Ultra-Processed Food Consumption and Obesity in Korean Adults
Article information
Abstract
Background
This study aimed to investigate the association between consumption of ultra-processed foods (UPF) and obesity in Korean adults.
Methods
We included the Cardiovascular and Metabolic Diseases Etiology Research Center cohort study baseline data of adults aged 30 to 64 years who completed a validated food frequency questionnaire. UPF was defined using the NOVA food classification. Multivariable linear and logistic regression analyses were performed to assess the association of dietary energy contribution of UPF with obesity indicators (body mass index [BMI], obesity, waist circumference [WC], and abdominal obesity).
Results
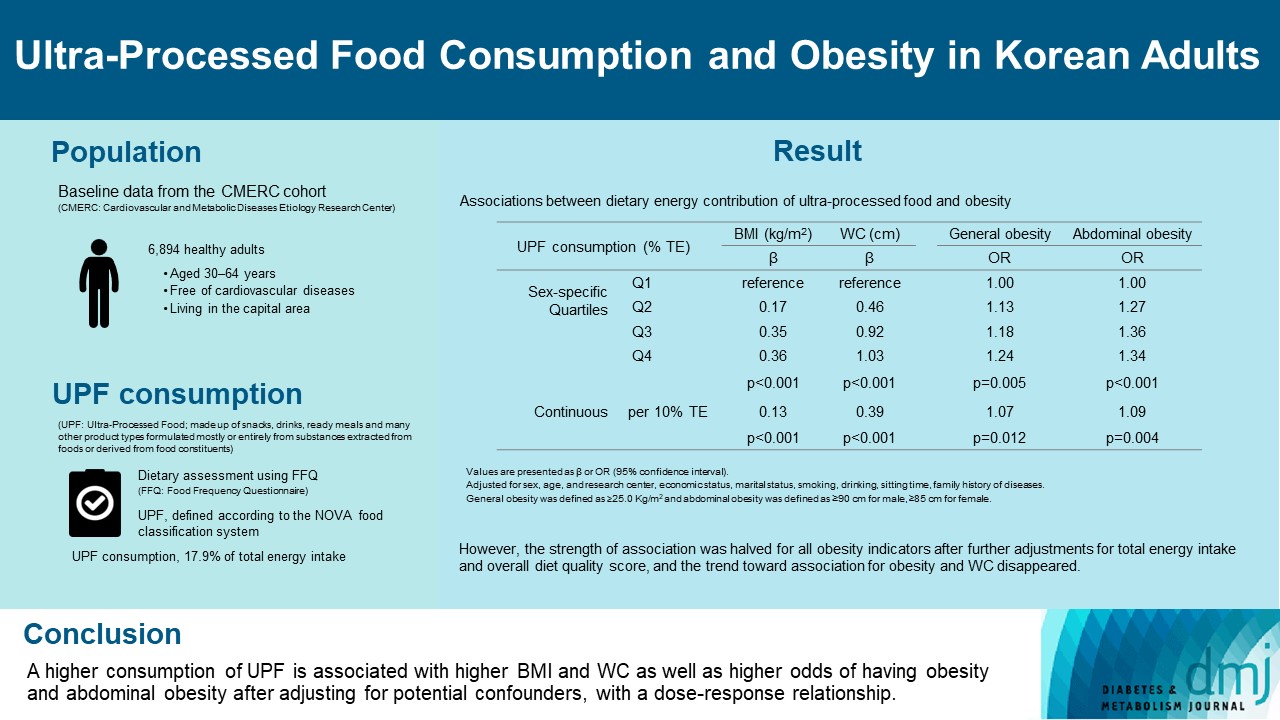
Consumption of UPF accounted for 17.9% of total energy intake and obesity and abdominal obesity prevalence was 35.4% and 30.2%, respectively. Compared with those in the lowest quartile of UPF consumption, adults in the highest quartile had greater BMI (β=0.36; 95% confidence interval [CI], 0.15 to 0.56), WC (β=1.03; 95% CI, 0.46 to 1.60), higher odds of having obesity (odds ratio [OR], 1.24; 95% CI, 1.07 to 1.45), and abdominal obesity (OR, 1.34; 95% CI, 1.14 to 1.57), after adjusting for sociodemographic characteristics, health-related behaviors, and family history of diseases. Dose-response associations between UPF consumption and obesity indicators were consistently found (all P trend <0.01). However, the strength of association was halved for all obesity indicators after further adjustments for total energy intake and overall diet quality score, and the trend toward association for obesity and WC disappeared.
Conclusion
Our finding supports the evidence that consumption of UPF is positively associated with obesity among Korean adults.
INTRODUCTION
Obesity is a major public health burden worldwide. In 2005, two in five adults worldwide were overweight or obese [1]. High body mass index (BMI) is an important risk factor for diabetes, hypertension, cardiovascular disease, cancer, and premature death [2,3]. Despite ongoing efforts to prevent or treat obesity, there has been a continuously rising prevalence of obesity among adults and children both in developed and developing countries [1,4-6]. It is projected that 57.8% of the world’s adult population will be either overweight or obese by 2030 [1].
Basically, obesity reflects energy imbalance wherein consumption exceeds expenditure; however, diverse factors are involved in the etiology of obesity, including genetic, biological, individual, and social factors [2]. Recently, there have been dramatic changes in the global food system [7,8] and the shifts in the food environment have substantially increased opportunities to purchase or consume highly processed foods [9-11]. These foods are typically high energy-dense, poor in nutrient content (high in sugars and fat, and low in dietary fiber), are easily consumed anytime and anywhere, leading to an excess energy intake [12,13]. The consumption of ultra-processed foods (UPFs) has been indicated as an emerging risk factor affecting human health [14,15]. Growing evidence suggests that higher household availability or consumption of UPF is associated with obesity and abdominal obesity [16-26]. However, mostly, these results were of studies conducted among European and American countries, where relatively high UPF is consumed [16,18,20-26]. Little is known regarding whether the consumption of UPF is associated with obesity among populations in Asian countries who consume relatively low levels of UPF.
According to our recent study, Koreans’ consumption of about one-fourth of the total energy from UPF in 2016 to 2018 [27] was lower than those for other countries, with UPF accounting for half of the total energy intake [16,28-30]. However, the past decade revealed a distinctive increase in UPF consumption in all subgroups regardless of sex, age, and economic status, among Koreans [27]. The food environment and sociodemographic structure (e.g., income, urbanization, household composition) have continuously changed and individual preference has changed towards the pursuit for convenience and taste [7,8,10]. In consideration of these findings, the proportion of UPF in the Korean diet is likely to increase. Thus, it seems necessary to provide evidence on the impact of the consumption of these foods on health.
This study aimed to investigate the association between consumption of UPF and obesity in Korean adults using a cross-sectional analysis of the Cardiovascular and Metabolic Diseases Etiology Research Center (CMERC) cohort study baseline data.
METHODS
Data source and population
This study used the baseline data from the CMERC cohort study, designed to identify new risk factors of cardiovascular and metabolic diseases and provide evidence for prevention strategies. The CMERC cohort study comprised healthy adults aged 30 to 64 years who were free of cardiovascular diseases and living in the capital area including Seoul in Korea. Cohort enrollment and baseline survey was completed during 2013 to 2018 at two research clinics. Baseline survey collected a vast amount of information on sociodemographic factors, medical history, health-related behaviors, mental health, social network and support, body size and composition, blood pressure, electrocardiogram, carotid ultrasonography, and biochemical indicators (blood and urine, among others). The CMERC cohort study protocol and procedure were reviewed and approved by the Institutional Review Boards of Severance Hospital, Yonsei University Health System, Seoul, Korea (approval No. 4-2013-0661) and Ajou University Hospital, Suwon, Korea (approval No. AJIRB-BMR-SUR-13-272). Each participant provided written informed consent before the baseline survey. Further details are described elsewhere [31,32].
Of the 8,097 adults who completed the baseline survey, we excluded 854 adults without dietary information (dietary assessment was excluded initially in the CMERC cohort study) and 349 with extreme dietary energy intake (males, <800 or >4,000; females, <500 or >3,500 kcal/day). Finally, 6,894 adults were included in this study.
Dietary assessment and ultra-processed food consumption
Dietary intake was assessed using a 112-item semi-quantitative food frequency questionnaire (FFQ) that was developed for Korean adults’ usual dietary assessment in the Korea National Health and Nutrition Examination Survey (KNHANES) [33]. Participants were asked to respond how often and how much, on average, they consumed each food item listed in the FFQ during the previous year. The frequency response section had nine categories (ranging from never to three times/day) and portion size had three to four categories (small, 0.5; medium, 1.0; and large, 1.5 or 2.0). Dietary intake was calculated using participants’ responses on the frequency and portion size and the nutrient content for each item. As previously evaluated [34], the FFQ presented acceptable reproducibility for nutrients and foods (average γ=0.54 and 0.57, respectively) and modest validity for nutrients (average γ=0.40). We collected dietary data and estimated participants’ usual intake following the guidelines applied in the KNHANES [35].
UPF were defined on the basis of the NOVA (not an acronym) food classification system [12,13], which classifies foods into four groups: (1) unprocessed or minimally processed foods (here, ‘minimally processed’ means processed but only to make unprocessed food suitable for consumption, cooking, or storage, such as cutting, drying, and grinding); (2) processed culinary ingredients (substances derived from group 1 foods, such as sugar, salt, fat and oils; usually used as seasoning when cooking group 1 foods); (3) processed foods (which are industrial products made by adding group 2 foods to group 1 foods to improve the durability and sensory qualities of group 1); and (4) UPF that are industrially formulated from food-driven substances and various cosmetic additives, with little or any whole food, using highly sophisticated processes. For corn, for example, corn powder, corn oil, canned corn, and popcorn or corn chips are classified as group 1, 2, 3, and UPF, respectively.
By applying the NOVA system, we classified 23 out of all FFQ items as UPF and calculated the contribution of UPF to the total energy intake (%TE). In this study, the following items were classified as UPF: ramyeon; loaf bread; bun with red bean paste (or cream) filling; cakes and pies; pizza; hamburger and sandwich; cereals; ham; Korean sausage (sundae); fish paste; milk (including plain milk and sugared milk drinks); yoghurt (liquid type, including plain and sugared yoghurt); yoghurt (curd type, including plain and sugared yoghurt); soybean milk; coffee (with added sugar or cream); carbonated beverages; fruit juice; rice drink; snacks; cookies and crackers; chocolates; ice creams; and distilled liquor (soju).
Anthropometric measurement and definition of obesity and abdominal obesity
Height and weight were measured to the nearest 0.1 cm and 0.1 kg using a stadiometer and scale, respectively. BMI was calculated as body weight (kg) divided by height squared (m2). Waist circumference (WC) was measured to the nearest 0.1 cm at the midpoint between the lower point of the rib cage and the upper point of the iliac crest during exhalation, using a plastic measuring tape. All participants fasted for at least 8 hours and wore a lightweight gown during the examination process. Obesity was defined as BMI ≥25.0 kg/m2 and abdominal obesity as WC ≥90 cm (males) and ≥85 cm (females) using the cutoffs recommended for Asians [36].
Assessment of other variables
Sociodemographic factors included sex, age, economic status, and marital status. Subjective economic status, surveyed using a multiple-choice question, was categorized into three groups (high, middle, and low). Marital status was categorized as married and unmarried (single, divorced, widowed). Health-related behaviors included smoking, drinking, and physical activity. Smoking status was categorized into nonsmoker, ex-smoker, and current smoker. Alcohol drinking was categorized into non-drinker, ex-drinker, and current drinker. Physical activity was assessed using the Korean version of International Physical Activity short form [37]. This study included sitting time (hours/day) as an index of physical inactivity. Each participant was also asked to report whether their parents and/or siblings were diagnosed with or died from hypertension, myocardial infarction, stroke, or diabetes. We defined the presence of family history of cardiovascular and metabolic diseases if their parents or siblings’ diagnosis or death history was reported.
To investigate an independent association of UPF consumption with obesity after additionally adjusting for overall diet quality, we also evaluated participants’ overall diet quality based on the Korean Healthy Eating Index (KHEI) that was developed to estimate overall diet quality of Korean adults [38]. The KHEI has 14 components covering adequate consumption (breakfast; mixed grains; total fruits; fresh fruits; total vegetables; vegetables excluding kimchi and pickled vegetables; meat, fish, eggs, and beans; milk and milk products), moderate consumption (saturated fatty acid; sodium; sweets and beverages), and balanced diet (carbohydrates; total fat; total energy). Each component scored 0 to 5 (or 10), and total score ranged from 0 to 100, with higher score implying higher quality diet. Using the food and nutrients’ dietary intakes assessed by the FFQ, we assigned points for the 13 KHEI components. The FFQ excluded breakfast frequency, but daily meal frequency was included as an additional question. Thus, participants reporting that they had two meals a day or less was considered skipping breakfast. More details on the KHEI are described elsewhere [38,39].
Statistical analysis
We first divided the study population into quartiles using the sex-specific distribution of dietary energy contribution of UPF (% TE) as follows: <13.9%, 13.9%–<21.2%, 21.2%–<28.4%, and ≥28.4% in male; and <9.0%, 9.0%–14.5%, 14.5%–21.2%, and ≥21.2% in female, respectively.
Participant sociodemographic characteristics, health-related behaviors, family history of diseases, and obesity indicators are presented as number (%) and mean±standard deviation. Differences in these characteristics across the sex-specific quartiles of dietary energy contribution of UPF were evaluated by chi-square test and analysis of variance (ANOVA). We also presented the mean intakes of total energy and nutrients and KHEI score across UPF consumption quartiles, and we evaluated differences in the mean dietary intakes across the quartiles, using ANOVA.
Linear and logistic regression analyses were performed to assess association of dietary energy contribution of UPF with obesity indicators. UPF consumption, the explanatory variable, was modelled both as a categorical (quartiles) and continuous variable (per 10% TE increase). Obesity indicators, the outcome variables, were modelled as BMI (kg/m2), WC (cm), obesity, and abdominal obesity. In both linear and logistic regression analyses, we built four multivariable models: model 1 was adjusted for sex, age, and research center; model 2 was adjusted for model 1 plus economic status, marital status, smoking drinking, sitting time, and family history of diseases; model 3 was adjusted for model 2 plus total energy intake; model 4 was adjusted for model 3 plus KHEI score. Odds ratios (OR) and 95% confidence intervals were calculated. Linear trend across quartiles of UPF consumption was tested by treating the quartile as an ordinal continuous variable. We also performed an exploratory subgroup analysis by sex, age group (30–49 and 50–64 years), and overall diet quality (low and high, using the participant median KHEI), using multivariable adjusted model 2. Data analyses were performed using SAS version 9.4 software (SAS institute, Cary, NC, USA) and P<0.05 was considered significant.
RESULTS
Participant characteristics
Table 1 shows the participant characteristics according to sexspecific quartiles of dietary energy contribution of UPF. The mean age of the 6,894 participants was 51.7 years (female, 65.5%). UPF accounted for 17.9% of the total energy intake (range, 6.7% to 31.1% in the lowest and highest quartile, respectively). Participants in the highest quartile were significantly younger than their lowest quartile counterparts (47.9 years vs. 54.7 years); and significantly more likely to be in the low economic status group (24.4% vs. 20.5%); unmarried (16.0% vs. 11.3%); current smoker (22.3% vs. 5.9%); current drinker (78.4% vs. 64.3%); and have longer sitting time (6.4 hours vs. 5.9 hours), respectively (all P<0.001). The highest quartile group had higher intakes of total energy and fat but lower intake of dietary fiber, vitamin A, thiamin, and vitamin C than their counterparts (all P<0.001). Their KHEI score was also lower than that of their lowest quartile counterparts (68.8 vs. 73.4, P<0.001). Regarding obesity, the mean BMI and WC were 24.1 kg/m2 and 82.3 cm, and the prevalence of obesity and abdominal obesity were 35.4% and 30.2%, respectively. There were no significant differences in the means and distribution of obesity indicators by quartiles of UPF consumption.
Associations between dietary energy contribution of UPF and obesity
Table 2 shows the results of associations of UPF consumption with obesity indicators. UPF consumption was associated with higher BMI and WC and higher odds of having obesity and abdominal obesity. In the multivariable analyses adjusted for sociodemographic characteristics, health-related behaviors, and family history of diseases (model 2), the highest quartile group of UPF consumption had 0.36 kg/m2 higher BMI, 1.03 cm higher WC, and 24% and 34% higher odds of having obesity and abdominal obesity, respectively, with dose-response associations (all P trend <0.01). Similar associations were observed when UPF consumption was treated as a continuous variable. Each 10%-point increase in dietary energy contribution of UPF was associated with 0.13 kg/m2 higher BMI, 0.39 cm higher WC, and 7% and 11% higher odds of having obesity and abdominal obesity, respectively. However, the magnitude of positive associations between UPF consumption and obesity indicators was substantially attenuated after adjusting for total energy intake and KHEI score and statistical significance was lost in some analyses (model 3 and 4).
Associations between dietary energy contribution of UPF and obesity by sex, age, and overall diet quality
Table 3 shows the results of subgroup analyses by sex, age group, and overall diet quality on the associations between UPF consumption and obesity indicators, after adjustment for sociodemographic characteristics, health-related behaviors, and family history of diseases. In both males and females, significant dose-response relations were found for all the obesity indicators (all P trend <0.05), except for obesity in males (P trend=0.120). For males and females, a 10%-increase in UPF consumption was associated with higher BMI (both 0.15 kg/m2) and WC (0.55 and 0.37 cm, respectively), and higher odds of having obesity (only in females, OR, 1.11) and abdominal obesity (OR, 1.13 in males; OR, 1.11 in females). Such positive associations of UPF consumption with obesity indicators were found in both age groups (30–49 and 50–64 years), but were slightly stronger among those aged 30 to 49 years than those aged 50 to 64 years. For example, the BMI per 10%-increase in UPF consumption was 0.16 kg/m2 higher in those aged 30 to 49 years than 0.13 kg/m2 in those aged 50 to 64 years. In subgroup analyses by overall dietary quality, each 10%-increase of UPF consumption among those with low dietary quality had a 0.14 kg/m2 higher BMI and 0.48 cm higher WC, 10% and 13% higher odds of having obesity and abdominal obesity. However, such associations were attenuated or disappeared in the high-quality diet group.
DISCUSSION
This cross-sectional analysis of the CMERC cohort study baseline data showed that higher consumption of UPF was associated with higher BMI and WC and higher odds of having obesity and abdominal obesity among Koreans after adjusting for sociodemographic characteristics, health-related behaviors, and family history of diseases. The strength of the association between UPF consumption and each obesity indicator was similar regardless of sex and age group, but there was a subtle difference in subgroup analysis by overall diet quality. The positive association of UPF consumption with each obesity indicator was more clearly observed in those with low quality diet. Furthermore, the association with each obesity indicator was attenuated after further adjustments for total energy intake and overall diet quality score, in addition to sociodemographic characteristics, health-related behaviors, and family history of diseases. Our findings, indicating that higher consumption of UPF was associated with higher obesity indicators, are in line with those of previous studies. An ecological study using nationally representative data from 19 European countries showed that household availability of UPF is positively associated with prevalence of obesity among adults [18]. Another study that analyzed associations between ultra-processed products sales per capita and population-level BMI trajectories across 80 countries found that increases in ultra-processed products volume sales per capita are positively associated with adult BMI trajectories [40]. Similar associations were also found in other studies using individual dietary assessment data [17,19-26]. A cross-sectional study of the Unite States adults aged 20 to 64 years participating in the 2005 to 2014 National Health and Nutrition Examination Survey demonstrated that those in the highest quintile of energy contribution of UPF for total energy (≥74.2%) had 1.61 kg/m2 higher BMI, 4.07 cm greater WC, 1.48 times higher odds of having obesity (BMI ≥25.0 kg/m2), and 1.62 times higher odds of abdominal obesity, compared with those in the lowest quintile (≤36.5%), after adjusting for sex, age, race, education, income, marriage, smoking, and physical activity [20]. Another study of Brazilians with relatively low dietary energy contribution of UPF found similar results of significantly higher BMI and WC (0.80 kg/m2 and 1.71 cm, respectively), and greater chances of having obesity (BMI ≥30.0 kg/m2; OR, 1.41) and abdominal obesity (OR, 1.41), when those in the lowest quartile (<16%) were compared with adults in the highest quartile of UPF consumption (>29%), independent of sex, age, race, income, smoking, physical activity, and comorbidities [19]. Evidence of the detrimental effects of UPF on obesity indicators were further strengthened by cohort studies [22,24-26] and clinical trials [41]. Higher consumption of UPF was strongly associated with increased weight gain and showed higher risks of overweight and obesity [22,24-26]. Some cohort studies investigated whether these associations with obesity indicators were independent of other dietary factors of obesity (e.g., dietary intake of total energy, dietary fiber, ω-3 fatty acid, fruit and vegetable, and overall diet quality) [22,24,26]. They showed that the associations remained unchanged even after further adjusting for other dietary factors. The adverse impact of UPF on obesity was also found in a 2-week crossover randomized clinical trial of healthy adults [41]. In this trial, participants’ energy intake was higher, and their body weight and body fat mass increased during the ultra-processed diet but decreased during the unprocessed diet.
The strength of the associations between UPF consumption and obesity was smaller than those observed in the United States, Canadian, and Australian adults [20,21,23]. This can be partially explained by that the UPF consumption in our study has a lower and narrow distribution, compared to those in the previous studies. In addition, in our study, the associations between UPF consumption and obesity were attenuated after adjusting for total energy intake and overall dietary quality. Total energy intake and overall dietary quality may, at least partially, medicate the association between UPF consumption and obesity.
The mechanisms explaining the association between UPF consumption and obesity are not fully elucidated, but it may be partly understood by their obesogenic nutritional features, physical and structural characteristics, food additives, and packaging materials. As widely known, the design of UPF as industrial products, are focused on profitability, convenience, and high palatability, and they are made from derived food and additive substances, with none or little whole food [12,13]. Thus, these foods generally have poor nutritional value, are high in energy, refined carbohydrate, added sugars, total fat and saturated fat, and low in dietary fiber, minerals, and vitamins [12,42]. Consumption of these foods is associated with excess caloric intake, poor dietary intakes, as well as a low diet quality [39,41,43]. Furthermore, UPF themselves may contribute to overeating; that is, foods that are easily consumed anywhere and at any time, and that may alter the consumers’ eating behavior, promote inattentive eating, thereby leading to overconsumption [44]. Furthermore, chronic consumption of foods high in refined sugars and fat causes changes in the brain’s reward pathway, leading to food addiction and overeating [45]. Moreover, through industrial processing, including moisture removal and heating, these foods undergo physical and structural changes (i.e., reduced total volume and softened textured). Therefore, these changes may lead to rapid eating rate, which interrupts the mechanisms controlling satiety, inducing excess caloric intake [44]. A recent review suggested that dietary exposure of food additives (e.g., flavor, sweetener, emulsifier, stabilizer, thickener, preservative) can increase glucose intolerance, and alter the gut microbiota, resulting in increased weight gain and increased risk of metabolic syndrome [46]. Another review demonstrated that food contaminants which are leached out from food packaging materials (e.g., phthalates, bisphenol-A) might disrupt the hormonal control regarding hunger and satiety, alter individual’s dietary intake, and thus lead to weight gain and fat storage [47].
The present study has several strengths. Most previous studies which examined the association between UPF consumption and obesity assessed dietary intake using 24-hour recall [17,20,21,23,24] or used food purchase or sales data as a proxy indicator of food consumption [18,40]. The dietary exposures measured in those studies may not represent individuals’ usual consumption of UPF, with the possibility of biased result. However, the present study collected dietary information using a validated FFQ [34] which is generally recommended for long-term diet assessment [48], and thus could investigate the association of the usual consumption of UPF with obesity. Moreover, some previous studies used estimates of obesity prevalence from national surveys [18], or defined obesity using national BMI estimates derived from population-based studies [40], or partially used self-reported height and weight [21]. However, we used anthropometric values measured by a standardized method, and the stadiometers and scales used during the entire study period at the two research clinics were calibrated regularly according to a standardized protocol [32]. Third, we identified UPF among FFQ items, according to the NOVA food classification. The NOVA classification system had been widely applied in the research of highly processed foods [12,13]. Finally, in most epidemiologic studies [17,18,20, 21,23,25], potential confounders were considered when examining the association of UPF and obesity, but no independent association was determined for other dietary factors known to be associated with obesity. Our study could examine whether the associations between UPF consumption and obesity persisted after further adjustment for total energy and overall dietary quality, and also provide the subgroup analysis results by sex, age groups, and overall diet quality.
Nevertheless, several limitations must be considered. As a cross-sectional study, our findings cannot support the causal relationship between UPF consumption and obesity indicators. Next, although the FFQ used is acceptable to assess usual dietary intake, it was not designed to classify foods based on the food processing status. Based on the Food and Agriculture Organization of the United Nations guidelines [49], diverse information such as ingredient list, preparation methods (for foods prepared at home or outside-the home), as well as brand and product name (for foods produced in industrial settings) are needed, in order to classify foods by the nature, extent, and purpose of food processing. Open-ended dietary assessment such as the 24-hour recall have been suggested as the most appropriate method to obtain information on food processing. However, the CMERC cohort study assessed dietary intake using FFQ, and thus UPF was identified by applying the NOVA classification to each food item description in the FFQ. Therefore, the present study cannot rule out the possibility of misclassification in assessing UPF consumption. In addition, self-reported dietary data are subject to misreporting from social desirability bias. This tendency is more frequently observed for foods considered unhealthy and in individuals with obesity [21], which would result in attenuation of the association of UPF consumption with obesity indicators among our population. Finally, although we considered diverse confounders, the potential of residual confounding exists.
In summary, our findings add to the growing evidence indicating that a higher consumption of UPF is associated with higher BMI and WC as well as higher odds of having obesity and abdominal obesity after adjusting for potential confounders, with a dose-response relationship. When additionally adjusting for dietary factors, such associations were attenuated. These findings show that even in a population with relatively low consumption of UPF, the consumption of UPF is associated with obesity. Currently, the UPF types are diverse, with increasing sales and transition towards more highly processed diets observed worldwide [7,8,11,27,28], although with variations across regions and countries. Further studies are needed to better understand the underlying mechanisms of UPF consumption and health outcomes as well as the impact of UPF on various health outcomes. Furthermore, strategies and actions to reduce UPF consumption and enhance the consumption of less processed foods should be considered.
Notes
CONFLICTS OF INTEREST
Dae Jung Kim has been associate editor of the Diabetes & Metabolism Journal since 2022. Hyeon Chang Kim has been statistical advisor of the Diabetes & Metabolism Journal since 2021. They were not involved in the review process of this article. Otherwise, there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Conception or design: J.S.S., H.C.K.
Acquisition, analysis, or interpretation of data: J.S.S., K.H.H., D.J.K., H.C.K.
Drafting the work or revising: J.S.S., H.C.K.
Final approval of the manuscript: J.S.S., K.H.H., D.J.K., H.C.K.
FUNDING
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A1-A01064904) and the Basic Research Program via the National Research Foundation of Korea funded by MSIT (NRF-2019R1-A4A1028155). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
None