Gestational Diabetes Mellitus and Its Implications across the Life Span
Article information
Abstract
Gestational diabetes mellitus (GDM) has historically been perceived as a medical complication of pregnancy that also serves as a harbinger of maternal risk of developing type 2 diabetes mellitus (T2DM) in the future. In recent decades, a growing body of evidence has detailed additional lifelong implications that extend beyond T2DM, including an elevated risk of ultimately developing cardiovascular disease. Furthermore, the risk factors that mediate this lifetime cardiovascular risk are evident not only after delivery but are present even before the pregnancy in which GDM is first diagnosed. The concept thus emerging from these data is that the diagnosis of GDM enables the identification of women who are already on an enhanced track of cardiometabolic risk that starts early in life. Studies of the offspring of pregnancies complicated by diabetes now suggest that the earliest underpinnings of this cardiometabolic risk profile may be determined in utero and may first manifest clinically in childhood. Accordingly, from this perspective, GDM is now seen as a chronic metabolic disorder that holds implications across the life span of both mother and child.
INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as glucose intolerance of varying severity with onset or first recognition during pregnancy. It is considered a medical complication of pregnancy that carries an increased risk of adverse obstetrical outcomes, including macrosomia, shoulder dystocia, birth injury, prematurity, perinatal mortality, and need for Caesarian section [1]. Accordingly, the screening of pregnant women for GDM and the antenatal treatment thereof has become a standard component of obstetrical care. However, the implications of GDM extend well beyond the pregnancy [2]. Most notably, it has long been known that GDM identifies women who are at risk of developing type 2 diabetes mellitus (T2DM) in the future. In recent years, there has been growing recognition that GDM also predicts higher risks of other chronic conditions, including cardiovascular disease (CVD) [2]. Moreover, the risk factors and determinants of these conditions can be detected even before the pregnancy in which GDM is diagnosed. Indeed, the early underpinnings of these risk factors are already evident in the children of GDM pregnancies. As we shall explore in this review, the concept emerging from these data is that, although it is identified and treated in pregnancy, GDM carries implications across the life span for mother and child (Fig. 1).
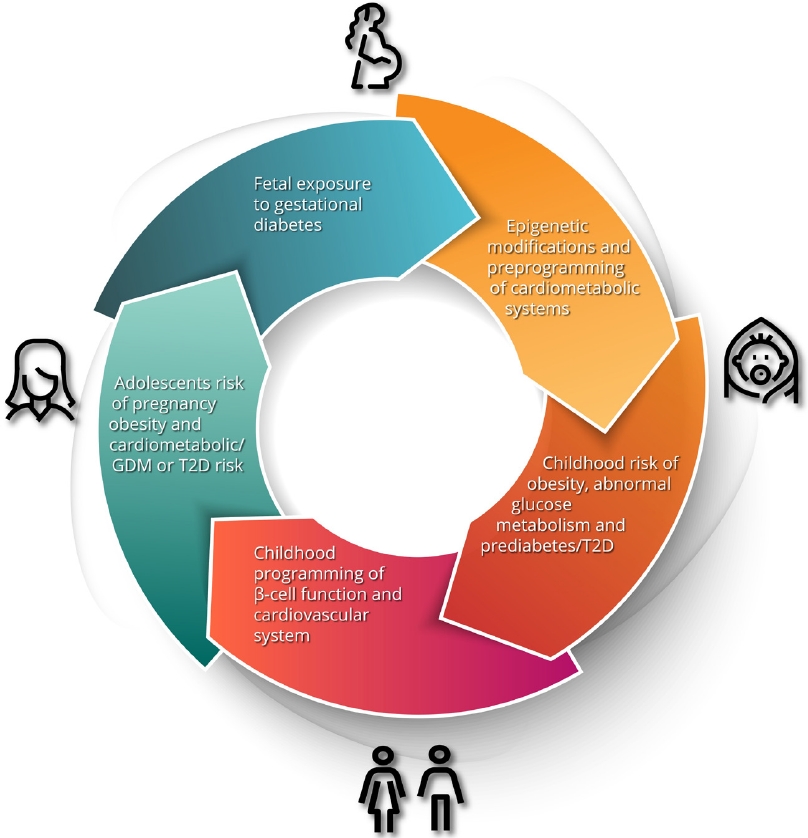
The intergenerational impact of gestational diabetes. Maternal hyperglycemia and resultant fetal hyperinsulinism results in developmental and epigenetic preprogramming of cardiometabolic risk in the offspring including obesity, dysglycemia, dyslipidemia, hypertension and early renal dysfunction. These risks progress to evident cardiometabolic abnormalities in adolescents and predispose young women to the development of gestational diabetes mellitus (GDM) in pregnancy, thereby restarting the cycle. T2D, type 2 diabetes mellitus.
MATERNAL HEALTH AFTER GDM PREGNANCY
Type 2 diabetes mellitus
Since the time of its initial description, GDM has been linked to T2DM. Indeed, in 1964, O’Sullivan and Mahan [3] reported that specific glycemic thresholds on the 3-hour 100 g oral glucose tolerance test (OGTT) in pregnancy could predict a woman’s risk of developing T2DM in the years thereafter, thereby establishing the initial diagnostic criteria for GDM. In the ensuing decades since then, studies have repeatedly demonstrated the truism that maternal glycemia in pregnancy is a predictor of future T2DM [2,4]. Of note, this relationship is evident irrespective of the diagnostic criteria that are applied for identifying GDM. When one considers that the varying sets of criteria (and their respective diagnostic thresholds) label different degrees of gestational glycemia as GDM, it becomes apparent that the relationship with future risk of T2DM must extend to the non-GDM range of glycemia (i.e., as identified by all but the least stringent GDM diagnostic criteria). For example, when compared to their normoglycemic peers, women with an abnormal OGTT that doesn’t meet the diagnosis of GDM by the stringent National Diabetes Database Criteria have an elevated risk of T2DM (albeit lesser than that of those with GDM) [5-7]. In fact, it is now recognized that the any degree of dysglycemia in pregnancy predicts a proportionate risk of progressing to T2DM in the future, with GDM representing the most severe element along both of these continua (i.e., the highest gestational glycemia and the highest risk of T2DM) [6-8]. The physiologic basis for this relationship can found in the underlying pathophysiology that links GDM and T2DM.
The pathophysiologic link in question is pancreatic β-cell dysfunction. Specifically, the progressive insulin resistance that characterizes normal human pregnancy from mid-gestation onwards poses a physiologic challenge (or stress test) for the β-cells, which must increase their secretion of insulin accordingly to maintain normoglycemia. Any insufficiency of β-cell compensation in response to this challenge will lead to dysglycemia in pregnancy, with GDM representing the most severe element thereof. Notably, women who develop GDM have a chronic β-cell defect that first becomes clinically apparent through the antenatal hyperglycemia that arises due to the insufficiency of their compensatory response [9]. Importantly, in addition to underlying the presentation of GDM in pregnancy, this β-cell defect is ultimately responsible for their development of T2DM in the future [10]. Specifically, women with GDM exhibit deterioration of β-cell function in the years after their pregnancy that is apparent even within the first year postpartum while their glucose tolerance may remain in the normal range [7,11]. Over time, however, this deterioration of β-cell function drives their progression from normal glucose tolerance to pre-diabetes and ultimately to T2DM [7,12,13]. Thus, the epidemiologic and clinical link between GDM and T2DM is rooted in a shared underlying pathophysiology of β-cell dysfunction.
Studies estimating the magnitude of risk of T2DM in women with a history of previous GDM have reported considerable variability, partly reflecting the impact of several methodologic factors. These factors include (1) between-study differences in GDM screening protocols and diagnostic criteria (thereby yielding differences in the severity of β-cell dysfunction and hence risk of T2DM); (2) variability in postpartum surveillance protocols and adherence thereto between-study populations; and (3) differences in the non-GDM control population against which women with GDM were compared. When considered collectively (albeit in the context of these limitations), this literature reveals that women with a history of GDM have a 7- to 10-fold higher risk of T2DM than that of their peers [14,15], reflecting an enormous risk increment for a major chronic disease in a population of young women of reproductive age.
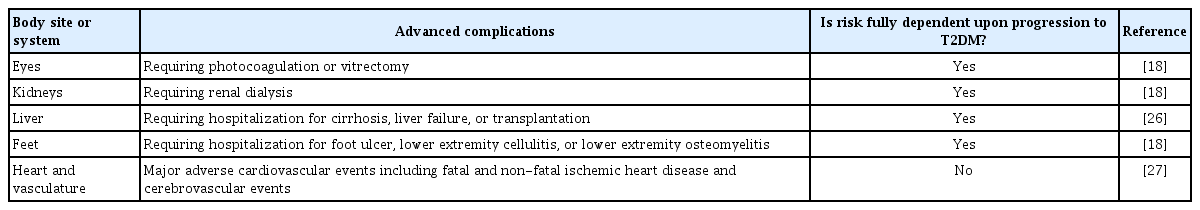
Advanced complications associated with T2DM
The relative youth of this high-risk patient population raises the possibility of developing T2DM at a comparatively early age, thereby resulting in longer exposure to the metabolic dysregulation of diabetes over the course of a lifetime. Since cumulative glycemic exposure is a determinant of risk for the vascular complications of T2DM, it is reasonable to anticipate that the incidence of such outcomes may be increased in women with previous GDM [16,17]. Indeed, consistent with this hypothesis, women with a history of GDM have an elevated risk of advanced retinopathy outcomes that is dependent upon the intercurrent development of diabetes [18]. Similarly, this patient population has higher incidence rates of advanced nephropathy (including initiation of dialysis) and hospitalization for foot infection (foot ulcer, lower extremity cellulitis, or lower extremity osteomyelitis, suggestive of neuropathy and/or peripheral vascular disease), with both risks dependent upon intercurrent T2DM [18]. While women with previous GDM do not appear to be at risk of these advanced complications in the absence of T2DM, increased estimated glomerular filtration rate has been reported in this patient population, potentially indicative of early glomerular hyperfiltration and associated renal dysfunction [19]. Similarly, increased microalbuminuria has been demonstrated in women with previous GDM upon progression to pre-diabetes [20], suggesting that renal changes and worsening glucose tolerance may be concomitant processes early in their respective natural histories.
A similar longitudinal relationship over time is evident between GDM and liver disease. Specifically, the presence of liver fat on abdominal ultrasound in early pregnancy has been shown to predict subsequent dysglycemia in 2nd trimester [21]. When compared to their peers in the years after delivery, women with previous GDM show higher rates of fatty liver, the greater severity of which is associated with glucose intolerance [22-25]. Ultimately, women with a history of GDM have an elevated long-term risk of advanced liver disease (hospitalization for cirrhosis, liver failure, or transplantation) that emerges only in those who progress to T2DM in the intervening years [26]. Thus, taken together, the risks of advanced ophthalmologic, nephropathic and hepatic complications in women with a history of GDM appear to be dependent upon the development of T2DM (Table 1), with both renal and liver dysfunction showing evidence of long-term progression of disease processes over time.
CVD with or without T2DM
A similar long-term relationship exists between GDM and CVD but with one key difference: the elevated risk of CVD outcomes is not fully attributable to T2DM (Table 1) [18,27]. Indeed, over the past decade, a series of studies has shown that women with a history of GDM have a higher incidence of CVD events (fatal and non-fatal ischemic heart disease and cerebrovascular events) than that of their peers [18,27-34]. A meta-analysis of these studies (involving >5 million women and >100,000 events) has revealed that, compared to their peers, women with GDM have a 2-fold higher risk of future CVD events (relative risk [RR], 1.98; 95% confidence interval [CI], 1.57 to 2.50) [27]. Of note, on meta-regression analysis, the rates of incident T2DM across studies did not affect this risk. Furthermore, even when restricting the meta-analysis to only women who did not develop T2DM, GDM predicts a 56% higher risk of future CVD events (RR, 1.56; 95% CI, 1.04 to 2.32) [27]. In addition to major adverse cardiovascular events, GDM has also been associated with higher rates of heart failure [34].
The recognition that incident CVD in women with previous GDM is not fully dependent upon intercurrent T2DM raises the question of the relevant determinants of cardiovascular risk in this patient population. In this context, it is notable that studies have consistently found that, compared to their peers, women with a history of GDM have a higher prevalence of cardiometabolic risk factors, including dyslipidemia, hypertension, overweight/obesity, and metabolic syndrome [35-39]. Moreover, this adverse cardiovascular risk factor profile is evident by 3-month postpartum [37,38]. Its presence so soon after delivery raises the question of whether the adverse risk factor profile is a consequence of the GDM pregnancy or alternatively if it might actually precede the diagnosis of GDM. To address this question, we must consider what is known about the health of women who go on to develop GDM.
Maternal health prior to GDM pregnancy
Various lines of evidence have suggested that, although GDM is typically diagnosed in late 2nd or early 3rd trimester, there are indeed findings that may precede the diagnosis. First, women who go on to develop GDM already exhibit metabolic changes in their amniotic fluid in 1st trimester, including altered levels of glucose, insulin and insulin-like growth factor-binding protein-1 [40]. Second, it has been shown that fetal overgrowth may precede the diagnosis of GDM [41]. Third, an increased likelihood of the subsequent development of GDM can be predicted by 1st trimester measurements of circulating biomarkers and analytes, including glycemic measures, fasting insulin, adiponectin, high density lipoprotein (HDL) cholesterol, triglycerides, C-reactive protein, tissue plasminogen activator antigen, and insulin-like growth factor-binding protein-2 [42]. It thus emerges that there exists a potentially detectable phenotype in early pregnancy that may predict higher risk of subsequent GDM, thereby leading to the question of whether such a presentation could be evident even before conception.
Prior to pregnancy, there are indeed differences between women who go on to develop GDM and those who do not. These differences include higher A1c, fasting glucose, low density lipoprotein (LDL) cholesterol and triglycerides, coupled with lower HDL cholesterol [43-46]. Moreover, in the 5 years prior to the index pregnancy, these cardiovascular risk factors exhibit divergent trajectories between women who go on to develop GDM and their peers, resulting in an amplification of the differences over time [46]. These divergent tracks may also be differentially affected by the pregnancy in women with and without GDM, resulting in further amplification of differences in the postpartum [47]. Ultimately, in enabling the diagnosis of GDM, pregnancy can be seen as a life event that facilitates the identification of a population of young women who are gradually developing a high-risk cardiometabolic phenotype over time [48]. Furthermore, the progressive worsening of their cardiometabolic risk factor burden in the years before pregnancy raises the question of how early in life this pathologic process might actually begin.
HEALTH OF THE OFFSPRING OF GDM PREGNANCY
Evidence of the existence of an unfavorable cardiometabolic profile prior to pregnancy suggests the increased risk for the development of pre-gestational T2DM, GDM, and post-gestational T2DM has early life origins. Following the landmark publication of Barker [49] introducing the concept of developmental origins of health and disease, there has been significant interest in understanding the intergenerational basis for cardiometabolic risk. The impact of in utero exposure to maternal diabetes to the offspring is 2-fold; the immediate perinatal complications and the longer-term risks of cardiometabolic disease. Although the focus of this review is maternal GDM, it is important to note that many of the original studies looking at offspring outcomes of maternal hyperglycemia have combined populations of maternal hyperglycemia, pre-gestational, and maternal GDM exposures.
Postnatal complications of exposure to gestational diabetes in utero
Maternal obesity, elevated gestational weight gain and hyperglycemia are known risk factors for fetal overnutrition, hyperinsulinism, production of insulin-like growth factor-1, and resultant overgrowth. Fetal hyperinsulinism itself has been associated with a disproportionate increase in fetal fat mass, altered fetal lung surfactant [50] resulting in neonatal respiratory distress syndrome, and neonatal hypoglycemia [51]. In addition, maternal hyperglycemia is associated with a relative fetal hypoxia and, devastatingly, fetal asphyxia, and stillbirth [52]. Other developmental impacts of maternal GDM include congenital anomalies of the heart, genitourinary tract, face (cleft lip +/– palate) and central nervous system. A recent large epidemiological study [53] reported an increased overall congenital anomaly risk associated with GDM exposure (odds ratio [OR], 1.28; 95% CI, 1.24 to 1.31), accounting for maternal age, ethnicity, education, smoking, parity, pre-pregnancy body mass index (BMI), hypertension, and infant sex. The perinatal and postnatal morbidity in offspring exposed to maternal hyperglycemia is evident across the spectrum of dysglycemia and is a harbinger of longer-term complications to follow.
Childhood cardiometabolic complications
The impact of fetal overnutrition and overgrowth appears to extend into infancy and childhood with increases in neonatal and infant fat mass and childhood risk of overweight and obesity. The early proposal by Pedersen [54] implicating intrauterine hyperglycemia in pregnancy in offspring obesity and T2DM risk has gained support in recent decades through epidemiological and prospective birth studies. Human observational studies have shown an increased birthweight and neonatal fat mass [55,56], abdominal adiposity [57], and overweight and obesity in those offspring exposed to maternal hyperglycemia and GDM [58-63]. A seminal study of sibships in the Akimal O’odam (Pima) population comparing offspring born before and after a diagnosis of maternal diabetes revealed an increased risk of childhood obesity with maternal diabetes exposure independent of shared genetics and environment [64]. Although reports of the impact of GDM exposure on BMI have been conflicting, more recent data exploring offspring body composition measures beyond weight and BMI from the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS) and Exploring Perinatal Outcomes in Children (EPOCH) study support the independent association between maternal diabetes exposure and elevated offspring adiposity. The HAPO FUS reported that exposure to GDM in pregnancy was associated with an increased odds of childhood obesity by 54%, elevated body fat by 35%, and elevated waist circumference by 34% at age 10 to 14 years [65]. The EPOCH study of multiethnic cohort of youth from Colorado reported a positive correlation of GDM exposure with offspring BMI, waist circumference, and visceral and subcutaneous adiposity; this relationship tracked from childhood (mean age 10.5 years) into adolescence (mean age 16.7 years) and was not significantly impacted by the postnatal environment [66]. Both studies showed attenuated but persistent effects when accounting for maternal pre-pregnancy BMI. Many, but not all [67] studies which have adjusted or accounted for maternal weight (pre-pregnancy BMI and/or gestational weight gain) have shown persistence of a significant childhood obesity risk with maternal diabetes exposure. The degree and timing of excessive childhood adiposity vary depending on the study methodology, suggesting variation in risk based on population, degree and timing of exposure to maternal dysglycemia, treatment of maternal dysglycemia, and other associated environmental exposures. Interestingly not all offspring exposed to diabetes in utero are large-for-gestational-age, have increased childhood adiposity or develop T2DM, suggesting that additional studies focusing on the mechanisms of intergenerational inheritance of risk and the interaction of these mechanisms with postnatal modifiers are urgently needed.
Childhood risk of developing insulin resistance and dysglycemia
In addition to the direct risk associated with obesity in the offspring, elevated maternal glycemia in pregnancy is a predictor of disorders of glucose metabolism including insulin resistance, reduced acute insulin response, impaired glucose tolerance [58,68,69] and T2DM [70] in the offspring. Studies in the Akimal O’odam population provided early evidence that maternal hyperglycemia increases both childhood obesity risk and insulin resistance and T2DM in the offspring [71]. More recent results from the HAPO FUS [69] comparing offspring born to mothers with and without GDM revealed higher plasma glucose levels at 30, 60, and 120 minutes during a 75 g OGTT in offspring born to mothers with GDM. This relationship remained after controlling for measures of current maternal and child obesity. In addition, compared to unexposed offspring, exposure to maternal GDM was associated with decreased insulin sensitivity and β-cell compensation, and an increased odds of developing impaired glucose tolerance (OR, 1.96; 95% CI, 1.41 to 2.73) at follow-up ages 10 to 14 years.
The SEARCH for Diabetes in Youth (SEARCH) study supports these observations as maternal diabetes and obesity exposure in utero accounted for 47% of the risk for developing childhood T2DM in offspring [72]. A large Canadian population-based prospective cohort study demonstrated both GDM and T2DM exposure were important independent risk factors for the development of T2DM and were associated with an accelerated time to diagnosis of T2DM in offspring [70]. Thus, exposure to maternal diabetes (T2DM and GDM) is associated with the development of pre-diabetes and diabetes in the offspring independent of the risk of childhood obesity and excess adiposity, suggesting a potential role of altered β-cell development and ultimately function in early life.
Although the underlying pathophysiology linking maternal diabetes exposure to offspring obesity and metabolic dysregulation remains unknown, accumulating evidence suggests that changes in DNA methylation resulting in altered gene expression link the prenatal environment to later-in-life disease development [73-75]. Altered DNA methylation of genes following exposure to maternal diabetes has been associated with childhood adiposity and T2DM [75-77]. Cord blood analysis in 14 Akimal O’odam offspring exposed to diabetes in pregnancy compared to 14 unexposed offspring revealed differentially methylated regions in promoters controlling insulin signaling and metabolic pathways [75,76,78]. Importantly, prenatal epigenetic marks can persist into adolescence and be associated with changes in glucose and lipid metabolism [76].
Long-term health consequences in offspring exposed to gestational diabetes
The independent impact of GDM exposure on offspring risk of CVD is difficult to determine owing to the frequent co-existence of obesity, central adiposity and dysglycemia of the offspring exposed. Indeed, the long-term cardiovascular risk to exposed offspring is likely multifactorial with increased adiposity, insulin resistance and hyperinsulinism, and T2DM playing important roles. However, studies have attempted to identify whether increased risk of specific cardiovascular risk factors such as hypertension and dyslipidemia can be linked to maternal diabetes exposure in utero. Early clinical studies in the Akimal O’odam population revealed an increase in systolic blood pressure by 11 mm Hg in offspring exposed to maternal diabetes independent of offspring sex, current measures of adiposity, and family history of diabetes [79]. A more recent large population prospective cohort study in Portugal [80] reported elevated systolic and diastolic blood pressures at age 10 in offspring born to mothers with GDM compared to unexposed peers. The relationship was mediated by childhood BMI and was sex-specific, with boys having higher risk than girls.
The risk of hypertension in offspring exposed to maternal diabetes may, in part, result from early renal dysfunction. Reports suggest in some populations up to approximately 50% of youth living with T2DM will develop micro- and macrovascular complications, including end-stage renal disease requiring dialysis, within 15 years of diagnosis (mean age 29 years old) [81,82]. In a cohort of predominantly First Nations adolescents with T2DM, approximately 30% had albuminuria 2-year postdiagnosis, suggesting that kidney disease occurs early and may be independent of persistent hyperglycemia [83]. Supporting evidence from a national surveillance study revealed that 5.4% of Canadian youth with T2DM had albuminuria in the first year of diagnosis and, compared to unexposed youth, were more likely to have been exposed to T2DM in utero [84]. Mechanistically, nephrogenesis is not complete until 36-week of gestation and intrauterine exposure to T2DM may be a critical determinant of nephron endowment [85], and later-in-life function. In mice, exposure to maternal diabetes in utero alters renal development by decreasing branching morphogenesis, impairing nephrogenesis and decreasing nephron endowment at birth [86], which has been shown to cause glomerular hypertrophy, hyperfiltration and subsequently, albuminuria [87].
Studies examining a more comprehensive panel of cardiometabolic risk factors include follow-up data from the EPOCH cohort [88,89] and a large Danish population-based cohort [90]. Short-term follow-up of children in EPOCH revealed associations of maternal GDM with elevated waist circumference, and an early biomarker of endothelial dysfunction, while longer-term follow-up in adolescence reported additional associations with unfavorable lipid profiles in girls (elevated total cholesterol and LDL cholesterol) and elevated systolic blood pressure in boys. Of interest, the relationship with lipid profiles in girls was not significant after adjustment for maternal GDM treatment, suggesting that hyperglycemia is the main culprit to fetal underpinnings of dyslipidemia. The Danish based cohort [90] showed that, after accounting for childhood BMI, exposure to GDM in utero was associated with insulin resistance, elevated fasting blood sugar, insulin and C-peptide levels, and central adiposity in offspring.
Fewer studies have examined hard outcomes such as cardiovascular events in offspring exposed to GDM. Two large population-based cohort studies have shown an increased odds of CVD in offspring at 35 to 40 years. The population-based administrative birth cohort study from Manitoba Canada revealed that intrauterine exposure to GDM is associated with 1.9-fold increased hazard for cardiovascular risk factors (hypertension, dyslipidemia, T2DM) and 1.42-fold increased risk of CVD (incident myocardial infarction, heart failure, cerebral vascular infarction) within 35 years of life compared to unexposed offspring. The authors also noted that increased CVD morbidity was driven by early-onset hypertension, T2DM, and dyslipidemia [91]. The other population-based study from Denmark [33,92] reported GDM exposure to be associated with increased rates of CVD (heart failure, hypertension, deep vein thrombosis, pulmonary embolism) in offspring at 40 years of follow-up (OR, 1.19; 95% CI, 1.07 to 1.32) which persisted when adjusted for maternal and paternal history of CVD. However, the strongest associations were found among the offspring of mothers with a history of diabetes complications or a history of CVD, suggesting a central role of chronic hyperglycemia in the mother in determining the elevated offspring risk.
A causal role for intrauterine exposures in increasing CVD risk is complicated by combined exposures to maternal diabetes and obesity and by postnatal exposures and resultant obesity in offspring. The causal link has been examined in a recent review of animal studies in which maternal GDM or hyperglycemia led to offspring cardiovascular complications including hypertrophy, increased left ventricle wall thickness, systolic and diastolic dysfunction, and high blood pressure in mice and/or rats which is driven in part by cardiac mitochondrial dysfunction [93].
Taken together, the current evidence is compelling for an intergenerational cycle of cardiometabolic dysfunction starting in utero and impacting health throughout the lifespan. The increased risk to offspring predisposes them to metabolic dysfunction and GDM in pregnancy which leads to poor cardiometabolic health in the next generation (Fig. 1). Evidence suggests that a lifecycle approach to prevention strategies is warranted, and reduction of risk will require intervention at several life stages.
CONCLUSIONS
The concept emerging from this literature is that, although diagnosed in pregnancy, GDM identifies women who have a burgeoning cardiometabolic risk factor profile that appears to first diverge from that of their peers early in life. The resultant lifelong exposure to an enhanced risk factor burden ultimately contributes to elevated risks of disease outcomes, including T2DM and CVD. Moreover, intrauterine exposure to this chronic metabolic dysfunction may set in place the replication of this cycle in the offspring of GDM pregnancies. Accordingly, the implications of GDM extend not only across the life span of the affected woman but also that of her child. Conversely, the effect of GDM on intergenerational risk transmission may also offer a unique opportunity for disease modification and possibly prevention. Specifically, it follows that preconception intervention to prevent the clinical manifestation of GDM in pregnancy may provide an opportunity to modify future cardiometabolic risk in both mother and child. As part of the Healthy Life Trajectories Initiative (HeLTI), we are currently conducting such trials of preconception lifestyle intervention to reduce maternal risk of GDM. Ultimately, this approach may enable interruption of the intergenerational transmission of cardiometabolic risk for the benefit of both mother and child.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None
Acknowledgements
Ravi Retnakaran holds the Boehringer Ingelheim Chair in Beta-cell Preservation, Function and Regeneration at Mount Sinai Hospital and his research program is supported by the Sun Life Financial Program to Prevent Diabetes in Women.