- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 38(6); 2014 > Article
-
Original ArticleClinical Care/Education Arterial Stiffness by Aerobic Exercise Is Related with Aerobic Capacity, Physical Activity Energy Expenditure and Total Fat but not with Insulin Sensitivity in Obese Female Patients with Type 2 Diabetes
- Ji Yeon Jung1, Kyung Wan Min2, Hee Jung Ahn, Hwi Ryun Kwon1, Jae Hyuk Lee2, Kang Seo Park2, Kyung Ah Han2
-
Diabetes & Metabolism Journal 2014;38(6):439-448.
DOI: https://doi.org/10.4093/dmj.2014.38.6.439
Published online: December 15, 2014
1Diabetes Center, Eulji General Hospital, Seoul, Korea.
2Department of Internal Medicine, Eulji University School of Medicine, Daejeon, Korea.
- Corresponding author: Kyung Ah Han. Department of Internal Medicine, Eulji University School of Medicine, 77 Gyeryong-ro 771beon-gil, Jung-gu, Daejeon 301-746, Korea. hka1114@yahoo.co.kr
- *Ji Yeon Jung and Kyung Wan Min contributed equally to this study as first authors.
Copyright © 2014 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Arterial stiffness is an important factor in atherosclerosis. Thus we examined whether aerobic exercise could reduce arterial stiffness in obese women with type 2 diabetes without diabetic complication.
-
Methods
- A total of 35 women with type 2 diabetes (body mass index, 26.6±2.8 kg/m2; age, 56.4±1.9 years; duration of diabetes, 4.7±4.8 years) were assigned to aerobic exercise group (AEG) or control group (CG). AEG completed a 12-week exercise program (3.6 to 5.2 metabolic equivalents, 3 day/week, 60 min/day), with their exercise activities monitored by accelerometers. We measured abdominal total fat area (TFA), visceral fat area (VFA), and subcutaneous fat area (SFA) by computed tomography, insulin sensitivity by insulin tolerance test (KITT), and augmentation index (AIx) by SphygmoCor at baseline and at the end of the 12-week program.
-
Results
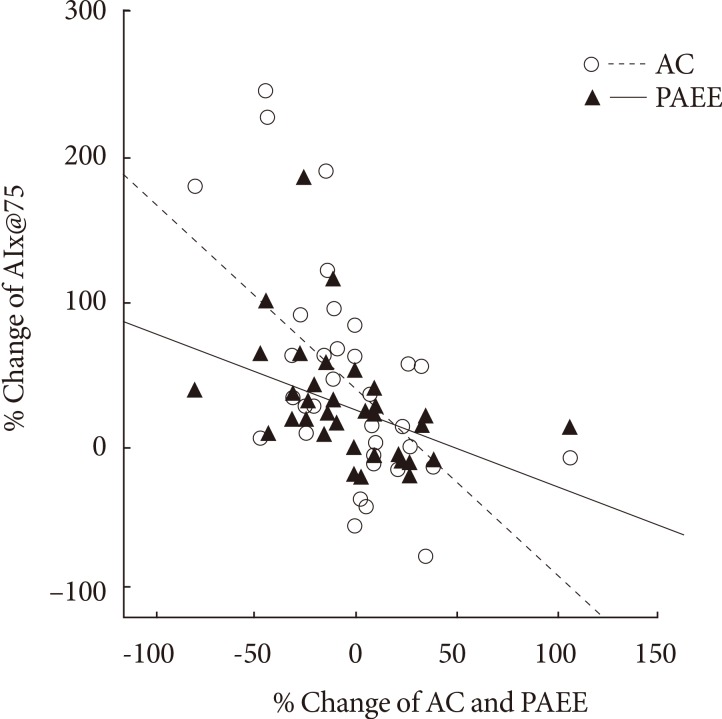
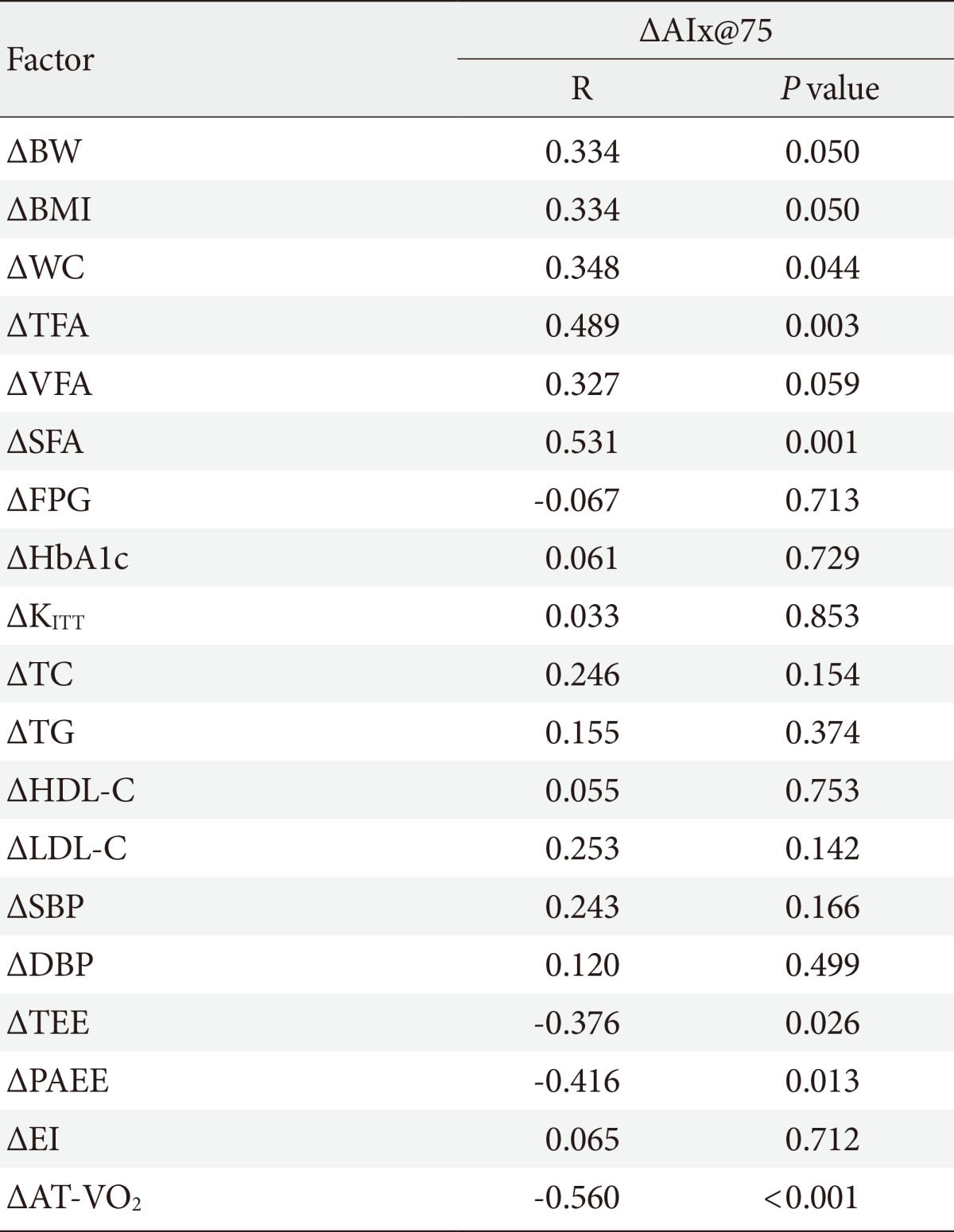
- The AIx was improved in the AEG compared with the CG (P<0.001). The percent change of AIx had significant correlation with the improvement of physical activity energy expenditure (PAEE), aerobic capacity, TFA, and SFA (r=-0.416, P=0.013; r=0.560, P<0.001; r=0.489, P=0.003; r=0.531, P=0.001, respectively), but not with insulin sensitivity, energy intake, or VFA.
-
Conclusion
- Improvement in aortic stiffness by aerobic exercise is related with the improvement of aerobic capacity, PAEE, and total fat but not with insulin sensitivity or energy intake in obese women with type 2 diabetes.
- Diabetes is major global public health concern that affects 347 million people have diabetes worldwide [1], which is correlated with increasing cardiovascular disease that is responsible for 52% deaths in type 2 diabetes [2]. In addition, people with type diabetes are also at a 2 to 4 times higher risk of developing cardiovascular diseases compared with the normal population [3]. The highest prevalence of cardiovascular disease occurred among people with diabetes when hyperglycemia is accompanied by other risk factors such as hypertension, dyslipidemia, hyperinsulinemia, and obesity [4]. Especially, obesity is an independent risk factor for cardiovascular diseases [5]. However, reducing weight in obese patients with type 2 diabetes has been shown to be effective in minimizing cardiovascular disease-related deaths [5]. Therefore, losing weight in obese patients with type 2 diabetes will be effective in preventing cardiovascular diseases. In patients with type 2 diabetes, increased arterial stiffness is regarded as an early stage of arteriosclerosis and it is also regarded as a risk factor and prognostic marker for cardiovascular diseases [6]. Recently, a method of measuring augmentation index (AIx) and central aortic pressure noninvasively by analyzing the pulse waves of peripheral artery was introduced [7], and a large randomized study reported that AIx and central aortic pressure are associated with the development of cardiovascular diseases [8].
- It is well known that adequate exercise, regardless of any changes in weight, independently improves cardiac function and arterial compliance, either preventing or stopping the progression of coronary artery diseases [9]. Kingwell et al. [10] compared the pulse wave velocity (PWV) of athletes and normal people and found that regular and constant exercise decreases PWV, and DeSouza et al. [11] showed that healthy adults who participated in moderate aerobic exercises for 2 to 3 months had improved arterial intima-media thickness and arterial compliance. The subjects of previous studies regarding arterial compliance only included normal population or patients with hypertension. However arterial stiffness may increase not only by hypertension but also by other risk factors of cardiovascular diseases such as old age, obesity, diabetes, and dyslipidemia. Furthermore, research regarding the correlation between reduced abdominal fat from exercises and improved arterial compliance in diabetic patients is still lacking.
- Therefore, the aims of this study were to (1) examine whether aerobic exercise can alleviate arterial stiffness in patients with type 2 diabetes; (2) identify the correlation between the changes in arterial stiffness and physical activity level, aerobic capacity, abdominal fat, and insulin resistance.
INTRODUCTION
- Study subjects
- A total of 35 type 2 diabetes patients in the Diabetes Center at Eulji Hospital were enrolled from March to July in 2011. Subjects that were 45 to 65 years old and overweight with a body mass index (BMI) over 23 kg/m2 or obese female patients with hemoglobin A1c (HbA1c) levels less than 10% were included in this study. At baseline, 24, 10, and 1 of the 35 patients were under exercise and diet, oral hypoglycemic agent, and insulin treatments, respectively. The exclusion criteria were: type 1 diabetes patients, congestive heart failure, uncontrollable arrhythmia, severe valvular disease, patients with malignant tumor, patients under renal replacement therapy, patients that were difficult to perform tracking observations, patients refusing to be investigated, smokers, and patients who changed the medicine that they were taking. The Institutional Review Board of the Clinical Research Institute at Eulji Hospital approved the study protocol and informed consent was obtained from each subject.
- Study design
- During the 2 weeks of screening period, we requested that participants maintain their daily routine physical activity and dietary intake before beginning of the study. Two weeks later, the subjects were randomized into aerobic exercise group (AEG) or control group (CG). AEG group was performed walking exercise 3 day/week for 60 min/day at an exercise intensity of higher than 4 according to an accelerometer, corresponding to 3 to 5 metabolic equivalents (METs) [8] for 12 weeks, which corresponds to an energy expenditure of approximately 500 kcal/day. During 12 weeks, the subjects were monitored every 2 weeks for accurate measurements of their compliance, energy intake (EI), and physical activity energy expenditure (PAEE).
- Measured categories and methods
- The subjects' height, weight, and waist circumference were measured at baseline and after the 12-week period. The height and weight were measured when subjects were only wearing light clothing, and BMI was calculated by dividing weight (kg) by the square of height (m2). Waist circumference was measured between the lower ribs and iliac crest after exhale with a tape measure, and blood pressure was measured after 10 minutes of rest while sitting with a mercury sphygmomanometer (Yamasu, Tokyo, Japan).
- As for biochemical tests, venous blood was collected 10 hours of fasting, which was centrifuged at 3,000 rpm for 15 minutes to separate the serum that was stored at -70℃ before being analyzed. Fasting glucose was measured by glucose oxidation method and HbA1c level was measured using high-performance liquid chromatography based on ion-exchange resin. Cholesterol, triglyceride, high density lipoprotein cholesterol, and low density lipoprotein cholesterol (LDL-C) were measured by enzyme-linked immunosorbent assay using an automatic chemistry analyzer (Hitachi 7170; Hitachi, Tokyo, Japan).
- As for biochemical tests, venous blood was collected 10 hours of fasting, which was centrifuged at 3,000 rpm for 15 minutes to separate the serum that was stored at -70℃ before being analyzed. Fasting glucose was measured by glucose oxidation method and HbA1c level was measured using high-performance liquid chromatography based on ion-exchange resin. Cholesterol, triglyceride, high density lipoprotein cholesterol, and low density lipoprotein cholesterol (LDL-C) were measured by enzyme-linked immunosorbent assay using an automatic chemistry analyzer (Hitachi 7170; Hitachi, Tokyo, Japan).
- Insulin resistance was calculated using insulin tolerance test (KITT) [12]. After 10 hours of fasting, an insulin tolerance test was performed. A 20-gauge catheter was inserted into a vein in one hand of the subject for blood collection and another catheter into the vein on the other side for insulin injection and glucose infusion at the end of the test. The appropriate insulin concentration (0.1 U per 1 kg) was calculated and prediluted insulin (Humulin R; Eli Lilly, Indianapolis, IN, USA) was injected into the antebrachial vein at resting state, while blood was collected from the vein in the opposite hand at baseline, 3, 6, 9, 12, and 15 minutes afterwards. One hundred milliliter of 20% glucose solution was infused 15 minutes after blood collection to prevent hypoglycemia, while the blood was immediately centrifuged to measure blood glucose levels. The glucose concentrations that were measured at different time points during insulin resistance test were entered into a computer software, where they were converted to each respective natural logarithm and the slope of regression line was measured using the values at 3 to 15 minutes. From this the half-life (t1/2) which is the time taken for baseline glucose level to drop by half was calculated and the rate constant for plasma glucose disappearance (KITT), the insulin resistance index, was obtained using the formula: KITT=0.693/(t1/2)×100 (%/min).
- AIx was acquired by measuring arterial stiffness and pulse wave reflection using SphygmoCor system (SphygmoCoR; AtCor Medical Pty Ltd., Sydney, Austrailia). The pulse pressure (PP) waveform of peripheral artery was measured with applanation tonometer. All measurements were taken after at least 5 minutes of rest while sitting with either right radial artery for right-handed subjects or left transradial coronary artery for left-handed subjects. When 40 continuous waveforms were recorded, a validated transform function was used to obtain the PP waveform of aorta, and PWV was used as an evaluation index for aorta stiffness. At least two measurements per subject were performed. These measurements were checked whether the quality index provided by the software was over 80% and whether the variations between aorta PP waveforms were minimal by three medical doctors, after which only the valid measurements were included.
- Definitions of major hemodynamic indices [13]: (1) Augmentation pressure (AP): maximum blood pressure (mm Hg) at the inflection point of systolic blood pressure; (2) AIx: AIx is this aortic AP expressed as a percentage of aortic PP: AIx=AP/PP×100 (%); (3) high AIx values denote severe aortic stiffness; (4) AIx adjusted to a hear rate of 75% (AIx@75): since AIx is affected by heart rate (HR), it is adjusted to a 'standard HR' of 75 bpm, the SphygomoCor Px software automatically decreases AIx by 4.8% per every 10 bpm rise in HR. This only applies to HR from 40 to 110 bpm.
- The CG did not participate in any exercise programs, while AEG performed 60 minutes of walking exercise at an exercise intensity between 3.6 and 5.2 METs (Lifecorder activity levels 4 to 6) [14] three times per week to consume 500 kcal every day for 12 weeks. Both groups received one dietary education program at the beginning of the intervention.
- To estimate the physical activity level of each subject, an accelerometer (Lifecorder; Suzuken Co., Nagoya, Japan) was used. The age, gender, height, weight, and measurement start date and time of each subject at baseline were entered. All subjects were requested to attach an accelerometer to their belt at all times except during sleep or shower during the intervention period and changes in weight were recorded every 2 weeks. Total energy expenditure (TEE), PAEE, and energy expenditure of each activity intensity levels were analyzed.
- The subjects were instructed to fill out a 3-day meal record (twice during weekdays and once during weekend) and visited the hospital every 4 weeks to review their intake records and investigate their overall dietary intake. All subjects participated in a prior education program about how to fill out the meal record, where they were instructed to record all the food that they have eaten in one day and their ingredients for each meal, as well as any dietary supplements or snacks. To increase the accuracy of the records, professional dietitians used life-size food models (Korea Mirage Replica Inc., Incheon, Korea), measuring cups, measuring spoons, and estimated food size by eye measurements of food photos (The Korean Dietetic Association, 1999) to review the meal records with the subjects when the dietary questionnaires were collected. Meal record data were analyzed with a computer-aided nutritional analysis program (CAN-Pro version 2.0; Korean Nutrition Society, Seoul, Korea) to be converted to dietary intake.
- The graded exercise test was performed using a stationary bicycle (ER 900, D-72475 Bits; JAGER, Wuerzburg, Germany), increasing the power by 20 W in every 2 minutes until the subjects could not continue exercising. Subjects' heart beats per minute, oxygen uptake, work done, and breathing rate were automatically recorded as numbers and graphs every 15 seconds, while blood pressure was recorded every 2 minutes by an automatic sphygmomanometer before, during, and after exercise. We measured anaerobic threshold using a Jaeger Oxycon Delta system.
- Computed tomography (GE, Milwaukee, WI, USA) of abdominal fat was measured based on methods used in previous studies, radiographic images were taken from the 4th to 5th lumber body to belly button with 10 mm above and below the range. Fat density was measured in Hounsfield unit in the range from -150 to -50 and was reconstructed. In other words, the total abdominal fat area within the abdominal cavity was calculated using a computer and the visceral fat area (VFA) was obtained by measuring the inner area with the abdomen and the dorsal peritoneum as the boundary. The subcutaneous fat area (SFA) was calculated by subtracting the total abdominal fat by VFA [15].
- Statistical analysis
- The mean and standard deviation for different variables in this study were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). The statistical differences in physical characteristics, biochemical test results, abdominal fat, and insulin resistance of both CG and AEG before and after the 12-week period were determined using paired t-test, and the differences between the groups were determined using independent t-test and analysis of covariance, and finally bivariate correlation test was used. Statistical significance was set at P<0.05.
METHODS
Anthropometry and biochemical tests
Exercise intervention
Measurements of physical activity level and energy intake
Graded exercise test
Computed tomography
- Baseline clinical characteristics
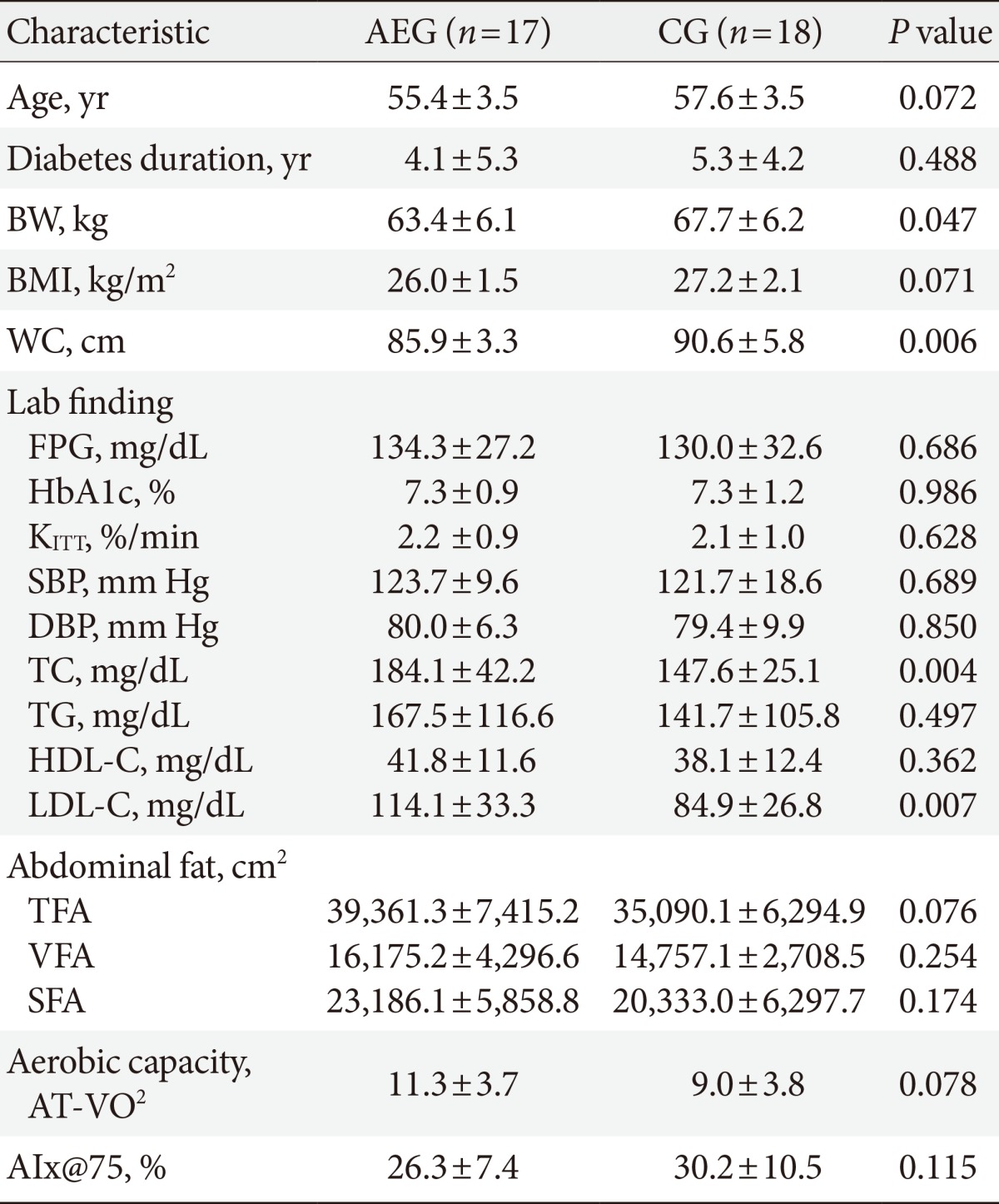
- A total of 35 (CG=18, AEG=17) subjects were enrolled for this study. The average age was 56.5±3.4, average diabetes duration was 4.7±4.8 years, and average BMI was 26.6±1.9 kg/m2. Although there were no significant differences in age (P=0.072), diabetes duration (P=0.488), and BMI (P=0.071) between the two groups (Table 1), the waist circumference was higher in CG compared with AEG (P=0.006). Furthermore, the average HbA1c (P=0.986), KITT (P=0.628) which is the insulin resistance index, and AIx@75 (P=0.115) were similar in both groups (Table 1). There were no changes in drug intake of subjects in attempt to prevent hypoglycemia during the 12-week period. At baseline, 13 and 9 subjects, 3 and 7 subjects, and 1 and 0 subjects in AEG and CG were receiving exercise and diet, oral hypoglycemic agent, and insulin therapies, respectively, but there were no significant differences in receiving different types of therapy between the groups.
- Changes in energy expenditure and energy intake
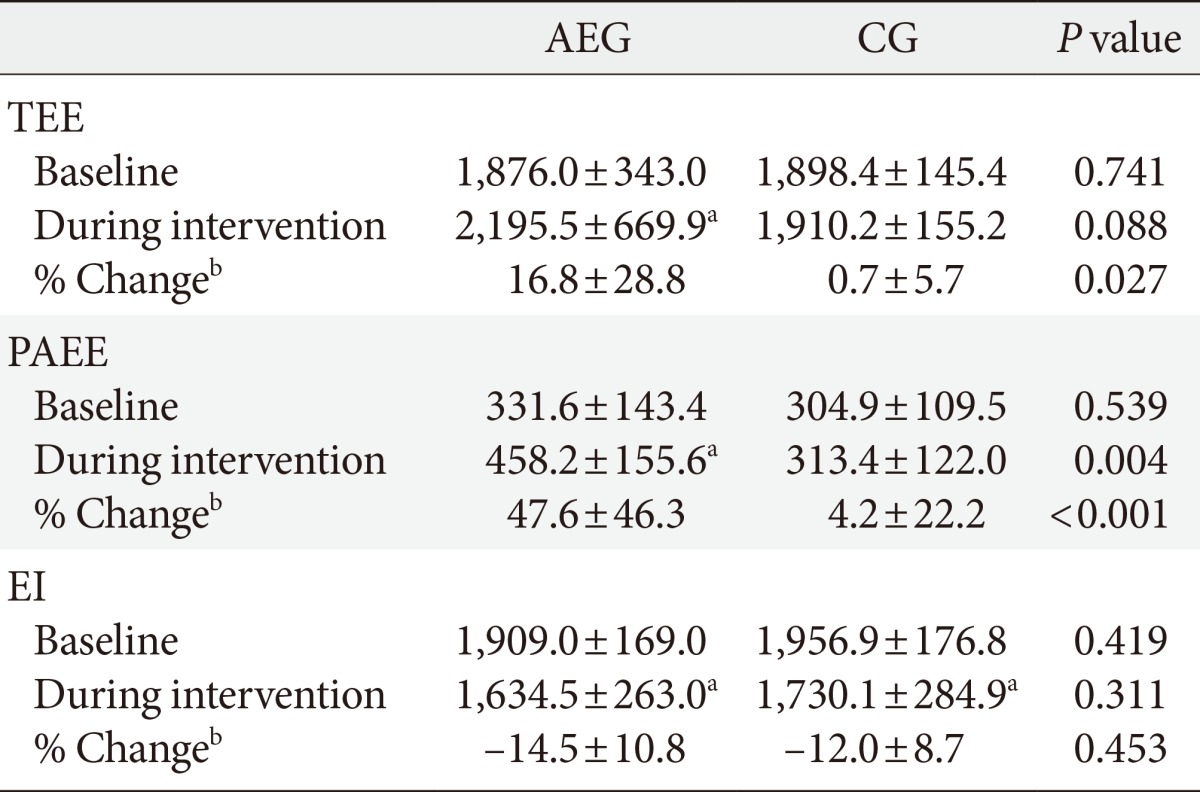
- There were no significant difference in the TEE (P=0.741), PAEE (P=0.539), and EI (P=0.419) at baseline between groups. After 12 weeks, the TEE (P=0.042) and PAEE (P=0.001) of AEG increased from baseline, but no differences were observed in CG (P=0.651, P=0.572, respectively). After 12 weeks, the changes in TEE (P=0.027) and PAEE (P=0.001) were significantly higher in AEG compared with CG (Tables 2 and 3).
- In CG, EI decreased significantly from 1,956.9±176.8 to 1,730.1±284.9 kcal/day (P<0.001), and AEG also showed a significant decrease in EI, from 1,909.0±169.0 to 1,634.5±253.0 kcal/day (P<0.001), but the changes in EI were not significantly different between the two groups (Tables 2 and 3).
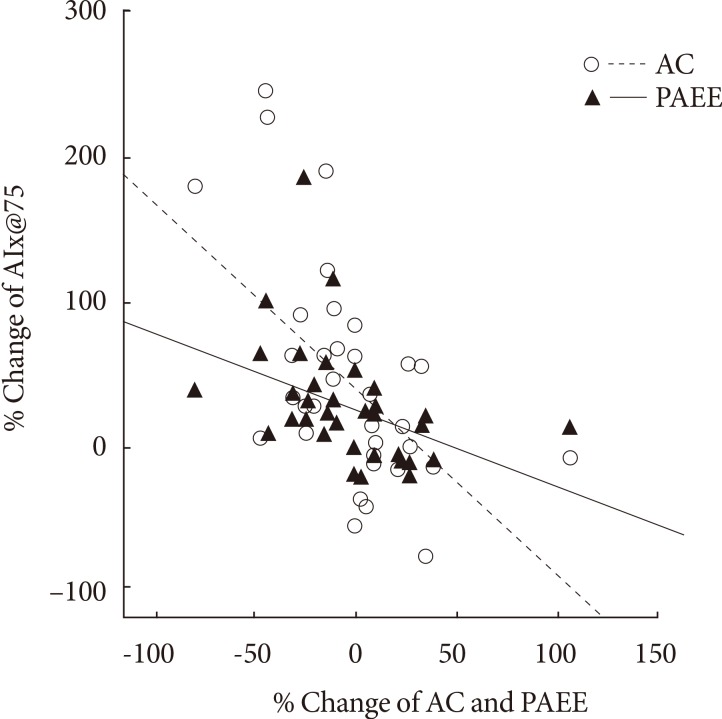
- While change in AIx@75 was associated with changes in TEE (r=-0.376, P=0.026) and PAEE (r=-0.416, P=0.013), it was not correlated with the change in EI (Table 4).
- Changes in anthropometric and biochemical indices
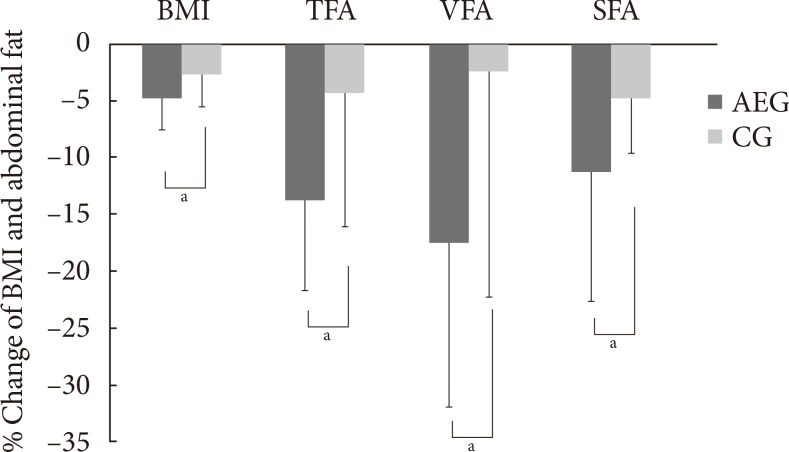
- After 12 weeks, both AEG and CG showed significant decreases in weight (P<0.001 and P=0.01, respectively), BMI (P<0.001 and P=0.02, respectively), and waist circumference (P<0.001 and P=0.04, respectively) from baseline (Table 3, Fig. 1).
- After 12 weeks, the decreases in weight, BMI, and waist circumference were significantly were greater in AEG compared with CG (P=0.039, P=0.039, and P=0.020, respectively), and the decreases in triglyceride level was also greater in AEG than in CG (P=0.034). However the change in insulin resistance was not significantly different between two groups (Table 3, Fig. 1).
- Although the change in AIx@75 was significantly correlated with change in waist circumference (r=0.348, P=0.044), it was not associated with insulin resistance improvement significantly (Table 4).
- Changes in abdominal fat
- After 12 weeks intervention, AEG showed significant decreases in total fat area (TFA; P<0.001), VFA (P<0.001), and SFA (P<0.001) from baseline. On the other hand, CG showed no significant differences in TFA (P=0.103) or VFA (P=0.356), but their SFA decreased significantly (P=0.047) from baseline. After 12 weeks, the changes in TFA (P=0.010), VFA (P=0.017), and SFA (P=0.044) decreased significantly in AEG compared with CG (Table 3, Fig. 1).
- Although the change in AIx@75 was significantly related to the changes in SFA (r=0.531, P=0.001) and TFA (r=0.489, P=0.003), it was not associated with VFA (Table 4).
- Changes in aerobic capacity and AIx
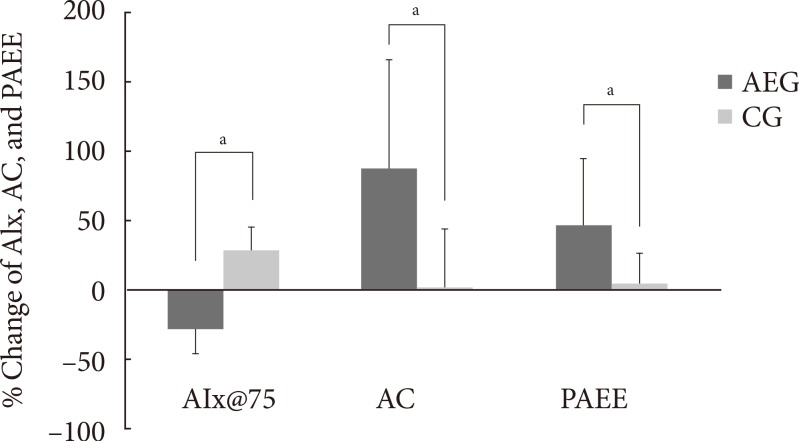
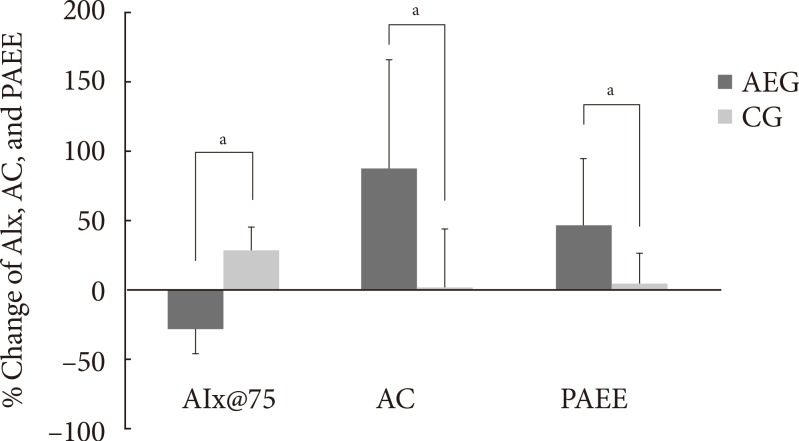
- After 12 weeks, the aerobic capacity significantly increased in AEG from baseline (P<0.001), while CG did not show any differences, and the change in aerobic capacity increased significantly in AEG compared with CG (P<0.001). The differences in aerobic capacity between the two groups was still significant even after adjusting for subjects' baseline age, weight, waist circumference, total cholesterol, LDL-C, and aerobic capacity (P=0.002) (Table 3, Fig. 2).
- After 12 weeks, the aortic AIx@75 decreased significantly in AEG from baseline (P<0.001), while it was increased in CG from baseline (P<0.001). The difference in changes of aortic AIx@75 of AEG and CG after 12 weeks was significant (P<0.001) (Table 3), and this significance was maintained even after adjusting for age, weight, waist circumference, total cholesterol, and LDL-C (P<0.001).
- The change in aortic AIx@75 had a significant correlation with the change in cardiorespiratory fitness (r=-0.560, P<0.001) (Table 4, Fig. 3).
RESULTS
- Increased arterial stiffness gives rise to left ventricular overload and increases the tension of blood vessel wall, consequently leading to increased risks of cardiovascular diseases. Arterial stiffness can be change after exercise. Indeed, Lee et al. [16] showed that an acute treadmill exercise (60% HRmax) in patients with hypertension can lead to decreased blood pressure and improved vascular compliance. Furthermore, improvements of vascular compliance was greater in the 12 weeks compared with at the 6 weeks among subjects with hypertension taking antihypertensive drugs, which indicated prolonged aerobic exercise showed much effectiveness although temporary aerobic exercise does improve vascular compliance [17]. Also, Mustata et al. [18] reported that aerobic exercise 60 min/day for 3 day/week for 12 weeks had an effect of significantly decreasing arterial stiffness in patients with predialysis chronic kidney disease. In line with previous studies, this study showed AIx was significantly decreased in AEG compared with CG. The possible reason for the restoration of vascular compliance after exercise is the augmented blood flow and blood pressure during exercise helps maintain the normal functions of aortic endothelium and efficiently supplies blood to arteriole [19]. In addition, the increased production of nitric oxide in vascular endothelium with exercise might be possible reason for the benefits of exercise-induced decrease in arterial stiffness [20].
- In general, arterial stiffness can be affected by age, obesity, hypertension, and diabetes, which are all risk factors for cardiovascular diseases [21]. Arterial stiffness has also shown to be correlated with insulin resistance because increased insulin resistance has been known to induce arterial stiffness through various mechanisms such as accelerated sympathetic nervous system, increased sodium uptake in kidneys, impaired vascular dilation, and accelerated renin-angiotensin-aldosterone system. These have been known to show significant correlation with AIx and fasting blood glucose [22], and Wilkinson et al. [23] showed that AIx is increased in diabetes. However, no differences in changes of fasting blood glucose, HbA1c, and KITT were observed between AEG and CG in this study, and no correlation between these variables and arterial stiffness was observed. A previous study reported that intense exercise is required to increase insulin sensitivity [24], while long-term period of exercise is required for moderate intense exercise [25,26]. Furthermore, Mustata et al. [18] reported that only 12 weeks of aerobic exercise is not sufficient to increase homeostasis model assessment of insulin resistance (HOMA-IR), and many other factors are involved in increasing HOMA-IR. However this study did not have enough exercise intensity, amount, and duration to increase insulin sensitivity, which might be a reason that changes in insulin sensitivity had no correlation with changes in AIx in this study. Further study that studies the association between enhanced insulin sensitivity and arterial stiffness with varying exercise intensity and duration is warranted.
- Arterial stiffness and abdominal fat are known to show significant correlation. An aquarobics exercise for 60 min/day for 3 day/week for 8 weeks among middle-aged obese women reduced body fat and increased vascular compliance [27], while Kim et al. [28] showed a significant relationship between arterial stiffness measured by PWV and waist circumference but not with weight and BMI. In line, this study also showed a correlation between changes in AIx and changes in waist circumference but not with weight and BMI. As such, weight and BMI has, until now, failed to show any consistent correlation with long-term arterial calcification and arterial stiffness because BMI does not only reflect body fat but also muscle mass [29]; therefore, the association between BMI and arterial stiffness can differ depending on the ratio of fat and muscle. Furthermore, we found that AEG had significantly higher reductions in total abdominal fat, visceral fat, and subcutaneous fat compared with CG, of which changes in total abdominal fat and subcutaneous fat showed significant correlation with changes in AIx, suggesting that abdominal fat has larger implications on AIx than weight. Although changes in visceral fat did not such a significant correlation, its P value was 0.059, which is very close to being significant, therefore further study with a larger cohort and longer duration may be required to confirm this results.
- Most studies regarding exercise reported that exercise alone does not have a large effect on weight reduction [30,31], but here we showed not only the significant reduction in weight of AEG (-3.1 kg) but also a significant reduction in subcutaneous fat of CG, unlike the previous studies. We speculate that the reason for such controversy is because both groups had reduced food intake at the end of study compared with baseline. However there was no difference in calorie intake in both groups. Arterial stiffness has been shown to be associated with increased aerobic exercise capacity through regular aerobic exercise [32]. Yoon et al. [33] showed that carotid-femoral artery PWV was negatively associated with VO2max which is an index of cardiorespiratory fitness among old-aged women (r=-0.533). Mustata et al. [18] also showed a significant association between the increased VO2max and AIx after 12 weeks of aerobic exercise with 40% to 60% intensity. This study also showed that AEG had increased aerobic capacity compared with CG, and the enhanced cardiorespiratory fitness showed a negative correlation with reduced arterial stiffness (r=-0.560), similarly to previous studies, and this had the highest correlation among other factors. As such, arterial compliance enhancement after exercise is known to be due to the increased aortic compliance, reduced peripheral blood vessel resistance, restoration of compliance, changes in plasma volume, and changes in endocrine system [34,35]. Aerobic exercise capacity is a strong predictor of total mortality due to cardiovascular diseases [36], and although this is greatly reduced in patients with type 2 diabetes [37], regular exercise can restore cardiorespiratory fitness and reduce risk factors for cardiovascular diseases. The American Diabetes Association particularly emphasizes the advantages of regular physical activities in diabetes prevention and treatment and recommends more than 30 minutes of moderate exercise every day [38]. They are also recommending more than 150 minutes of moderate exercise (50% to 70% of HRmax) every week and more than three times per week of aerobic exercise, especially to control blood glucose levels, maintain weight, and reduce risks of cardiovascular diseases [39]. In addition, this study showed that changes in AIx does not have a significant relationship with changes in calorie intake but does have a negative correlation with TEE and PAEE.
- The limitations of this study were that the cohort size was small and that this was only a preliminary study. Also, this study does not provide any clues regarding how exercise intensity, amount, and duration can affect weight reduction and insulin resistance. Therefore further study with a larger cohort with differing exercise intensity, amount, and duration is needed.
- In conclusion, 12 weeks of moderate aerobic exercise can reduce weight and abdominal fat, alleviate arterial stiffness, and improves aerobic capacity but does not decrease insulin resistance. In addition, changes in arterial stiffness was more associated with waist circumference and changes in abdominal fat rather than weight, and with changes in PAEE rather than changes in EI. These suggested that moderate intensity exercise improved arterial stiffness with relation to cardiorespiratory fitness and also with central obesity in type 2 diabetes.
DISCUSSION
- 1. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 27 million participants. Lancet 2011;378:31-40. ArticlePubMed
- 2. Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001;44(Suppl 2):S14-S21. ArticlePubMedPDF
- 3. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993;16:434-444. ArticlePubMedPDF
- 4. Alexander CM, Landsman PB, Teutsch SM, Haffner SM. Third National Health and Nutrition Examination Survey (NHANES III). National Cholesterol Education Program (NCEP). NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003;52:1210-1214. ArticlePubMedPDF
- 5. O'Brien MJ, Alos VA, Davey A, Bueno A, Whitaker RC. Acculturation and the prevalence of diabetes in US Latino Adults, National Health and Nutrition Examination Survey 2007-2010. Prev Chronic Dis 2014;11:E176ArticlePubMedPMC
- 6. Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation 2002;106:2085-2090. ArticlePubMed
- 7. Hope SA, Tay DB, Meredith IT, Cameron JD. Use of arterial transfer functions for the derivation of aortic waveform characteristics. J Hypertens 2003;21:1299-1305. ArticlePubMed
- 8. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M. CAFE Investigators. Anglo-Scandinavian Cardiac Outcomes Trial Investigators. CAFE Steering Committee and Writing Committee. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 2006;113:1213-1225. ArticlePubMed
- 9. Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003;289:1799-1804. ArticlePubMed
- 10. Kingwell BA, Cameron JD, Gillies KJ, Jennings GL, Dart AM. Arterial compliance may influence baroreflex function in athletes and hypertensives. Am J Physiol 1995;268(1 Pt 2):H411-H418. ArticlePubMed
- 11. DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 2000;102:1351-1357. ArticlePubMed
- 12. Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, Corgnati A, Muggeo M. Estimates of in vivo insulin action in man: comparison of insulin tolerance tests with euglycemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab 1989;68:374-378. ArticlePubMed
- 13. Izzo JL Jr. Pulse contour analysis and augmentation index: it's time to move beyond cuff blood pressure measurement. Am J Hypertens 2005;18(1 Pt 2):1S-2S. PubMed
- 14. Kumahara H, Schutz Y, Ayabe M, Yoshioka M, Yoshitake Y, Shindo M, Ishii K, Tanaka H. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr 2004;91:235-243. ArticlePubMed
- 15. Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr 1982;36:172-177. ArticlePubMed
- 16. Lee JH, Hwang BY, Nam SN. The change of post treadmill exercise on blood pressure and vascular compliance in essential hypertension patients. Korean J Phys Educ 2004;43:511-520.
- 17. Lee JH. Effects of aerobic exercise on arterial pulse wave velocity and vessel pressure in patients with anti-hypertensives agents [master's thesis]. Seoul: Hanyang University; 2005.
- 18. Mustata S, Groeneveld S, Davidson W, Ford G, Kiland K, Manns B. Effects of exercise training on physical impairment, arterial stiffness and health-related quality of life in patients with chronic kidney disease: a pilot study. Int Urol Nephrol 2011;43:1133-1141. ArticlePubMedPDF
- 19. Stewart KJ, Sung J, Silber HA, Fleg JL, Kelemen MD, Turner KL, Bacher AC, Dobrosielski DA, DeRegis JR, Shapiro EP, Ouyang P. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens 2004;17:314-320. ArticlePubMed
- 20. Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation 2010;122:1221-1238. ArticlePubMed
- 21. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588-2605. ArticlePubMed
- 22. Lee JI, Sohn TS, Kwon HS, Lee JM, Chang SA, Cha BY, Son HS. The changes of central aortic pulse wave analysis in metabolic syndrome. Korean Diabetes J 2008;32:522-528.Article
- 23. Wilkinson IB, MacCallum H, Rooijmans DF, Murray GD, Cockcroft JR, McKnight JA, Webb DJ. Increased augmentation index and systolic stress in type 1 diabetes mellitus. QJM 2000;93:441-448. ArticlePubMed
- 24. Jung JY, Han KA, Ahn HJ, Kwon HR, Lee JH, Park KS, Min KW. Effects of aerobic exercise intensity on abdominal and thigh adipose tissue and skeletal muscle attenuation in over-weight women with type 2 diabetes mellitus. Diabetes Metab J 2012;36:211-221. ArticlePubMedPMC
- 25. Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 2003;52:1888-1896. ArticlePubMedPDF
- 26. DiPietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol (1985) 2006;100:142-149. ArticlePubMed
- 27. Ka SH. The impact of aquarobics on the body composition and vascular compliance of middle-aged obese women [dissertation]. Daejeon: Chungnam National University; 2006.
- 28. Kim TS, Seo YY, Lee SH, Hong YH, Kim DY, Won HS, Yang MS, Shin HH. Correlation between pulse wave velocity and cardiovascular risk factors in Korean women. J Korean Soc Lipidol Atheroscler 2008;18:239-246.
- 29. Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, Lakatta EG, Kuller LH. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health Study. Am J Hypertens 2002;15(1 Pt 1):16-23. ArticlePubMed
- 30. Despres JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition 1993;9:452-459. PubMed
- 31. Dekker MJ, Lee S, Hudson R, Kilpatrick K, Graham TE, Ross R, Robinson LE. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism 2007;56:332-338. ArticlePubMed
- 32. Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 1993;88(4 Pt 1):1456-1462. ArticlePubMed
- 33. Yoon ES, Jung SJ, Jae SY. Association between cardiorespiratory fitness and arterial stiffness in older women. Exerc Sci 2009;18:307-316.Article
- 34. Chandler MP, DiCarlo SE. Acute exercise and gender alter cardiac autonomic tonus differently in hypertensive and normotensive rats. Am J Physiol 1998;274(2 Pt 2):R510-R516. ArticlePubMed
- 35. Shen W, Zhang X, Zhao G, Wolin MS, Sessa W, Hintze TH. Nitric oxide production and NO synthase gene expression contribute to vascular regulation during exercise. Med Sci Sports Exerc 1995;27:1125-1134. ArticlePubMed
- 36. Cole CR, Foody JM, Blackstone EH, Lauer MS. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann Intern Med 2000;132:552-555. ArticlePubMed
- 37. Poirier P, Garneau C, Bogaty P, Nadeau A, Marois L, Brochu C, Gingras C, Fortin C, Jobin J, Dumesnil JG. Impact of left ventricular diastolic dysfunction on maximal treadmill performance in normotensive subjects with well-controlled type 2 diabetes mellitus. Am J Cardiol 2000;85:473-477. ArticlePubMed
- 38. Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, Clark NG. American Diabetes Association. North American Association for the Study of Obesity. American Society for Clinical Nutrition. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004;27:2067-2073. ArticlePubMed
- 39. Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:1433-1438. PubMed
REFERENCES

Values are presented as mean±standard deviation. P values are obtained by independent t-test.
AEG, aerobic exercise group; CG, control group; BW, body weight; BMI, body mass index; WC, waist circumference; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; KITT, insulin sensitivity by insulin tolerance test; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TFA, total fat area; VFA, visceral fat area; SFA, subcutaneous fat area; AT-VO2, anaerobic threshold-oxygen consumption; AIx@75, augmentation index 75%.
Values are presented as mean±standard deviation. P values are obtained by independent t-test.
AEG, aerobic exercise group; CG, control group; TEE, total energy expenditure; PAEE, physical activity energy expenditure; EI, energy intake.
aP<0.05 compared to baseline within group, bChange (percent change) was calculated as (12 weeks value-baseline value)×100/baseline value
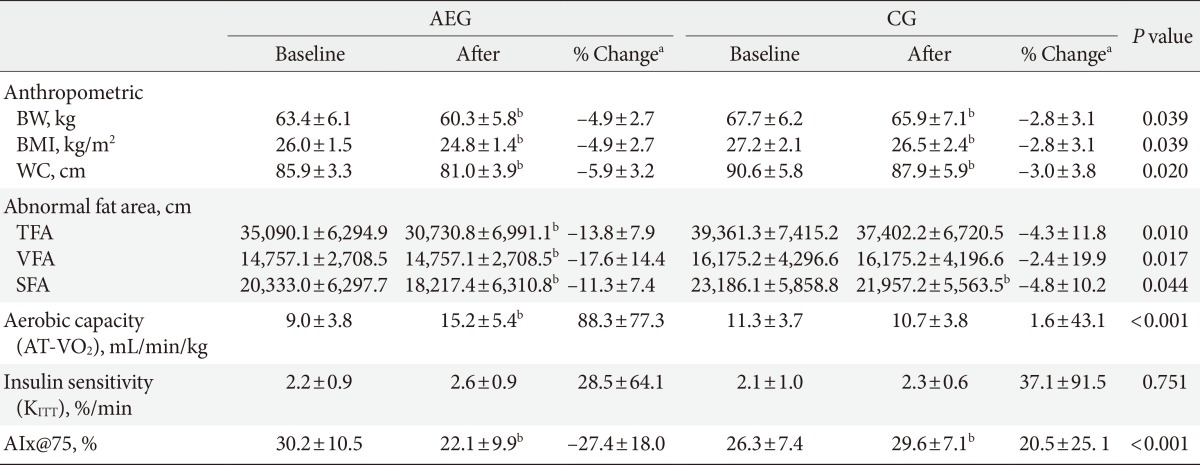
Values are presented as mean±standard deviation. P values are obtained by independent t-test between groups.
AIx, augmentation index; AEG, aerobic exercise group; CG, control group; BW, body weight; BMI, body mass index; WC, waist circumference; TFA, total fat area; VFA, visceral fat area; SFA, subcutaneous fat area; AT-VO2, anaerobic threshold-oxygen consumption; KITT, insulin sensitivity by insulin tolerance test; AIx@75, augmentation index 75%.
a% Change (percent change) was calculated as (12 weeks value-baseline value)×100/baseline value, bP<0.05 significance from paired t-test within group.
AIx@75, augmentation index 75%; BW, body weight; BMI, body mass index; WC, waist circumference; TFA, total fat area; VFA, visceral fat area; SFA, subcutaneous fat area; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; KITT, insulin sensitivity by insulin tolerance test; TC, total cholesterol; TG, triglyceride; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; TEE, total energy expenditure; PAEE, physical activity energy expenditure; EI, energy intake; AT-VO2, anaerobic threshold-oxygen consumption.
Figure & Data
References
Citations

- Relationship between objectively measured physical activity and subclinical cardiovascular disease: a systematic review
Aparna Narendrula, Ellen Brinza, Christine Horvat Davey, Chris T Longenecker, Allison R Webel
BMJ Open Sport & Exercise Medicine.2024; 10(1): e001596. CrossRef - Aerobic training reduces pancreatic fat content and improves β‐cell function: A randomized controlled trial using IDEAL‐IQ magnetic resonance imaging
Min Li, Qidong Zheng, Joshua D. Miller, Panpan Zuo, Xiaodan Yuan, Jitao Feng, Chao Liu, Shan Bao, Qingqing Lou
Diabetes/Metabolism Research and Reviews.2022;[Epub] CrossRef - Effect of aerobic exercise on waist circumference in adults with overweight or obesity: A systematic review and meta‐analysis
Alex Armstrong, Klaus Jungbluth Rodriguez, Angelo Sabag, Yorgi Mavros, Helen M. Parker, Shelley E. Keating, Nathan A. Johnson
Obesity Reviews.2022;[Epub] CrossRef - Aortic waveform responses to insulin in late versus early chronotype with metabolic syndrome
Mary‐Margaret E. Remchak, Emily M. Heiston, Anna Ballantyne, Brielle L. Dotson, Steven K. Malin
Physiological Reports.2022;[Epub] CrossRef - Exercise and ectopic fat in type 2 diabetes: A systematic review and meta-analysis
A. Sabag, K.L. Way, S.E. Keating, R.N. Sultana, H.T. O’Connor, M.K. Baker, V.H. Chuter, J. George, N.A. Johnson
Diabetes & Metabolism.2017; 43(3): 195. CrossRef - Arterial Stiffness Measured with the Cuff Oscillometric Method Is Predictive of Exercise Capacity in Patients with Cardiac Diseases
Yasushi Tazawa, Nobuyoshi Mori, Yoshiko Ogawa, Osamu Ito, Masahiro Kohzuki
The Tohoku Journal of Experimental Medicine.2016; 239(2): 127. CrossRef

 KDA
KDA PubReader
PubReader Cite
Cite