubMedPMC
- 19. He C, Zhu H, Li H, Zou MH, Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes 2013;62:1270-1281. ArticlePubMedPMCPDF
- 20. Duan X, Ponomareva L, Veeranki S, Choubey D. IFI16 induction by glucose restriction in human fibroblasts contributes to autophagy through activation of the ATM/AMPK/p53 pathway. PLoS One 2011;6:e19532ArticlePubMedPMC
- 21. Marambio P, Toro B, Sanhueza C, Troncoso R, Parra V, Verdejo H, Garcia L, Quiroga C, Munafo D, Diaz-Elizondo J, Bravo R, Gonzalez MJ, Diaz-Araya G, Pedrozo Z, Chiong M, Colombo MI, Lavandero S. Glucose deprivation causes oxidative stress and stimulates aggresome formation and autophagy in cultured cardiac myocytes. Biochim Biophys Acta 2010;1802:509-518. ArticlePubMed
- 22. Carroll B, Korolchuk VI, Sarkar S. Amino acids and autophagy: cross-talk and co-operation to control cellular homeostasis. Amino Acids 2015;47:2065-2088. ArticlePubMedPDF
- 23. Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer 2009;16:1103-1123. ArticlePubMed
- 24. Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766-1777. ArticlePubMedPDF
- 25. Taubes G. Cancer research. Cancer prevention with a diabetes pill? Science 2012;335:29ArticlePubMed
- 26. Chung HH, Moon JS, Yoon JS, Lee HW, Won KC. The relationship between metformin and cancer in patients with type 2 diabetes. Diabetes Metab J 2013;37:125-131. ArticlePubMedPMC
- 27. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854-865. ArticlePubMed
- 28. El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem 2000;275:223-228. ArticlePubMed
- 29. Musi N. AMP-activated protein kinase and type 2 diabetes. Curr Med Chem 2006;13:583-589. ArticlePubMed
- 30. Cheng J, Huang T, Li Y, Guo Y, Zhu Y, Wang Q, Tan X, Chen W, Zhang Y, Cheng W, Yamamoto T, Jing X, Huang J. AMP-activated protein kinase suppresses the in vitro and in vivo proliferation of hepatocellular carcinoma. PLoS One 2014;9:e93256ArticlePubMedPMC
- 31. Xiao X, He Q, Lu C, Werle KD, Zhao RX, Chen J, Davis BC, Cui R, Liang J, Xu ZX. Metformin impairs the growth of liver kinase B1-intact cervical cancer cells. Gynecol Oncol 2012;127:249-255. ArticlePubMedPMC
- 32. Vytla VS, Ochs RS. Metformin increases mitochondrial energy formation in L6 muscle cell cultures. J Biol Chem 2013;288:20369-20377. ArticlePubMedPMC
- 33. Feng Y, Ke C, Tang Q, Dong H, Zheng X, Lin W, Ke J, Huang J, Yeung SC, Zhang H. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis 2014;5:e1088ArticlePubMedPMCPDF
- 34. Sanchez-Alvarez R, Martinez-Outschoorn UE, Lamb R, Hulit J, Howell A, Gandara R, Sartini M, Rubin E, Lisanti MP, Sotgia F. Mitochondrial dysfunction in breast cancer cells prevents tumor growth: understanding chemoprevention with metformin. Cell Cycle 2013;12:172-182. ArticlePubMedPMC
- 35. Din FV, Valanciute A, Houde VP, Zibrova D, Green KA, Sakamoto K, Alessi DR, Dunlop MG. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology 2012;142:1504-1515.e3. ArticlePubMedPMC
- 36. Takahashi A, Kimura F, Yamanaka A, Takebayashi A, Kita N, Takahashi K, Murakami T. Metformin impairs growth of endometrial cancer cells via cell cycle arrest and concomitant autophagy and apoptosis. Cancer Cell Int 2014;14:53ArticlePubMedPMC
- 37. Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y, Giorgetti-Peraldi S, Cormont M, Bertolotto C, Deckert M, Auberger P, Tanti JF, Bost F. Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res 2010;70:2465-2475. ArticlePubMedPDF
- 38. Queiroz EA, Puukila S, Eichler R, Sampaio SC, Forsyth HL, Lees SJ, Barbosa AM, Dekker RF, Fortes ZB, Khaper N. Metformin induces apoptosis and cell cycle arrest mediated by oxidative stress, AMPK and FOXO3a in MCF-7 breast cancer cells. PLoS One 2014;9:e98207ArticlePubMedPMC
- 39. Zhang Z, Zhang C, Ding Y, Zhao Q, Yang L, Ling J, Liu L, Ji H, Zhang Y. The activation of p38 and JNK by ROS, contribute to OLO-2-mediated intrinsic apoptosis in human hepatocellular carcinoma cells. Food Chem Toxicol 2014;63:38-47. ArticlePubMed
REFERENCES
Fig. 1
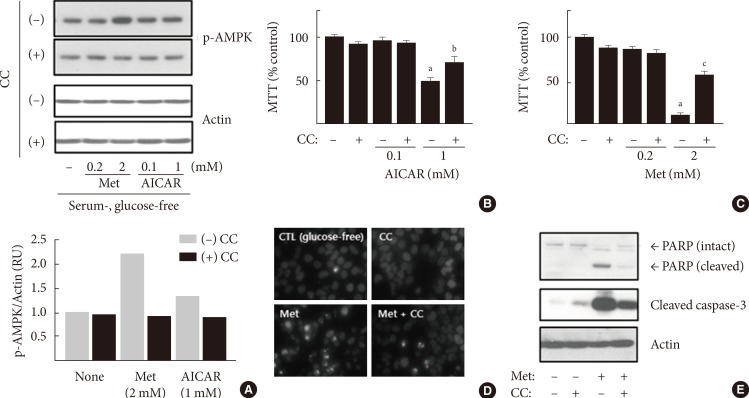
Metformin (Met) inhibits cell viability and induces apoptosis in H4IIE cells. (A) H4IIE cells were pre-incubated in serum-free Dulbecco's minimal essential medium (DMEM, 1 g/L glucose) for 24 hours and then pretreated with compound C (CC, 10 µM) in glucose-free DMEM (GFM) for 30 minutes. Cells were further treated with Met and 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) for 4 hours (A) or 24 hours (B-E). The levels of phospho-AMP-activated protein kinase (p-AMPK) and actin were detected by Western blotting analyses, and the density of p-AMPK was normalized against actin. Each bar represents the average value of the duplicated experiments (n=2) (A). Cell viability was analyzed using the 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) assay, and each bar represents the mean±standard error (n=3) (B, C). The onset of apoptosis was detected using H33342 staining to observe nuclear chromatin condensation (D) and Western blotting analyses for poly ADP ribose polymerase (PARP) and cleaved caspase-3 (E). CTL, control. aP<0.001 vs. the non-treated control, bP<0.05 vs. cells treated with 1 mM AICAR, cP<0.001 vs. cells treated with 2 mM Met.

Fig. 2

Inhibition of AMP-activated protein kinase stimulates the formation of acidic intracellular vesicles, a marker of the onset of autophagy, and protected metformin-induced apoptosis in glucose-free DMEM (GFM), but not in amino acid (AA)-free medium. H4IIE cells were pre-incubated in serum-free Dulbecco's minimal essential medium (DMEM, 1 g/L glucose) for 24 hours and then pretreated with compound C (CC, 10 µM) in fresh GFM (A, B), AA-free Hank's-balanced salt solution (1 g/L glucose) (C, D), or AA-containing DMEM (1 g/L glucose) (D) for 30 minutes. Cells were further treated with 2 mM metformin (Met) and 1 mM 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR, AIC) for 24 hours. After treatment, cells were stained with AO and H33342 (A-C) and subjected to the 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) assay (D). Arrows (B) indicates condensed chromatin (apoptotic bodies). Each bar represents the mean±standard error (n=3) (D). CTL, control. aP<0.05 vs. the non-treated control (dark bar), bP<0.05 vs. cells treated with 2 mM Met (dark bar), cP<0.01 vs. the non-treated control (white bar).

Fig. 3
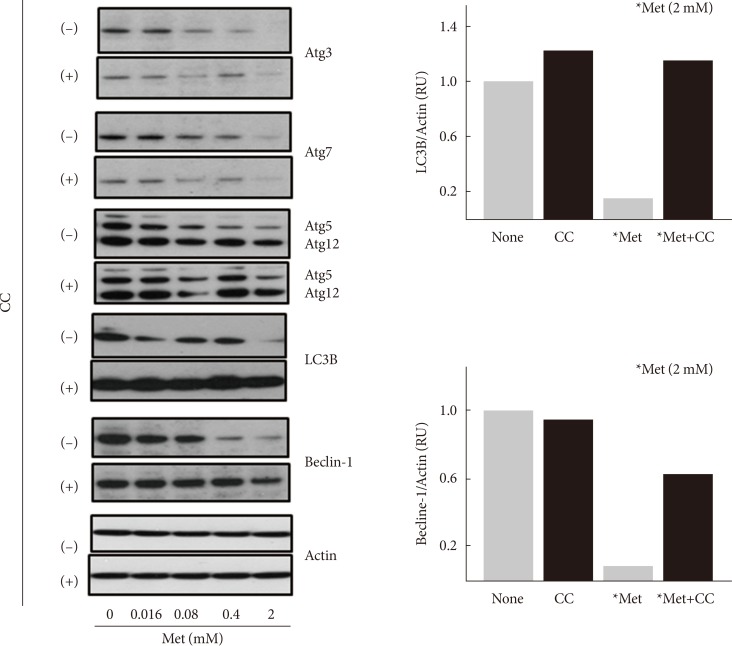
Metformin decreases the expression levels of autophagy-related proteins. H4IIE cells were pre-incubated in serum-free Dulbecco's minimal essential medium (DMEM, 1 g/L glucose) for 24 hours and then pretreated with compound C (CC, 10 µM) in glucose-free DMEM (GFM) for 30 minutes. Cells were further treated with metformin (Met, 0 to 2 mM) for 24 hours and subjected to Western blotting analyses. The density of light chain 3B (LC3B) or beclin-1 was normalized against actin. Each bar represents the average value of the duplicated experiments (n=2).

Fig. 4

Inhibition of p38 mitogen-activated protein kinase (p38MAPK) as well as AMP-activated protein kinase protected cells from apoptosis induced by metformin (Met). H4IIE cells were pre-incubated in serum-free Dulbecco's minimal essential medium (DMEM, 1 g/L glucose) for 24 hours and then pretreated with various inhibitors against signaling proteins (20 µM compound C [CC], 50 µM SB202190, 50 µM SP600125, 10 µM U0126, 100 nM rapamycin, and 100 nM wortmannin) for 30 minutes. Cells were further treated 1 mM Met for 24 hours. After the treatments, the protein levels of cleaved caspase-3, beclin-1, light chain 3B (LC3B), and actin were detected using Western blotting analyses (A, C, D), and cell viability was analyzed using the 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) assay (B, E). Each bar represents the mean±standard error (n=3) (B, E). Rapa, rapamycin; SB, SB202190; SP, SP600125; U, U0126; Wt, wortmannin. aP<0.01 vs. cells treated with 2 mM Met (n=3) (B).

Figure & Data
References
Citations
Citations to this article as recorded by 

- Metformin Induces Lipogenesis and Apoptosis in H4IIE Hepatocellular
Carcinoma Cells
Deokbae Park, Sookyoung Lee, Hyejin Boo
Development & Reproduction.2023; 27(2): 77. CrossRef - Novel phloretin-based combinations targeting glucose metabolism in hepatocellular carcinoma through GLUT2/PEPCK axis of action: in silico molecular modelling and in vivo studies
Alaa Elmetwalli, Neamat H. Kamosh, Rania El Safty, Amany I. Youssef, Mohammed M. Salama, Khaled M. Abd El-Razek, Tarek El-Sewedy
Medical Oncology.2023;[Epub] CrossRef - Targeted Pyroptosis Is a Potential Therapeutic Strategy for Cancer
Hao Wu, Dianlun Qian, Xiangfeng Bai, Shibo Sun, Jayaprakash Narayana Kolla
Journal of Oncology.2022; 2022: 1. CrossRef - The effects of metformin on autophagy
Guangli Lu, Zhen Wu, Jia Shang, Zhenxing Xie, Chaoran Chen, Chuning zhang
Biomedicine & Pharmacotherapy.2021; 137: 111286. CrossRef - Protective Effect of Metformin against Hydrogen Peroxide-Induced Oxidative Damage in Human Retinal Pigment Epithelial (RPE) Cells by Enhancing Autophagy through Activation of AMPK Pathway
Xia Zhao, Linlin Liu, Yizhou Jiang, Marta Silva, Xuechu Zhen, Wenhua Zheng
Oxidative Medicine and Cellular Longevity.2020; 2020: 1. CrossRef Metformin Induces Autophagy via the AMPK-mTOR Signaling Pathway in Human Hepatocellular Carcinoma Cells
Chun Gao, Long Fang, Hui Zhang, Wei-Shuo Zhang, Xiao-Ou Li, Shi-Yu Du
Cancer Management and Research.2020; Volume 12: 5803. CrossRef- Metabolomics profiling of metformin-mediated metabolic reprogramming bypassing AMPKα
Min Yan, Huan Qi, Tian Xia, Xinjie Zhao, Wen Wang, Zhichao Wang, Chang Lu, Zhen Ning, Huan Chen, Tongming Li, Dinesh Singh Tekcham, Xiumei Liu, Jing Liu, Di Chen, Xiaolong Liu, Guowang Xu, Hai-long Piao
Metabolism.2019; 91: 18. CrossRef - Metformin Induces Oxidative Stress-Mediated Apoptosis without the Blockade of Glycolysis in H4IIE Hepatocellular Carcinoma Cells
Deokbae Park
Biological and Pharmaceutical Bulletin.2019; 42(12): 2002. CrossRef - Activation of AMPK prevents monocrotaline-induced pulmonary arterial hypertension by suppression of NF-κB-mediated autophagy activation
Cui Zhai, Wenhua Shi, Wei Feng, Yanting Zhu, Jian Wang, Shaojun Li, Xin Yan, Qingting Wang, Qianqian Zhang, Limin Chai, Cong Li, Pengtao Liu, Manxiang Li
Life Sciences.2018; 208: 87. CrossRef - Metformin and epothilone A treatment up regulate pro-apoptotic PARP-1, Casp-3 and H2AX genes and decrease of AKT kinase level to control cell death of human hepatocellular carcinoma and ovary adenocarcinoma cells
Aneta Rogalska, Barbara Bukowska, Agnieszka Marczak
Toxicology in Vitro.2018; 47: 48. CrossRef - Quantitative assessment of cell fate decision between autophagy and apoptosis
Bing Liu, Zoltán N. Oltvai, Hülya Bayır, Gary A. Silverman, Stephen C. Pak, David H. Perlmutter, Ivet Bahar
Scientific Reports.2017;[Epub] CrossRef - Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients
Shujuan Ma, Yixiang Zheng, Yanni Xiao, Pengcheng Zhou, Hongzhuan Tan
Medicine.2017; 96(19): e6888. CrossRef - ROS Production and ERK Activity Are Involved in the Effects of d-β-Hydroxybutyrate and Metformin in a Glucose Deficient Condition
Santosh Lamichhane, Tonking Bastola, Ramesh Pariyar, Eun-Sol Lee, Ho-Sub Lee, Dae Lee, Jungwon Seo
International Journal of Molecular Sciences.2017; 18(3): 674. CrossRef - Metformin represses glucose starvation induced autophagic response in microvascular endothelial cells and promotes cell death
Samson Mathews Samuel, Suparna Ghosh, Yasser Majeed, Gnanapragasam Arunachalam, Mohamed M. Emara, Hong Ding, Chris R. Triggle
Biochemical Pharmacology.2017; 132: 118. CrossRef - NHX-5, an Endosomal Na+/H+ Exchanger, Is Associated with Metformin Action
Jeongho Kim, Hye-Yeon Lee, Jheesoo Ahn, Moonjung Hyun, Inhwan Lee, Kyung-Jin Min, Young-Jai You
Journal of Biological Chemistry.2016; 291(35): 18591. CrossRef - Metformin in pancreatic cancer treatment: from clinical trials through basic research to biomarker quantification
Archana Bhaw-Luximon, Dhanjay Jhurry
Journal of Cancer Research and Clinical Oncology.2016; 142(10): 2159. CrossRef - Metformina: stary lek w nowej aplikacji
Anna Dmoszyńska, Monika Podhorecka, Krzysztof Giannopoulos
Acta Haematologica Polonica.2016; 47(2): 139. CrossRef

 KDA
KDA PubReader
PubReader Cite
Cite