Selective Immunoglobulin A Deficiency in Type 1 Diabetes Mellitus: A Prevalence Study in Western Sicily (Italy)
Article information
Abstract
Background
The association between type 1 diabetes and immunoglobulin A deficiency (IgA-D) has long been recognized in many populations. The aim of this study was to assess the prevalence of IgA-D in patients with type 1 diabetes mellitus all coming from a defined geographical area and to investigate the clinical features of these subjects.
Methods
The records of 150 consecutive patients with type 1 diabetes mellitus referred in a period of one year were analyzed. A detailed history was obtained for each patient. Information was collected concerning age, gender, time of onset of diabetes, and presence of other autoimmune diseases.
Results
Out of 150 patients with type 1 diabetes, eight (5.3%) had a diagnosis of IgA-D. There were one female and seven male; all these patients were diagnosed by screening: none of them had history of recurrent infections. Autoimmune thyroiditis was coexisting in five patients (62%). Although other associated autoimmune disorders were found in a number of patients, there was no different prevalence rate in IgA deficient patients.
Conclusion
This study shows the prevalence of IgA-D in Sicilian patients with type 1 diabetes as 5.3% which is much higher than reported in other Italian studies. Moreover, our data show a high prevalence of IgA-D in male gender and describe thyroiditis as the most frequent autoimmune disease present in these patients. Finally, in our case report, IgA-D diagnosis always followed routine IgA measurement when case finding for celiac disease with no history of recurrent infections in each patient.
INTRODUCTION
Selective immunoglobulin A deficiency (IgA-D) is the most common primary immunodeficiency disease but its prevalence varies widely within different geographical regions [1,2,3]. The association between type 1 diabetes and IgA-D has long been recognized in many populations: in several studies of children and adults with type 1 diabetes, a high prevalence of IgA-D has been found [1,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Although no cases were observed in some studies [4,11,15], the prevalence rates of IgA-D in subjects with type 1 diabetes are estimated to be between 0.4% and 5.4% which is more than 10 times the prevalence in the general population.
Many cases of IgA-D are asymptomatic or have features that are only recognized retrospectively; however, in diabetic patients, because of the availability of serological screening for celiac disease (CD), more cases of IgA-D are now being diagnosed.
There are few reports in literature regarding IgA-D in type 1 diabetes mellitus in Italian population [7,10,15]; furthermore, data published derived largely from studies designed to investigate the association between CD and diabetes rather than IgA-D and conducted principally in the paediatric age group [7,8,9,10].
The aims of this study were to estimate the prevalence of IgA-D among a population of children and adults with type 1 diabetes all coming from a defined geographical area in Western Sicily, to examine the association of other diseases with IgA-D and diabetes and to investigate how IgA-D presents among subjects with type 1 diabetes in term of its clinical feature and time course of development.
METHODS
We retrospectively studied the patients with type 1 diabetes mellitus, children and adults, referred to the Diabetes Unit of the "Paolo Borsellino" Hospital, Marsala (Italy), during a period of 1 year. All patients with type 1 diabetes, as diagnosed by the American Diabetes Association criteria [23], attended the outpatient clinic of our division every 3 to 4 months as part of their routine follow-up. Our current clinical practice is to obtain thyroid stimulating hormone, thyroid hormone levels, serum immunoglobulin A (IgA) levels, thyroperoxidase antibodies, and tissue transglutaminase antibodies (TTG-Ab) every 1 to 2 years or with symptoms of thyroid or gastrointestinal dysfunction.
We have reviewed the clinical records of all type 1 diabetic patients who were consecutively admitted to our Unit, most frequently at its ambulatory facility, from July 2013 to June 2014.
Clinical records were hand-written charts and/or computer stored documents. A detailed history was obtained for each patient. Information was collected concerning age, gender, time of onset of diabetes, and presence of other autoimmune diseases. Serum IgA and other immunoglobulin levels were measured using an immunoturbidimetric assay.
We defined IgA-D based on a IgA value <0.07 g/L, with normal immunoglobulin M (IgM) and immunoglobulin G (IgG) levels, in individuals >4 years of age, in accordance with the recommendations of the International Union of Immunological Societies Expert Committee on Primary Immunodeficiences [24]. Diagnosis of CD was considered in symptomatic or asymptomatic patients with elevated IgA TTG-Ab and with positive duodenal biopsy.
Data were expressed as mean±standard deviation and/or as percentage. For comparison between groups, Student t-test and the chi-square test were used according to the characteristics of the variables analyzed. Statistical significance was posted at level P<0.05.
RESULTS
During the period of the survey 150 type 1 diabetic patients presented to consultations at our metabolic service. Among these patients 73 were males (48.6%) and 77 were females; the mean age at referral was 23.4±11.2 years while age at diabetes onset was 14.7±9.9 years.
Out of these patients, eight (5.3%) met the diagnostic criteria for IgA-D. There were seven males (87%) and one female; mean age at referral was 25.8±14.2 years (range, 8 to 52 years) and age at their diabetes onset was 15.3±12.7 years (range, 6 to 44 years). Demographic and clinical characteristics of all subjects with and without IgA-D are shown in Table 1. IgA-D was significantly prevalent in male gender (P<0.05).

Demographic characteristics and laboratory findings of type 1 diabetic probands with and without IgA-D
IgA-D patients had an age at referral and an age at diabetes onset not significantly different versus others. IgG and IgM levels were not significant different between groups as well as C-peptide and hemoglobin A1c (HbA1c) levels. In all the patients, IgA-D was diagnosed by screening; on the basis of clinical charts, none of them had a history of recurrent infections.
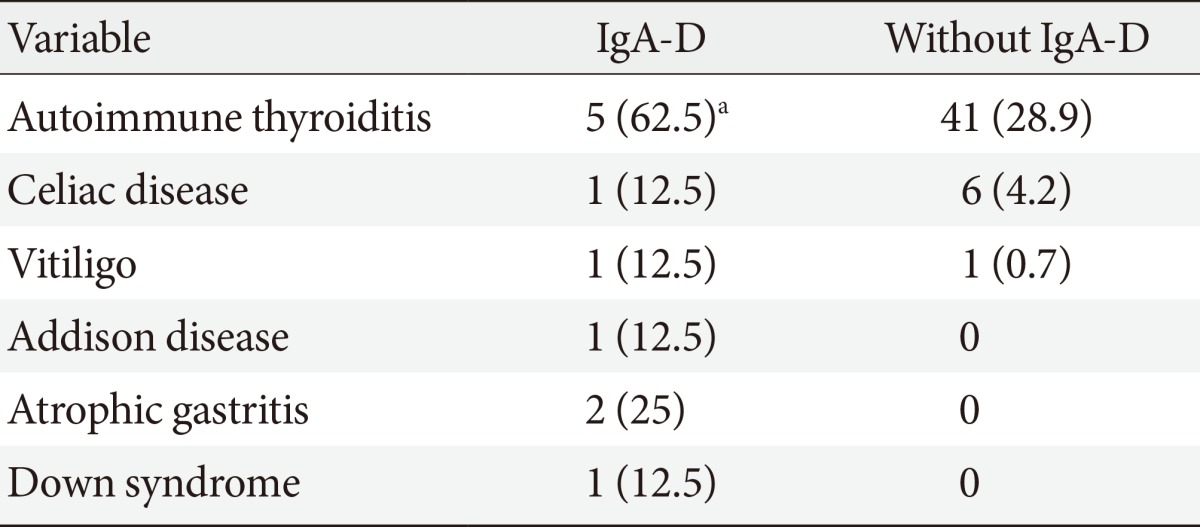
Table 2 gives associated diseases. Prevalence of autoimmune thyroiditis was significantly higher in IgA-D patients: it was present in the female and in four male patients with IgA-D (62%); thyroid status was euthyroidism in three subjects and overt hypothyroidism in two subjects. No significant difference was present for other autoimmune diseases (Table 2).
It is noteworthy that in three male subjects, IgA-D was coexisting with more than one autoimmune disease: the first patient was affected by Down syndrome and also by hypothyroidism and CD; the second patient was affected by Addison disease and was also hypothyroid; the third patient was affected by atrophic gastritis and vitiligo.
DISCUSSION
An increased prevalence of IgA-D has been documented in a number of autoimmune diseases [1,2,3]; particularly, IgA-D has been associated with CD leading to false negative screening serological testing. It is well known that CD is more prevalent in type 1 diabetic patients [25,26,27]: for this reason, various scientific societies have recommended that IgA testing is necessary when case-finding for CD in type 1 diabetes mellitus.
Many studies have examined the association between IgA-D and type 1 diabetes mellitus: although a higher prevalence of IgA-D has been reported in screened diabetes clinic populations, frequencies largely differ among reports [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22].
In our study, performed in the Western part of Sicily (Italy), the presence of IgA-D was found in eight out of the 150 subjects (children and adult patients) with type 1 diabetes who had been followed up regularly at our diabetes unit in a 1-year period; therefore, we found IgA-D in 5.3% of our patients, a higher percentage than reported in the review of Wang et al. [1] and in the majority of similar studies.
Moreover, prevalence of IgA-D in our case report is higher than previously described by Cerutti et al. [7] by Picarelli et al. [15] in Italy, although in another Italian study regarding children with type 1 diabetes, the prevalence rate of IgA-D was similar [10]. However, our results are hardly comparable with those of previous studies: study design, age and number of patients, diagnosis criteria of IgA-D, genetic and geographical background can explain the differences across studies; on the other hand, data published have emanated largely from paediatric referral departments rather than general diabetes centres and principally derived from studies designed to investigate association between CD and type 1 diabetes rather than IgA-D.
Our data show IgA-D as more prevalent in male subjects with type 1 diabetes than in females. To our knowledge there are no data in the literature on the above topic. In a recent large population-based study on the association between IgA-D and other autoimmune conditions, 57% of all IgA deficient patients were women, but gender distribution in diabetic subjects were not reported [3]. At the same manner, the majority of the available studies on this topic, do not describe gender distribution of the subjects with both type 1 diabetes and IgA-D; only in the recent work of Sayarifard et al. [21], the two subjects with IgA-D were reported both male. It is known that type 1 diabetes is equally prevalent among males and females in most populations. This unequal gender distribution of IgA-D prevalence in patients with diabetes suggests that etiological risk factors for developing this immunodeficiency could not be the same as for type 1 diabetes.
In our case reports, IgA-D diagnosis typically follows the diagnosis of diabetes: in our patients, diagnosis occurs through the screening for CD performed at diabetes onset or in the follow-up.
Theoretically, precocious identifying of IgA-D in diabetic patients can prevent the vicious cycle of recurrent infections and decrease risk for morbidity and metabolic decompensation. However, in a large proportion of patients with type 1 diabetes, IgA-D is asymptomatic, subclinical or may be characterized by atypical features, recognized only retrospectively. Obviously it is easy to suppose that in asymptomatic patients it is difficult to miss a diagnosis of the disease because screening protocols are by now universally recommended and performed. Accordingly, none of our patients with IgA-D presented classical symptoms of the disease: in particular they did not present any history of recurrent gastrointestinal or respiratory infections and their HbA1c levels were similar to that of subjects without IgA-D: these outcomes question the practice of routine IgA measurement in these patients. These findings are in agreement with the study by Sayarifard et al. [21] that indicated IgA-D did not correlate with either increased rate of infections or HbA1c levels. On the other hand, IgA-D has been linked to an excess of mortality in the first 10 to 15 years after diagnosis [28]. Therefore, although controversial, precocious identifying of IgA-D in diabetic patients and long-term monitoring of their immune and clinical status as well as prompt and appropriate treatment of infectious processes could contribute to decrease morbidity and mortality in this group of patients. Thus, the recommendation for patients with type 1 diabetes is to screen at the time of diabetes diagnosis even in the absence of clinical signs.
An interesting finding in our study was the coexistence of IgA-D and thyroid autoimmunity in many type 1 diabetic patients. We have found a very high prevalence of autoimmune thyroiditis in the group of patients with type 1 diabetes and IgA-D (62%).
It is well known that patients with type 1 diabetes as well as patients with selective IgA-D generally show a higher prevalence of autoimmune thyroid disease, but at this time no data were published with respect to the prevalence of autoimmune thyroid disease in subjects with both type 1 diabetes and IgA-D.
Finally, it is noteworthy the presence of one case of Down syndrome in our IgA-D patients. It is well known that the prevalence of autoimmune diseases such as autoimmune thyroiditis, type 1 diabetes mellitus, and CD is higher in patients with Down syndrome than in the general population; however, no case reports in the literature describe the simultaneous occurrence of type 1 diabetes, CD, IgA-D, and hypothyroidism in children with Down syndrome as in our patient.
The present study clearly has several limitations. The lack of a control group of nondiabetic subjects in the same geographical area is a major limitation of the study as well as the small number of subjects enrolled. The age of the patients was highly variable and the duration of diabetes ranged widely. However, it is difficult to accumulate an adequate number of subjects, since patients with concurrent type 1 diabetes and IgA-D are relatively rare. Another major limitation of the study is the retrospective nature of design. However, in order to clarify the exact role of IgA-D coexistence in the evolution and treatment of type 1 diabetes, these findings represent preliminary data that require further prospective studies with long observation periods and larger cohorts.
In conclusion, our data confirm, in a Sicilian population, the not unusual association between IgA-D and type 1 diabetes, particularly in male gender, with a prevalence rate of 5.3%. Thyroid disease is the other autoimmune disease that coexists with a high prevalence in our patients. IgA-D diagnosis typically follows diagnosis of type 1 diabetes and occurs through the screening protocols for CD. Since patients with diabetes often have few or no symptoms associated with IgA-D, screening remains the best tool for detecting all clinically silent forms. Although further studies are needed for better understanding the effects of IgA-D on diabetes control and on morbidity and mortality, the high prevalence of IgA-D in subjects with type 1 diabetes, in accordance with the large data published in the literature, underlines the need of a systematic screening of IgA-D at diabetes diagnosis in order to avoid false negative in CD search and to promptly start appropriate prevention strategies to a theoretical major infections susceptibility.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.