CycloZ Improves Hyperglycemia and Lipid Metabolism by Modulating Lysine Acetylation in KK-Ay Mice
Article information
Abstract
Background
CycloZ, a combination of cyclo-His-Pro and zinc, has anti-diabetic activity. However, its exact mode of action remains to be elucidated.
Methods
KK-Ay mice, a type 2 diabetes mellitus (T2DM) model, were administered CycloZ either as a preventive intervention, or as a therapy. Glycemic control was evaluated using the oral glucose tolerance test (OGTT), and glycosylated hemoglobin (HbA1c) levels. Liver and visceral adipose tissues (VATs) were used for histological evaluation, gene expression analysis, and protein expression analysis.
Results
CycloZ administration improved glycemic control in KK-Ay mice in both prophylactic and therapeutic studies. Lysine acetylation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha, liver kinase B1, and nuclear factor-κB p65 was decreased in the liver and VATs in CycloZ-treated mice. In addition, CycloZ treatment improved mitochondrial function, lipid oxidation, and inflammation in the liver and VATs of mice. CycloZ treatment also increased the level of β-nicotinamide adenine dinucleotide (NAD+), which affected the activity of deacetylases, such as sirtuin 1 (Sirt1).
Conclusion
Our findings suggest that the beneficial effects of CycloZ on diabetes and obesity occur through increased NAD+ synthesis, which modulates Sirt1 deacetylase activity in the liver and VATs. Given that the mode of action of an NAD+ booster or Sirt1 deacetylase activator is different from that of traditional T2DM drugs, CycloZ would be considered a novel therapeutic option for the treatment of T2DM.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a metabolic disorder characterized by hyperglycemia and insulin resistance in multiple organs [1]. A healthy lifestyle that includes exercise, a proper diet, and body weight control may be beneficial in managing the disease; however, as the disease progresses, treatment with oral medications or insulin therapy is often necessary [2]. Currently available oral drugs such as hypoglycemic agents or insulin sensitizers, including sulfonylureas (SU), biguanides, thiazolidinediones (TZD), dipeptidyl peptidase-4 (DPP-4) inhibitors, and sodium-glucose cotransporter 2 (SGLT2) inhibitors have been used to control blood glucose levels for a long time [3]. However, some drugs have limited efficacy and can induce various adverse effects [4,5]. For example, patients taking SU have an increased risk of weight gain and hypoglycemia, and while those taking biguanides are exposed to a potential risk of lactic acidosis. TZDs are not recommended for patients with existing edema, heart failure, and acute liver diseases. The most common adverse reactions to DPP-4 inhibitors are upper respiratory tract infections [5,6], and while SGLT2 inhibitors are associated with urinary tract and genital infections [7]. Therefore, it is necessary to develop safer and more effective drugs with different mechanisms of action.
Lysine acetylation plays an important role in maintaining energy homeostasis in various metabolic pathways [8]. In particular, several enzymes involved in glucose and lipid metabolism are regulated by the acetylation of lysine residues [9-11]. The sirtuin family of enzymes is composed of increased the level of β-nicotinamide adenine dinucleotide (NAD+)-dependent deacetylases that regulate the activities of many other enzymes. Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), a positive regulator of mitochondrial biogenesis, is regulated by sirtuin 1 (Sirt1) [12,13]. Sirt1 also modulates the acetylation of liver kinase B1 (LKB1), a major AMP-activated kinase (AMPK) kinase involved in lipid synthesis and fatty acid oxidation [13]. Sirtuin has also been considered a new target for the treatment of chronic metabolic diseases [10] and it has been reported that the enhancement of Sirt1 activity reverts the pathologic effects of T2DM [14-16].
Young KK-Ay mice have been used as a mild hyperglycemia model for prophylactic treatment of T2DM with obesity [17]. Hyperglycemia and hyperinsulinemia in KK-Ay mice worsen with age [18]; therefore, aged KK-Ay mice with advanced hyperglycemia were used in a separate study to examine the therapeutic effect of CycloZ in a severe diabetes model.
CycloZ is a combination of cyclo-His-Pro (CHP) and zinc. CHP is a cyclic dipeptide found in many tissues and has been observed to have several biological functions, such as protection against oxidative stress and anti-inflammatory activity [19]. Cyclization confers higher stability and protection against peptidases and is required for its active transport in the intestine [20] and through the blood-brain barrier [21], explaining the wide distribution of CHP throughout the body [22]. CycloZ has also been reported to improve glycemic control in diabetes via an additive mechanism [23]. However, the anti-diabetic mechanism of CycloZ is still poorly understood and needs to be better defined [24,25].
METHODS
Animals and administration
5 weeks of age male KK-Ay mice purchased from CLEA Japan Inc. (Nishishinbashi, Japan) were housed in individual cages in air-conditioned room at temperature of 23°C±3°C with a 12 hours light/dark cycle, and were free to access distilled water and laboratory chow diet. All animal experiments were approved by accordance with Ethics Review Committee of the Pohang Advanced Bio Convergence Center, Republic of Korea (ABCC201712). All animals were used for experiments after 1 week of adaptation. For prophylactic study, animals were divided into two groups. Control group was administered water as a vehicle. The KK-Ay mice were orally gavaged CycloZ (Cyclo His-Pro [5 mg/kg] and zinc gluconate [70 mg/kg]) daily for 20 weeks. For therapeutic study, the animals were divided into a control group and an experimental group at 12 weeks of age. The KK-Ay mice were orally gavaged water or CycloZ daily for 8 weeks, respectively. At the end of each experiments, all mice were anesthetized with isoflurane using RC2 Rodent Circuit Controller Anesthesia System (Vetequip, Pleasanton, CA, USA). Blood was collected by cardiac puncture and plasma was separated. Isolated adipose tissues, liver and plasma were stored at –80°C until analysis.
Oral glucose tolerance test and glycosylated hemoglobin measurement
For oral glucose tolerance test (OGTT), mice were fasted for 16 hours and orally gavaged 2 g/kg glucose. At the time of 15, 30, 60, 90, 120 minutes after glucose administration, blood was taken from tail vein. Blood glucose level was measured immediately using a blood glucose meter (AGM-4000, Allmedicus, Anyang, Korea). To measuring glycosylated hemoglobin (HbA1c), blood was taken from tail vein. HbA1c was measured using DCA vantage® analyzer (Siemens, Munich, Germany).
Analysis of blood biochemical parameters
Whole blood was collected by cardiac puncture and plasma was separated by centrifugation at 2,000 ×g for 10 minutes. The plasma was then stored at –80°C until analysis. Aspartate aminotransferase (AST), alanine aminotransferase, alkaline phosphatase, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, creatinine, and blood urea nitrogen (BUN) were measured by a biochemistry analyzer (BS-390, Mindray Bio-medical Electronics Co. Ltd., Shenzhen, China). free fatty acid was quantified by Enzy-Chrom Free Fatty Acid Assay Kit (EFFA-100, BioAssay System, Hayward, CA, USA).
RNA extraction, cDNA synthesis, and mRNA expression analysis
Total RNA was extracted from the tissues and cells using NucleoZOL reagent (740404.200, Macherey-Nagel, Allentown, PA, USA). An 1 μg of total RNA was used for cDNA synthesis using iScript cDNA synthesis kit (1708891, Bio-Rad, Hercules, CA, USA). Real-time quantitative polymerase chain reaction (RT-qPCR) was performed using gene specific primers (Supplementary Table 1) and IQ SYBR Green Supermix (BR1708882, Bio-Rad). Amplification of RT-qPCR was performed as we previously described [26]. with minor modification. Briefly, RTqPCR was performed by following reaction cycle of amplification (95°C for 10 seconds, 60°C for 10 seconds, 72°C for 30 seconds). The expression level was normalized to those of β-actin or glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Quantification of NAD+/β-nicotinamide adenine dinucleotide
NAD+ and β-nicotinamide adenine dinucleotide (NADH) ratio were measured by NAD+/NADH Quantification Colorimetric Kit (K-337-100, Biovision, Milpitas, CA, USA) from the tissue lysate following the manufacturer’s protocol. Briefly, 10 mg of tissues were homogenized with the provided extraction buffer. The 50 μL of extracted samples were transferred into 96-well microplate for measuring total NAD+ concentration. To decompose NAD, the rest of extracted samples were heated to 60°C for 30 minutes. Consequently, 50 μL of decomposed samples were transferred into 96-well microplate. After development, the plate was measured at 450 nm.
Western blot
For the protein experiments, tissues and cells were lysed with radioimmunoprecipitation assay (RIPA) buffer (89901, Thermo Scientific, Waltham, MA, USA) including Halt Protease and Phosphatase Inhibitor Cocktail (78440, Thermo Scientific). For the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), Bolt 4% to 12% Bis-Tris Plus Gels (Thermo Scientific) were used and the transfer was performed using Trans-Blot Turbo system (Bio-Rad). The following antibodies were used to detect the target proteins, PGC-1α (NBP1-04676, Novusbio, Centennial, CO, USA), Ac-Lysine (9814S, Cell Signaling Technology [CST], Danvers, MA, USA), phospho-AMPK (5831S, CST), AMPK (2535S, CST), phospho-Akt (9271S, CST), Akt (9272S, CST), LC3 I/II (4108S, CST), GAPDH (2118S, CST), adiponectin (2789S, CST), and Sirt1 (07-131, EMD Millipore, Burlington, MA, USA).
Histochemistry
The liver and the adipose tissues were fixed with neutral buffered 10% formalin solution (HT-501128, Sigma, St. Louis, MO, USA) and embedded in paraffin blocks. Serial (4 μm-thick) sections were deparaffinized, dehydrated, and stained with hematoxylin and eosin (H&E). Immunohistochemical analysis was subjected using antibodies against tumor necrosis factor α (TNFα, Abcam, Cambridge, UK; ab1793), macrophage chemoattractant protein 1 (MCP-1, Abcam, ab25124), F4/80 (Abcam, ab111101), and CD11b (Abcam, ab133357) as described previously [27]. Stained sections were confirmed by light microscopy (Olympus BX53 upright microscope, Olympus, Tokyo, Japan).
Enzyme-linked immunosorbent assay
To measure concentration of insulin in plasma, Mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (80-INSMSE01, ALPCO, Salem, NH, USA) was used. To measure concentration of TNFα and MCP-1 in tissues, Mouse TNFα Quantikine ELISA Kit (MTA00B, R&D Systems, Minneapolis, MN, USA) and mouse MCP-1 Quantikine ELISA Kit (MJE00B, R&D Systems) were used. The procedure was conducted according to the manufacturer's protocol.
Oxygen consumption rate measurement
The oxygen consumption rate (OCR) was measured using an XF96 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA, USA) according to the manufacturer’s protocol. Alpha mouse liver 12 (AML12) cells were seeded in XF-96 tissue culture plates at a density of 1×104 cells/well. The next day, the medium was replaced with XF base medium (pH 7.4, Seahorse Biosciences, North Billerica, MA, USA) supplemented with 25 mM D-glucose (G7528, Sigma-Aldrich), 1 mM sodium pyruvate (S8636, Sigma-Aldrich), and 1 X GlutaMAXTM (35050, Gibco, Waltham, MA, USA), followed by appropriate drugs treatment. To assess OCR, the compounds and metabolites used in this study were as follow: insulin (100 nM, I5556, Sigma-Aldrich), oligomycin A (1 μM, 75351, Sigma-Aldrich), carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (CCCP, 2 μM, C2920, Sigma-Aldrich), rotenone (1 μM, R8875, Sigma-Aldrich). To normalize by cell number, 4´,6-diamidino-2-phenylindole (DAPI)-stained cells were counted automatically by ImageXpress Micro Confocal Microscopy (Molecular Devices, San Jose, CA, USA).
MitoTracker staining
The 0.5×105 cells were plated on glass coverslips in 12 well plate and incubated for 24 hours: 200 μM of palmitate was treated with or without CycloZ in serum free media for 24 hours; 500 nM of MitoTracker Deep Red FM (M22426, Invitrogen, Carlsbad, CA, USA), diluted in serum free media, was treated and incubated for 30 minutes. Cells were washed with phosphate-buffered saline and fixed in 4% paraformaldehyde at room-temperature for 40 minutes. After Hoechst staining, the coverslips were mounted on slide glass. Images (7 to 10) per group were taken using Leica confocal laser scanning microscope. Fluorescence in randomly selected five areas in each image was measured using Leica LAS AF program (Leica, Wetzlar, Germany).
Primer information
Primer sequences used for RT-qPCR amplification were listed in Supplementary Table 1.
Statistical analysis
Statistical analysis was performed with the Prism software (GraphPad Prism 6, GraphPad Software Inc., San Diego, CA, USA). All data expressed as mean±standard error of the mean. Significance of differences between two groups were analyzed by Student’s t-test (two-tailed), multiple comparisons were determined by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. P<0.05 was considered statistically significant. We applied Grubb’s test for exclusion of outliers.
RESULTS
CycloZ administration ameliorates T2DM and obesity in KK-Ay mice
We verified the additive effects of CycloZ in counteracting diabetes and obesity in KK-Ay mice (Supplementary Fig. 1A and B). Compared with individual compound treatment, CycloZ administration is more efficacious in improving glucose tolerance, as evaluated through the results of the OGTT and HbA1c measurements.
The weight gain of mice in the CycloZ-treated group gradually decreased compared to that of the control group at the end of the experiment without a noticeable difference in food intake (Fig. 1A, Supplementary Fig. 1C). CycloZ administration significantly suppressed the increase in the masses of the liver and visceral adipose tissue (VAT), such as epididymal adipose tissue (EAT), mesenteric adipose tissue (MAT), but not subcutaneous adipose tissue (Fig. 1B). CycloZ was safe and well-tolerated, as reflected by the absence of changes in AST, BUN, and creatinine (Supplementary Table 2).
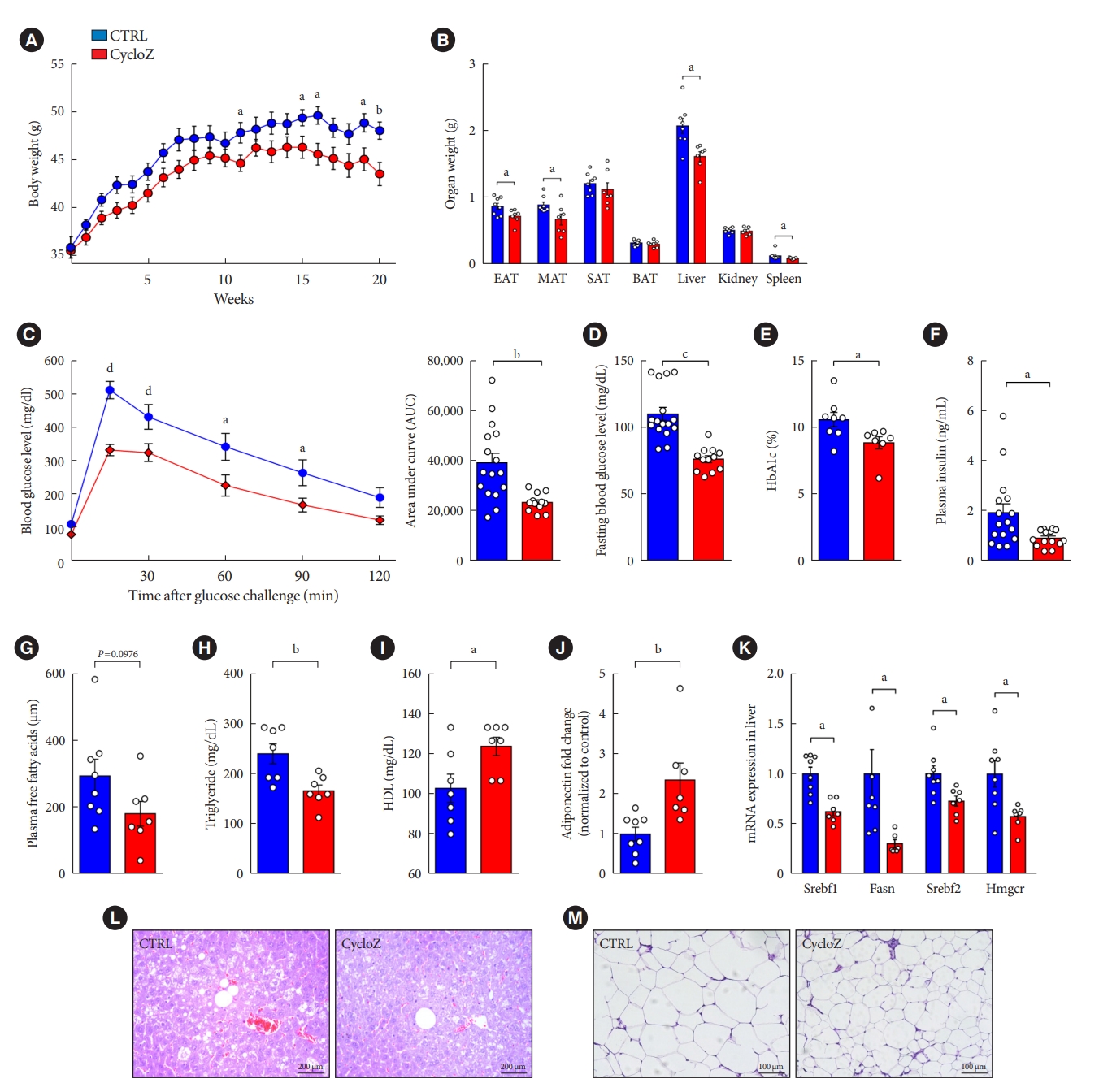
CycloZ administration ameliorates type 2 diabetes mellitus and obese phenotypes in KK-Ay mice. (A) Body weight changes during 20 weeks of administration (n=7–8). (B) Weight of each organ after sacrifice (n=7–8). (C) Oral glucose tolerance test for 2 hours after 16 hours fasting and glucose administration (2 g/kg) at 10 weeks of treatment (n=14–16). (D) Blood glucose level after 16 hours fasting at 10 weeks of treatment (n=14–16). (E) Glycosylated hemoglobin (HbA1c) levels in KK-Ay mice at 11 weeks of treatment (n=7–8). (F) Plasma insulin concentration (n=14–16). (G) Plasma free fatty acid concentration (n=7–8). (H) Plasma triglyceride concentration (n=7–8). (I) Plasma high-density lipoprotein (HDL) concentration (n=7–8). (J) Plasma adiponectin level was measured by Western blot (n=7–8). (K) mRNAs expression related to fatty acid and cholesterol synthesis in liver (n=7–8). (L, M) H&E staining of liver and epididymal adipose tissue (EAT). Data shown represent mean±standard error of the mean. Unpaired Student’s t-tests. CTRL, control; MAT, mesenteric adipose tissue; SAT, subcutaneous adipose tissue; BAT, brown adipose tissue; Srebf, sterol regulatory element-binding transcription factor; Srebp, sterol regulatory-element binding protein; Fasn, fatty acid synthase; Hmgcr, 3-hydroxy-3-methylglutaryl-CoA reductase. aP≤0.05, bP≤0.01, cP≤0.001, dP≤0.0001.
CycloZ improved glucose tolerance in KK-Ay mice, as determined by the results of the OGTT (Fig. 1C). In addition, fasting blood glucose, HbA1c levels, and plasma insulin concentrations were significantly decreased by CycloZ administration (Fig. 1D-F). These results demonstrated that CycloZ improved glucose metabolism and insulin sensitivity.
CycloZ treatment also decreased the weights of the liver and VATs in mice. The blood lipid profile of the mice was also examined using a biochemical analyzer. It was observed that, in CycloZ-treated mice, free fatty acid and triglyceride levels were decreased, HDL-C levels were increased (Fig. 1G-I), but total cholesterol levels did not change (Supplementary Fig. 1D and E). These changes in lipid levels were explained by the decrease in the mRNA expression of genes related to fatty acid and cholesterol synthesis, including sterol regulatory-element binding transcription factor 1 (Srebf1), fatty acid synthase (Fasn), Srebf2, and 3-hydroxy-3-methylglutaryl-CoA reductase (Hmgcr) (Fig. 1K). CycloZ administration also ameliorated hepatic lipid deposition, whereas the control group showed fatty liver pathology, such as steatosis (Fig. 1L). Adiponectin level were significantly increased in the blood of CycloZ-treated mice (Fig. 1J). Moreover, the area of adipocytes in the EAT was significantly decreased in the CycloZ-treated group compared with that in the control group (Fig. 1M, Supplementary Fig. 1F). These results suggest that the weight loss induced by CycloZ administration is due to the fat reduction induced by regulating lipid and cholesterol metabolisms in the liver and VATs.
CycloZ improves inflammation and reduces immune cell infiltration in the liver and VATs of KK-Ay mice
Because obesity-induced insulin resistance in the liver and VATs is closely related to chronic inflammation in many tissues, we investigated whether CycloZ reduces the production of pro-inflammatory cytokines (TNFα and MCP-1) and monocyte infiltration (F4/80 and CD11b). The expression of inflammatory cytokine genes in the liver and MATs, as well as the expression level of F4/80 and MCP-1, were strongly reduced by CycloZ administration (Fig. 2A). Consistently, CycloZ treatment also resulted in a significant reduction in TNFα and MCP-1 protein levels in the liver and EAT (Fig. 2B and C). Furthermore, expression of TNFα, MCP-1, F4/80, and CD11b in the liver and EAT was investigated via immunohistochemistry (Fig. 2D and E). Both inflammatory cytokine production and monocyte infiltration were abolished by CycloZ administration. Taken together, these data suggest that the improvement in tissue insulin resistance after CycloZ administration was accompanied by reduced inflammation.

CycloZ recovers inflammation and immune cell infiltration in liver and visceral adipose tissues of KK-Ay mice. (A) mRNAs expression level related inflammatory cytokines and infiltrated monocyte in liver and mesenteric adipose tissue (n=7–8). (B, C) Tumor necrosis factor α (TNFα) and macrophage chemoattractant protein 1 (MCP-1) protein levels in liver (B) and epididymal adipose tissue (EAT) (C) (n=7–8). (D, E) Expression of TNFα, MCP-1, F4/80, and CD11b in liver (D) and EAT (E) was measured by immunohistochemistry. Data shown represent mean±standard error of the mean. Unpaired Student’s t-tests. CTRL, control; Il-1b, interleukin 1 beta; WAT, white aidpose tissue. aP≤0.05, bP≤0.01, cP≤0.0001.
CycloZ administration improves mitochondrial biogenesis and inflammation by modulating protein acetylation levels
Infiltrating macrophages play an important role in developing of insulin resistance in metabolic organs. Their activity is partly regulated by the deacetylation of transcription factors, such as the p65 subunit of nuclear factor-κB (NF-κB) [28]. Our results showed that CycloZ robustly reduced the acetylation of p65 in the liver and EATs of CycloZ-treated mice (Fig. 3A).

CycloZ improves mitochondrial function via sirtuin 1 deacetylase. (A) Levels of acetylated lysine on p65 in liver and white adipose tissue (WAT) (n=14–16). (B) Acetylated lysine levels on peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) in liver and epididymal adipose tissue (EAT) (n=14–16). (C) Acetylated lysine levels on liver kinase B1 (LKB1) in liver and EAT (n=14–16). (D) Phosphorylated AMP-activated kinase (AMPK) (T172) in liver and EAT (n=7–8). (E) mRNAs expression level related to mitochondrial biogenesis in liver and mesenteric adipose tissue (MAT) (n=7–8). (F) mRNAs expression level related to lipid oxidation in liver and MAT (n=7–8). (G) Oxygen consumption rate (OCR) was measured in alpha mouse liver 12 (AML12) after 16-hour treatment of CycloZ with and without palmitate. (H) MitoTracker staining for measuring mitochondrial mass. Data shown represent mean±standard error of the mean. Unpaired Student’s t-tests. CTRL, control; IP, immunoprecipitation; Ac-K, acetylated-lysine; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Foxo1, forkhead box O1; Esrra, estrogen related receptor alpha; Nrf1, nuclear respiratory factor 1; Tfam, transcription factor A, mitochondrial; Ucp1, uncoupling protein 1; Ppara, peroxisome proliferator-activated receptor alpha; Cpt1a, carnitine palmitoyltransferase 1A; Ppargc1a, PPARG coactivator 1 alpha; CCCP, carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone; Rot/AA, rotenone/antimycin A; BSA, bovine serum albumin; PAL, palmitic acid. aP≤0.05, bP≤0.01, cP≤0.001, dP≤0.0001.
Lysine acetylation regulates the activities of many metabolic enzymes and transcription factors. In previous studies, the global lysine acetylation profile was found to be increased in the kidneys and hearts of patients with diabetes [29,30]. In the study, we found that the global acetyl-lysine level in the liver of mice in the CycloZ-treated group was significantly reduced compare to that in the control group (Supplementary Fig. 2A). Moreover, the acetylation of PGC-1α and LKB1 was significantly reduced in the liver and EATs of CycloZ-treated mice (Fig. 3B and C). In addition to its deacetylation, PGC-1α requires AMPK-mediated phosphorylation for activation. Therefore, we examined whether CycloZ affected the AMPK-PGC-1α pathway. Our results showed that AMPK phosphorylation was increased in the livers and EATs of CycloZ-treated mice (Fig. 3D).
Next, we examined the expression of PGC-1α-related genes. The expression of forkhead box O1 (Foxo1), estrogen-related receptor alpha (Esrra), transcription factor A, mitochondrial (Tfam), nuclear respiratory factor 1 (Nrf1), and uncoupling protein 1 (Ucp1), which are required for the mitochondrial biogenesis, were increased in the livers and MATs of mice in the CycloZ-treated group compared to those in the control group (Fig. 3E). Similarly, the expressions of peroxisome proliferator-activated receptor alpha (Ppara), carnitine palmitoyltransferase 1A (Cpt1a), and PPARG coactivator 1 alpha (Ppargc1a), which are related to lipid oxidation in the liver, were also increased in the CycloZ-treated group, and acyl-CoA oxidase 1 (Acox1), medium-chain acyl-CoA dehydrogenase (Mcad), Pparα, Cpt1a, and Ppargc1a levels were increased in the MATs of CycloZ-treated mice (Fig. 3F). Moreover, increased mitochondrial biogenesis upon CycloZ administration was demonstrated by the increased mitochondrial DNA (mtDNA) content in the livers of CycloZ-treated mice (Supplementary Fig. 2B).
This suggests that CycloZ improves mitochondrial biogenesis, which increases mitochondrial function, and the activation of lipid oxidation. Therefore, we investigated mitochondrial respiration and, OCR in AML12 mouse hepatocytes. Compared with the palmitate-treated group, the CycloZ-treated group showed significantly enhanced OCR, ATP-linked respiration, and maximal respiration capacity (Fig. 3G). MitoTracker Deep Red FM staining showed an increased mitochondrial mass in CycloZ-treated AML12 cells (Fig. 3H). These results demonstrate that CycloZ enhances mitochondrial biogenesis and function by modulating acetylation status.
CycloZ administration regulates the expression of genes involved in NAD+ synthesis
We observed the deacetylation of the non-histone transcription factors, PGC-1α, LKB1, and p65 in the liver and VATs. The sirtuin family of deacetylases is known to regulate the acetylation of the above proteins. Sirt1 has the broadest spectrum of substrates and affects various physiological pathways, including energy metabolism [31]. Since Sirt1 uses NAD+ as a cosubstrate to remove acetyl groups, the cellular NAD+/NADH ratio reflects Sirt1 enzyme activity [10,32]. Therefore, we hypothesized that CycloZ increases the deacetylation activity of Sirt1 by increasing NAD+ levels or the NAD+/NADH ratio.
We found that the NAD+/NADH ratio was elevated by CycloZ administration in the liver and EAT, but not in the muscle (Fig. 4A, Supplementary Fig. 2C). The increase in NAD+/NADH ratio in the liver and EAT was due to an increase in the total amount of NAD+ (Fig. 4B). To investigate the reason for this increase in NAD+ level, we examined the expression of genes involved in NAD+ synthesis. The results showed that the expression of several genes involved in NAD biosynthesis was significantly upregulated upon CycloZ administration relative to the control group (Fig. 4C and D). These results suggest that CycloZ increased NAD+ levels by modulating gene expression related to NAD+ synthesis.
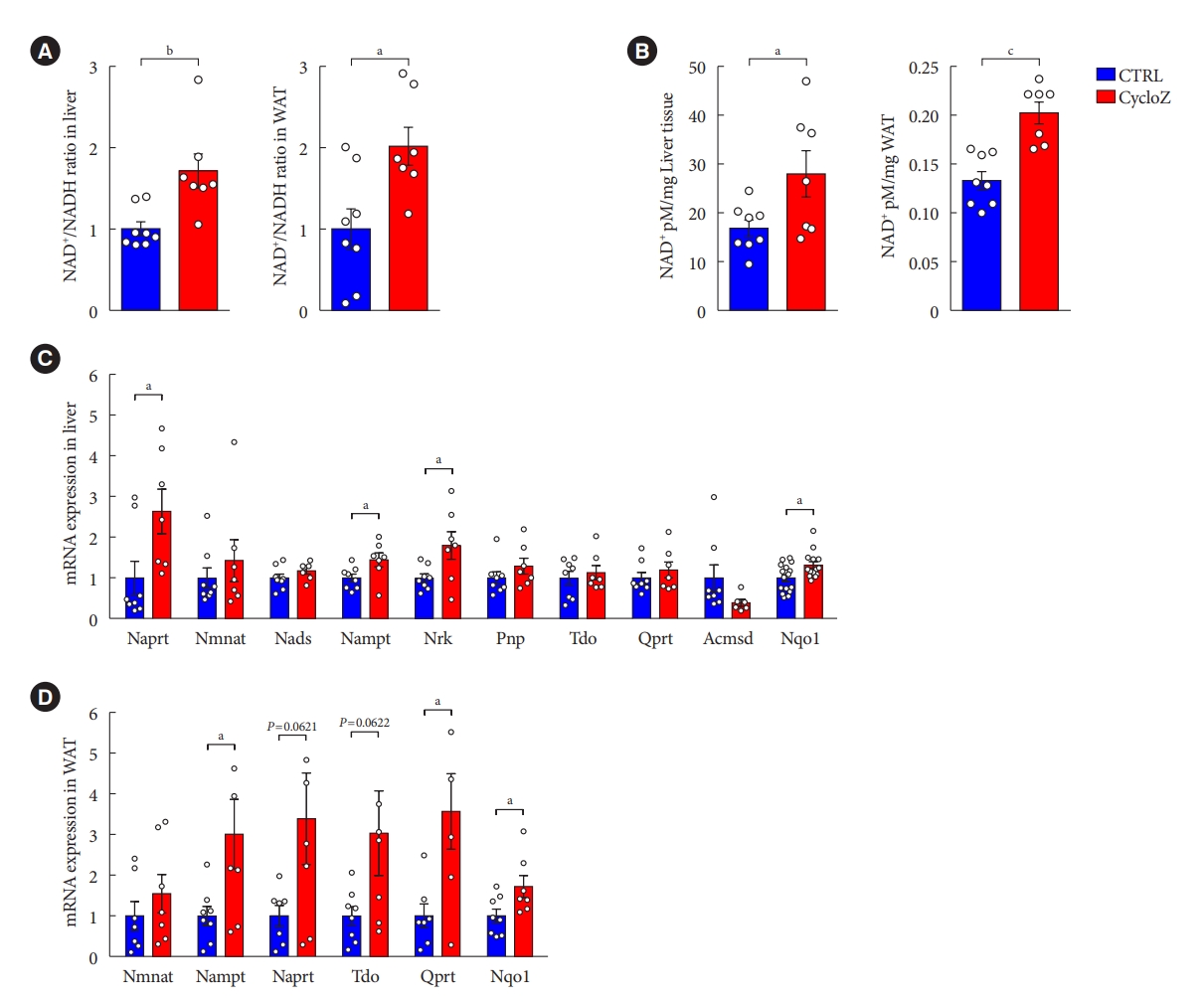
CycloZ administration increases the level of β-nicotinamide adenine dinucleotide (NAD+) content by modulating the expression of genes involved in NAD+ synthesis. (A) NAD+/nicotinamide adenine dinucleotide (NADH) ratio in liver and epididymal adipose tissue (EAT) (n=7–8). (B) Quantification of NAD+ in liver and EAT. (n=7–8). (C, D) mRNAs expression levels related to NAD+ synthesis in liver (C) and mesenteric adipose tissue (D) (n=7–8). Data shown represent mean±standard error of the mean. Unpaired Student’s t-tests. CTRL, control; WAT, white adipose tissue; Naprt, nicotinate phosphoribosyltransferase; Nmnat, nicotinamide mononucleotide adenyltransferase; Nads, NAD synthase; Nampt, nicotinamide phosphoribosyltransferase; Nrk, nicotinamide riboside kinase; Pnp, purine nucleoside phosphorylase; Tdo, tryptophan 2,3-dioxygenase; Qprt, quinolinate phosphoribosyltransferase; Acmsd, aminocarboxymuconate semialdehyde decarboxylase; Nqo1, NAD(P)H quinone dehydrogenase 1. aP≤0.05, bP≤0.01, cP≤0.001.
Therapeutic CycloZ administration improves glucose control in a mouse model of severe T2DM
The in vivo data we reported so far showed the prophylactic effects of CycloZ in KK-Ay mice that were administered the drug at early time points in their progression to hyperglycemia. In the clinical setting, patients with T2DM are only started on drugs when they are diagnosed with pre-diabetes or diabetes. Therefore, it is necessary to examine the therapeutic effect of CycloZ in the later stages of diabetes, which is typically characterized by severe hyperglycemia. According to a study by Iwatsuka et al. [18], KK-Ay mice exhibit an age-dependent increase in hyperglycemia and insulin resistance. By measuring HbA1c levels in KK-Ay mice, we found that hyperglycemia became more severe at 12 weeks of age compared to that of 8 weeks of age (Supplementary Fig. 3A). Therefore, we investigated the therapeutic effect of CycloZ by administering it for 8 weeks in mice that are 12 weeks of age. Glucose tolerance (Fig. 5A) and HbA1c levels (Fig. 5B) were significantly improved by CycloZ administration. Moreover, we also observed the reduced acetylation of PGC-1α and LKB1 and increased AMPK phosphorylation were also evident and fully consistent with the results of the prophylactic study (Fig. 5C and D, Supplementary Fig. 3G). Likewise, the expression of mRNAs related to mitochondrial biogenesis and function were also increased in the liver after the therapeutic administration of CycloZ (Supplementary Fig. 3E and F). In addition, the amount of NAD+, NAD+/NADH ratio and expression of genes involved in NAD+ synthesis were also increased upon CycloZ administration under these conditions (Fig. 5E and F). These results suggest that CycloZ administration is still effective even in a more severe diabetes model.
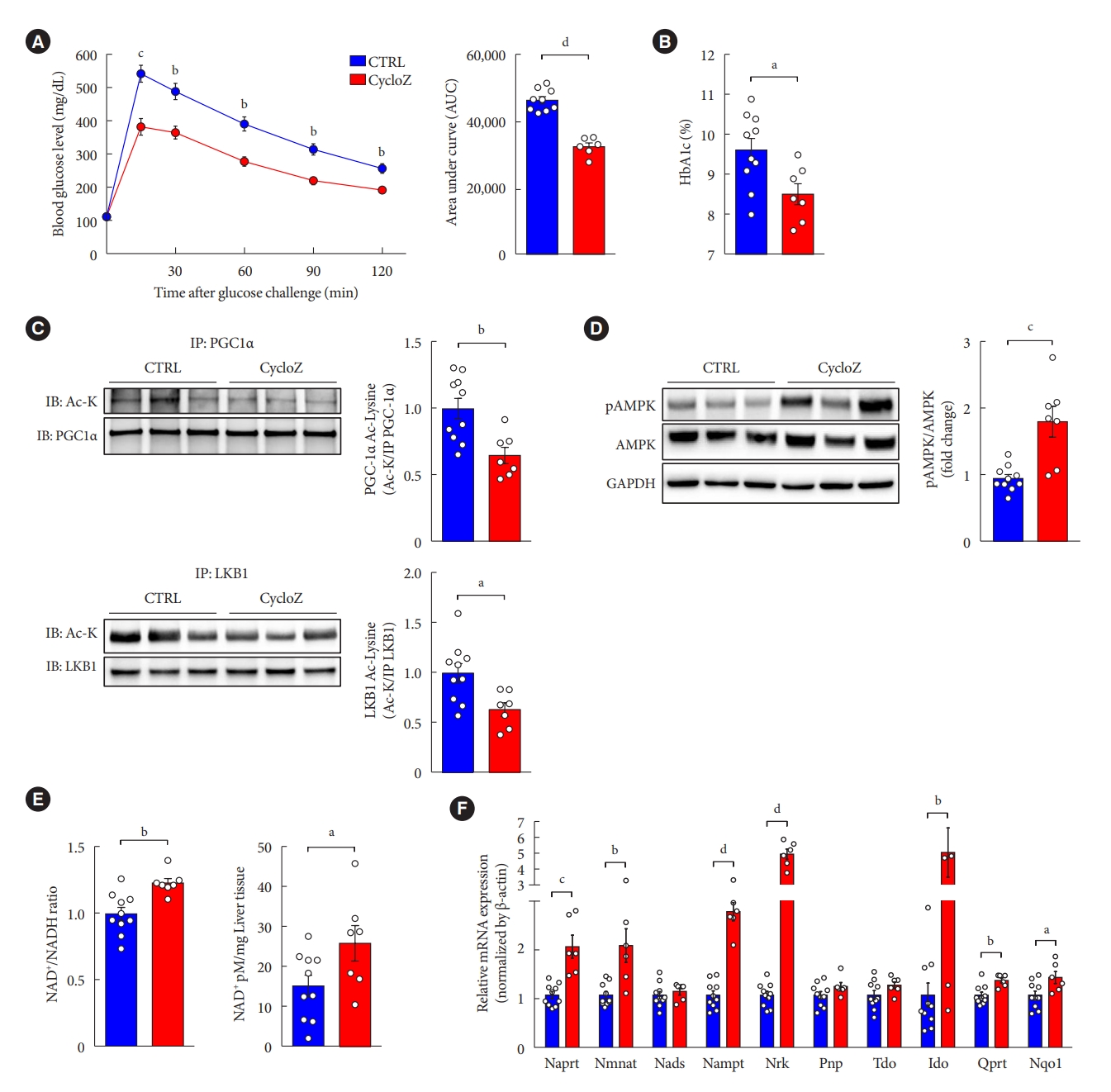
CycloZ administration improves glucose control in a model of severe established type 2 diabetes mellitus. (A) Oral glucose tolerance test for 2 hours after 16 hours fasting and glucose administration (2 g/kg) at 10 weeks of treatment (n=8–10). (B) Glycosylated hemoglobin (HbA1c) level at 11 weeks of treatment (n=8–10). (C) Acetylated lysine levels on peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and liver kinase B1 (LKB1) in liver (n=8–10). (D) Phosphorylated AMP-activated kinase (AMPK) (T172) in liver (n=8–10). (E) Increased the level of β-nicotinamide adenine dinucleotide (NAD+)/nicotinamide adenine dinucleotide (NADH) ratio and quantification of NAD+ in liver (n=8–10). (F) NAD+ synthesis related mRNAs expression levels in liver (n=8–10). Data shown represent mean±standard error of the mean. Unpaired Student’s t-tests. CTRL, control; IP, immunoprecipitation; IB, iimmunoblotting; Ac-K, acetylated-Lysine; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Naprt, nicotinate phosphoribosyltransferase; Nmnat, nicotinate/nicotinamide mononucleotide adenyltransferase; Nads, NAD synthase; Nampt, nicotinamide phosphoribosyltransferase; Nrk, Nik related kinase; Pnp, purine nucleoside phosphorylase; Tdo, tryptophan 2,3-dioxygenase; Qprt, quinolinate phosphoribosyltransferase; Nqo1, NAD(P)H quinone dehydrogenase 1. aP≤0.05, bP≤0.01, cP≤0.001, dP≤0.0001.
DISCUSSION
The metabolism of some trace elements such as zinc, copper, chromium, and manganese, has been reported to be altered in patients with T2DM [33]. Among these elements, zinc levels are is particularly reduced in the blood and various organs, including the liver, muscle, adipose tissue, and pancreas [33,34]. In animal studies, zinc deficiency exacerbates insulin resistance by modulating the activity of enzymes involved in insulin signaling, such as protein tyrosine phosphatase 1B (PTP1B), phosphatase and tensin homolog (PTEN), and tribbles homolog 3 (TRB3) [35]. Studies on the mode of action of CycloZ have mainly focused on its effect on increasing zinc levels. Indeed, CycloZ was reported to chelates zinc ions and promote zinc absorption in the body [36]. In addition, another study reported that CycloZ promotes insulin receptor recycling and ameliorates insulin resistance by increasing the intracellular activity of the insulin-degrading enzyme (IDE), a zinc-dependent metalloprotease that degrades insulin [37]. This was consistent with another study that proposed that increased blood IDE level is a risk factor for diabetes and that the inhibition of extracellular IDE can potentially treat diabetes [38]. In clinical studies, it has been reported that zinc supplementation had little to no benefit in T2DM [39,40].
In the present study, we propose a novel mode of action for the anti-diabetic and anti-obesity effects of CycloZ through the modulation of protein acetylation in the liver and VATs of KK-Ay mice. Increase VATs mass have been suggested as one of the major risk factors for various metabolic diseases [41]. In this study, we found a significant reduction in liver and VAT mass in CycloZ-administered mice. Moreover, PGC-1α deacetylation upon CycloZ administration induced the transcriptional regulation of genes related to mitochondrial function in the liver and VATs. It has been known that a close relationship exists between PGC-1α activity and the development of T2DM, which is associated with mitochondria biogenesis and glucose/fatty acid metabolism [42]. Specifically, the reduced activity of PGC-1α (such as in PGC-1α G482S) has been linked to altered lipid oxidation [43], and the expression of PGC-1α in the adipose tissue is downregulated in patient with T2DM [44].
Chronic inflammation with abnormally increased cytokine levels and immune cell infiltration is observed in many metabolic disorders, contributing to disease progression. In the present study, we observed a reduction in the inflammation of the liver and VATs of CycloZ-treated KK-Ay mice. Previous studies have shown that CHP reduces inflammation by regulating NF-κB and Nrf2 signaling or inhibiting NLR family pyrin domain containing 3 (NLRP3) inflammasome production [19,45]. Interestingly, we found that the acetylation of p65, a key NF-κB subunit, decreased upon CycloZ administration. Sirt1 may also have played an important role in reducing inflammation by deacetylating transcription factors such as p65, resulting in the transcriptional repression of various inflammatory genes.
Notably, we showed that CycloZ regulated the expression of enzymes involved in NAD+ synthesis. Nicotinamide (NAM) is a major contributor to NAD+ synthesis in mammalian cells. In the classical salvage pathway, nicotinamide phosphoribosyltransferase (NAMPT), a rate-limiting enzyme, converts NAM into nicotinamide mononucleotide (NMN) [46]. The mRNA expression of Nampt was increased in both the liver and VATs of CycloZ-treated mice. Since NAMPT is a rate-limiting enzyme, CycloZ treatment increased NAD+ levels, subsequently increasing Sirt1 activity. The NAD+/NADH ratio and the amount of NAD+ were increased only in the liver and VATs but not in the muscle (Supplementary Fig. 2B), suggesting that CycloZ may modulate NAD+ synthesis in a tissue-specific manner. We observed increased Sirt1 mRNA and protein expression in the liver and EAT of mice (Supplementary Fig. 2D and E). SRT1720 and resveratrol, which are known Sirt1 activators, have been reported to increase Sirt1 expression and enzyme activity [47,48]. In our study, it can be inferred that Sirt1 activity may have increased because the NAD+ level and NAD+/NADH ratio, which are essential for Sirt1 activity, were increased. However, further research on whether CycloZ regulates Sirt1 expression is needed to uncover the underlying molecular mechanisms. Together, these data suggest that CycloZ decreases protein acetylation by increasing NAD+ levels and regulating the activity of NAD+-dependent deacetylases such as sirtuins. This warrants future studies on other sirtuin substrates, such as acetyl-CoA synthetase 1 (AceCS1) and histone proteins, and further characterization of the activity of acetyltransferases that control the acetylation status of enzymes related to metabolism.
In general, KK-Ay mice are used as a model of mild hyperglycemia to study the prophylactic effects of drugs on T2DM with obesity [17]. However, results from therapeutic studies are essential for the clinical translation of experimental observation. Hyperglycemia in KK-Ay mice worsens with age [18]; therefore, aged KK-Ay mice with advanced hyperglycemia, an animal model of more severe diabetes, were used to examine the therapeutic effect of CycloZ in this study. CycloZ also had a significant anti-diabetic effect in this severe diabetes model, even in the therapeutic mode of administration. Contrary to the results of the prophylactic study, there were no significant changes in body weight and fat weight in mice after CycloZ administration, but their liver weights decreased (Supplementary Fig. 3B-D). According to these results, CycloZ also works effectively on liver fat metabolism and may be used to treat fatty liver disease. Furthermore, CycloZ was well-tolerated in a long-term study in KK-Ay mice (20 weeks) as well as in a 39-week-long toxicology study in beagle dogs (unpublished data). Likewise, initial clinical studies of CycloZ showed no safety issues (NCT00878605, NCT02784275, and NCT03560-271), suggesting that it may have clinical applications in metabolic diseases.
In conclusion, CycloZ activated the Sirt1/PGC-1α/LKB1/AMPK signaling axis, exhibiting anti-diabetic and anti-obesity properties and an excellent safety profile. Based on these data, we suggest that CycloZ acts as a novel NAD+ booster and Sirt1 deacetylase activator, a mechanism of action different from traditional T2DM drugs.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2022.0244.
Primer sequence data
Plasma biochemical analysis data
(A) Oral glucose tolerance test (OGTT) for 2 hours after 16 hours fasting and glucose administration (2 g/kg) at 10 weeks of treatment with zinc (Zn) gluconate (10 mg/kg) and cyclo-His-Pro (CHP) (5 mg/kg) individually or in combination (n=6–8). (B) Glycosylated hemoglobin (HbA1c) levels in KK-Ay mice at 11 weeks of treatment (n=6–8). (C) Accumulated calorie intake during 20 weeks of treatment (n=7–8). (D, E) Low-density lipoprotein (LDL) cholesterol (CHOL) and total CHOL level in plasma (n=7–8). (F) Adipocyte size frequency of white adipose tissue (n=7–8). Data shown represent mean±standard error of the mean. Unpaired Student’s t-tests. CTRL, control; NS, not significant. aP≤0.05, bP≤0.01, cP≤0.001.
(A) Global acetyl-lysine in liver tissue (n=7–8). (B) Mitochondrial DNA (mtDNA) was measured by mitochondrial D-loops polymerase chain reaction in liver (n=14–16). (C) Increased the level of β-nicotinamide adenine dinucleotide (NAD+)/nicotinamide adenine dinucleotide (NADH) ratio in skeletal muscle (n=7–8). (D) Sirtuin 1 (Sirt1) protein expression in the liver and epididymal adipose tissue (EAT) (n=14–16). (E) Sirt1 mRNA expression in the liver and EAT (n=14–16). Data shown represent mean±standard error of the mean. Unpaired Student’s t-tests. CTRL, control; IB, immunoblotting; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; AU, arbitrary unit; NS, not significant. aP≤0.05, bP≤0.01.
(A) Glycosylated hemoglobin (HbA1c) levels in KK-Ay mice at 8, 12, 16, 20 weeks of age (n=4). (B) Body weight change during 8 weeks of treatment (n=8–10). (C) Accumulated calorie intake during 8 weeks of treatment (n=8–10). (D) Weight of each organ after sacrifice (n=8–10). (E, F) mRNAs expression level related mitochondrial biogenesis and lipid oxidation in liver (n=8–10). (G) Global acetyl-lysine in liver. Data shown represent mean±standard error of the mean. Unpaired Student’s t-tests. CTRL, control; NS, not significant; EAT, epididymal adipose tissue; MAT, mesenteric adipose tissue; SAT, subcutaneous adipose tissue; Foxo1, forkhead box O1; Tfam, transcription factor A, mitochondrial; Esrra, estrogen-related receptor alpha; Nrf1, nuclear respiratory factor 1; Ppargc1a, PPARG coactivator 1 alpha; Sirt1, sirtuin 1; Acox1, acyl-CoA oxidase 1; Ppara, peroxisome proliferator-activated receptor alpha; mcad, medium-chain acyl-CoA dehydrogenase; IB, immunoblotting; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. aP≤0.05, bP≤0.01, cP≤0.001, dP≤0.0001.
Notes
CONFLICTS OF INTEREST
Jongsu Jeon, Dohyun Lee, Bobae Kim, Onyu Park, and Seoyeong Baek are employed by NovMetaPharma. Hoe-Yune Jung and Johan Auwerx are board members of NovMetaPharma.
AUTHOR CONTRIBUTIONS
Conception or design: J.A., K.T.K., H.Y.J.
Acquisition, analysis, or interpretation of data: J.J., D.L., B.K., B.Y.P., C.J.O., O.P., S.B., C.W.L., H.Y.J.
Drafting the work or revising: M.J.K., J.H.J., I.K.L., D.R., S.F., J.A., K.T.K., H.Y.J.
Final approval of the manuscript: J.J., D.L., J.A., K.T.K., H.Y.J.
FUNDING
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI16C1501).
Acknowledgements
None
