Effect of Different Types of Diagnostic Criteria for Gestational Diabetes Mellitus on Adverse Neonatal Outcomes: A Systematic Review, Meta-Analysis, and Meta-Regression
Article information
Abstract
Background
Evidence supporting various diagnostic criteria for diagnose gestational diabetes mellitus (GDM) are consensus-based, needs for additional evidence related to outcomes. Therefore, the aim of this systematic-review and meta-analysis was to assess the impact of different GDM diagnostic-criteria on the risk of adverse-neonatal-outcomes.
Methods
Electronic databases including Scopus, PubMed, and Web of Sciences were searched to retrieve English original, population-based studies with the universal GDM screening approach, up to January-2020. GDM diagnostic criteria were classified in seven groups and International Association of the Diabetes and Pregnancy Study Groups (IADPSG) was considered as reference one. We used the Mantel–Haenszel method to calculate the pooled odds of events. The possibility of publication bias was examined by Begg’s test.
Results
A total of 55 population-based studies consisting of 1,604,391 pregnant women with GDM and 7,770,855 non-GDM counterparts were included. Results showed that in all diagnostic-criteria subgroups, the risk of adverse neonatal outcomes including macrosomia, hyperbilirubinemia, respiratory distress syndrome, neonatal hypoglycemia, neonatal intensive care unit admission, preterm birth, and birth-trauma were significantly higher than the non-GDM counterparts were significantly higher than non-GDM counterparts. Meta-regression analysis revealed that the magnitude of neonatal risks in all diagnostic-criteria subgroups are similar.
Conclusion
Our results showed that the risk of adverse-neonatal-outcome increased among women with GDM, but the magnitude of risk was not different among those women who were diagnosed through more or less intensive strategies. These findings may help health-care-providers and policy-makers to select the most cost-effective approach for the screening of GDM among pregnant women.
INTRODUCTION
Gestational diabetes mellitus (GDM) is a globally rising health problem [1]. According to American Diabetes Association (ADA), GDM is defined as “diabetes diagnosed in the second or third trimester of pregnancy that was not clearly overt diabetes prior to gestation” [2]. The prevalence of GDM among various population is varied between 4% and 15% [3].
GDM results from impaired secretory response of pancreatic β-cell to increased maternal insulin demands during pregnancy [4]. Variety of factors including family history of diabetes, previous history of macrocosmic babies, higher first trimester body mass index and older maternal age lead to increased risk of developing GDM [5,6]. It is well acknowledge that GDM is associated with an increased short and long-term risk of complications for mothers and their babies [7–10].
Despite health consensus recommend various diagnostic criteria for GDM, there is no consensus about the optimal screening and diagnosis criteria [11]. In 2010 (a decade ago), the International Association of the Diabetes and Pregnancy Study Group (IADPSG) [12] provided stringent threshold for GDM diagnosis by one-step 75 g oral glucose tolerance tests based on the results of the observational Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study [13], later on, some other expert professional organizations including International Federation of Gynecology and Obstetrics (FIGO) [14], ADA, and World Health Organization (WHO) [15] supported the recommendation of IADPSG. However, the American College of Obstetricians and Gynecologists (ACOG) has always endorsed the two-step approach to GDM [16]. Furthermore some countries follow the national guideline with different diagnostic approach and glucose thresholds [17–24]. The main point is that the evidence supporting these endorsements are consensus-based, and both main organizations of IADPSG and ACOG note the need for additional evidence related to outcomes [2,25].
In addition, although there is a clear linear relationship between maternal hyperglycemia and maternal and perinatal outcomes, the effects of identifying and treating milder cases of gestational diabetes on these outcomes are not known yet [25–27]. By conducting this meta-analysis, we tried to fill the gap of knowledge, based on available evidence to find the impact of different gestational-diabetes (GDM) diagnostic-criteria on the risk of adverse-neonatal-outcomes.
METHODS
This study approved by ethics committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.ENDOCRINE.REC.1399.076). Informed consent was waived by the board.
The present review study was reported based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [28] to examine the following objectives:
To examine the pooled odds of adverse neonatal outcomes among participants with GDM compared to non-GDM groups
To examine the pooled odds of adverse neonatal outcomes among participants with GDM compared to non-GDM counterparts, based on the various screening and diagnostic criteria for GDM
Inclusion criteria
Studies were entered into the analysis if they were: (1) universally screened the GDM; (2) provided accurate screening and diagnosis criteria; (3) being population-based design; (4) provided the one of the short-term neonatal outcomes of in both pregnant women with GDM and non-GDM. Studies were excluded if they were: reviews, letter to editor, meeting abstracts, case reports. There are no restrictions regarding country, age, race and other demographic characteristics of counterparts.
Search strategy
A comprehensive systematic search up to January 2020 was performed in the electronic databases including PubMed, Web of Sciences, and Scopus to retrieve relevant English publications based on the combination the keywords as follows: adverse pregnancy outcome, pregnancy outcome, pregnancy complication, small for gestational age (SGA), macrosomia, large for gestational age (LGA), neonatal distress, respiratory distress syndrome (RDS), neonatal RDS, neonatal intensive care unit (NICU) admission, NICU, preterm, hyperbilirubinemia, stillbirth, neonatal hypoglycemia, birth trauma, shoulder dystocia, bone fracture, GDM, gestational diabetes, pregnancy-induced diabetes, glucose intolerances, and impaired glucose tolerances.
Study selection and data extraction
The title and abstract of records screened by two investigators (M.S.G. and S.B.G.) independently for determining final eligibility criteria. Any disagreements were discussed by two researchers and an another investigator (F.R.T.) until consensus was achieved. Two authors (M.S.G. and R.B.Y.) applied data extraction. Data were extracted from full text of studies including name of first author, country, years of publication, sample size, percent/number of events related to the each outcomes, diagnostic criteria for GDM, and population characteristics.
Study subgroups
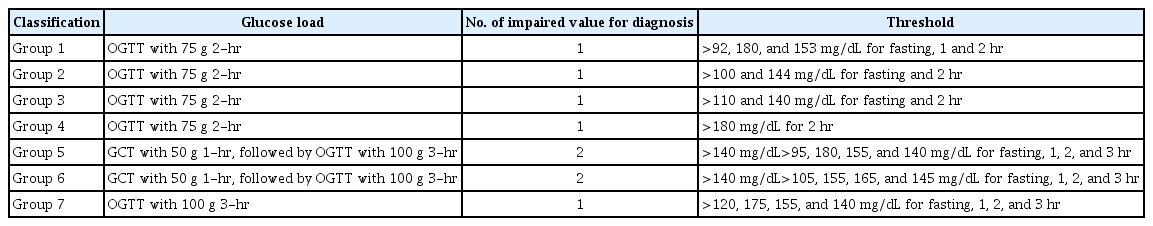
All included studies were classified in seven subgroups based on the screening and diagnosis approaches and closest value of blood glucose thresholds (Table 1).
Outcome measures
The main outcomes in this meta-analysis were nine separate neonatal short outcomes of SGA, preterm birth, LGA, stillbirth, macrosomia, hyperbilirubinemia, RDS, neonatal hypoglycemia, NICU admission, and one composite outcome of neonatal birth trauma (including bone fracture, shoulder dystocia, birth injury, and Erb’s palsy).
Quality assessment and risk of bias
The methodological quality assessment of included studies was performed by two investigators (M.S.G. and S.B.G.) independently using the Newcastle-Ottawa Scale [10]. This scale is categorized into three dimensions including selection, comparability and outcomes. The scoring system (range, 0 to 9) is used to provide final judgment regarding the quality of included studies. Scores above 6, 3–5, and below 3 were interpreted as high, moderate, and low quality, respectively. The Cochrane Collaboration’s tool was used for assessing the risk of bias of included studies [29]. Risk of bias of cross-sectional studies was performed in five domains including: bias in selection of cases and controls, control of prognostic variable and development of outcome also in cohort studies the risk of bias evaluation was performed in seven domains including selection, assessment of exposure and outcome, presence of outcome of interest at start of study, control of prognostic variables, presence or absence of prognostic factors and adequacy regarding follow-up of cohorts. The authors classified their judgment on the quality of each study into high risk, unclear risk, or low risk of bias.
Data analysis
All data analyses were performed in the STATA version 13 (STATA Inc., College Station, TX, USA). We used the Mantel–Haenszel method to calculate the pooled odds ratios (ORs) of events. The heterogeneity between studies was assessed using I2 and Cochrane’s Q test. Heterogeneous and non-heterogeneous results were analyzed using the random-effects and fixed effect model respectively [30]. The possibility of publication bias in the present study was examined by Begg’s test. The trim and fill method was used to deal with publication bias [31]. Meta-regression was performed to investigate any potential source of heterogeneity among GDM diagnosis criteria (IADPSG as a reference group). All results reported in significance level of 0.05 with 95% confidence intervals (CIs).
RESULTS
Search results, study characteristics, and quality assessment
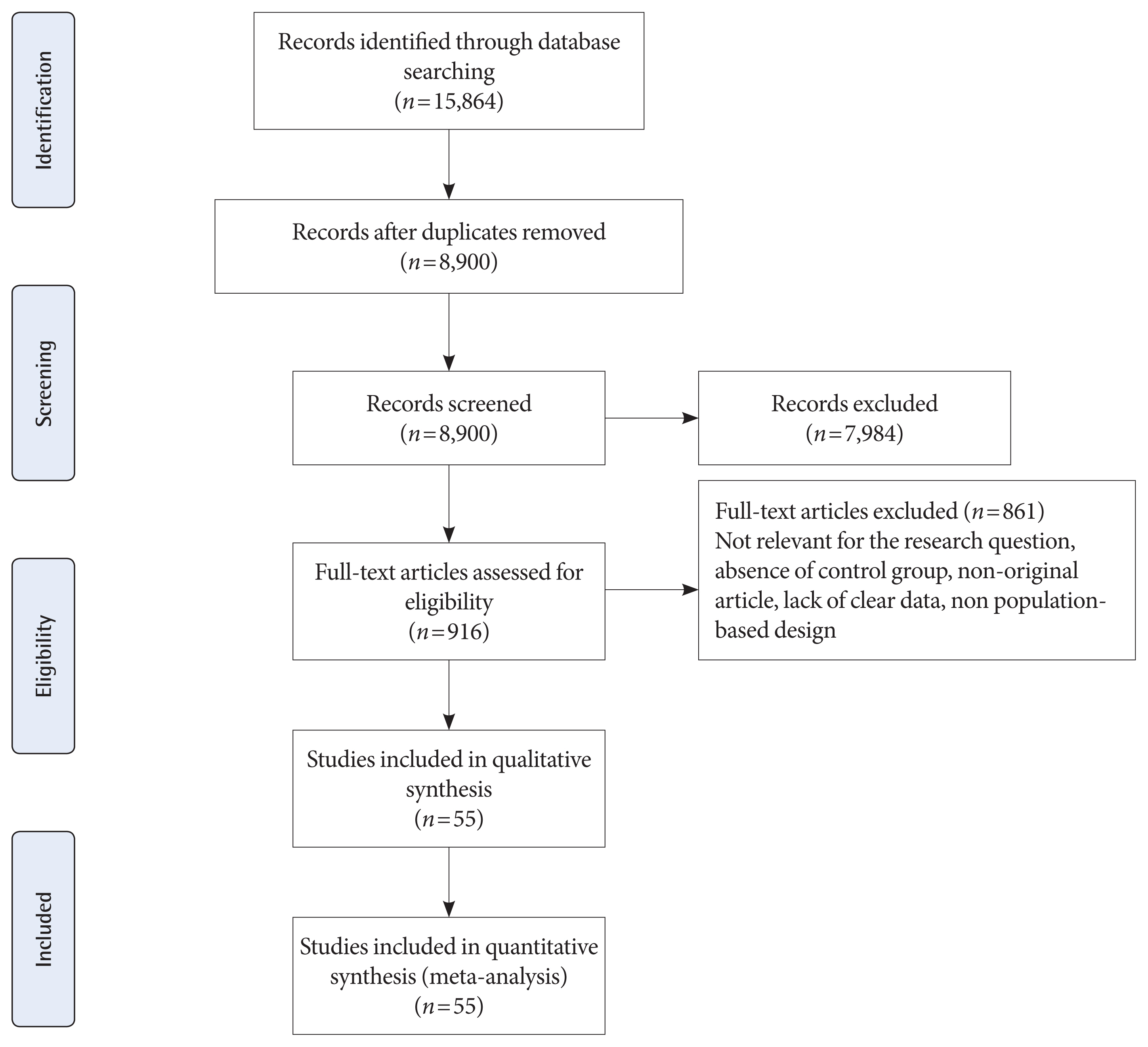
Fig. 1 shows the flow diagram of studies retrieval and study selection. In this review, 55 studies provide information of adverse neonatal outcomes of 1604,391 participants with GDM and 7,770,855 non-GDM participants. Details of the characteristics of included studies are presented in Supplementary Table 1. All studies were classified as high quality (Supplementary Tables 2 and 3) [32–86]. A total of 53 studies were prospective or retrospective cohorts [32–64,66–82,84–86] and two were cross-sectional studies [66,84]. A total of 19 studies classified as group 1 [32,36,48,49,53,57,59,60,65–67,69–71,77–79,81,85] which used IADPSG criteria; six as group 2 [38,51,67,77,83, 86]; three as group 3 [41,58,74]; two as group 4 [37,67]; 22 as group 5 [33–35,39,42,44,45,47,50,52–54,56,59,61,69,72,73,75, 76,82,85]; eight as group 6 [43,46,54,55,61–63,68], and six as group 7 [40,44,64,65,80,84]. It should be noted that 10 studies used more than one GDM classification [44,53,54,59,61,65,67,69,77,85]. Seventeen studies were conducted in America including USA [32,34,42,49,54,55,61,68,75,76,85] and Canada [35,45,46,69,72,73]; six in Australia [38,51,83,86], New Zealand [67], and Cook Islands [66]; 13 in Asia including Iran [59,60], China [36,53,71,84], Saudi Arabia [78], India [41,58], Korea [52], Qatar [48], and Japan [44,57]; 18 in Europe, including Italy [81], Sweden [37,80], Ireland [64,70,79], UK [74,77], Israel [43,56,62,82], Croatia [65], Spain [63], Norway [40], and Finland [33,39,47]; and one in Mediterranean countries including Malta, Greece, Serbia, Italy, France, Portugal, Morocco, Tunisia, Algeria, Syria, and Lebanon [50].
Meta-analysis and meta-regression results
Figs. 2–4 and Supplementary Figs. 1–6 present the forest plot of outcome measures obtained from Mantel–Haenszel method. Table 2 shows the overall pooled OR (95% CI) of adverse neonatal outcomes, its heterogeneity and publication bias estimation among various subgroups of GDM diagnosis criteria, compared to non-GDM groups.
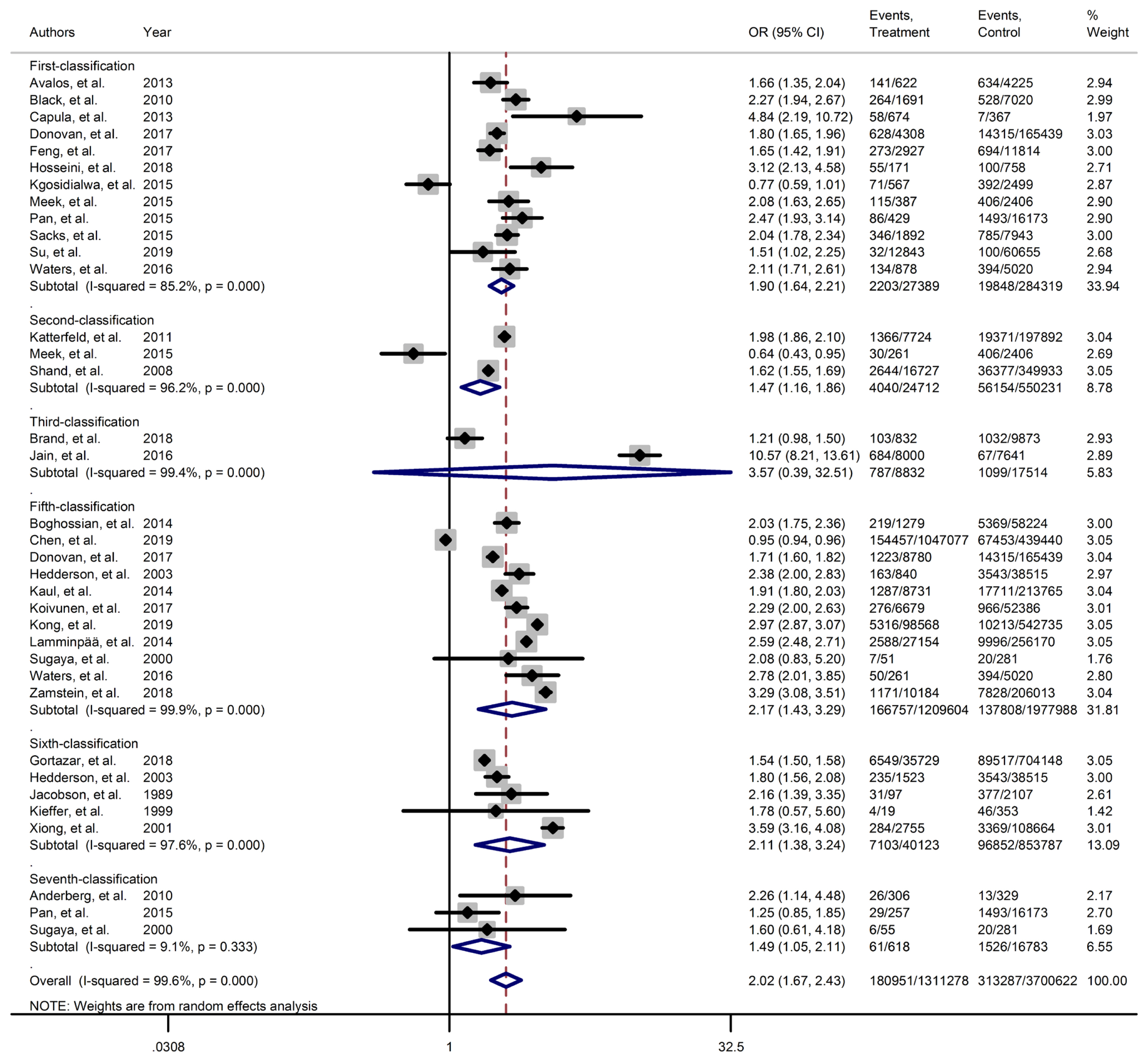
Forest plot of large for gestational age obtained from Mantel–Haenszel method. Effect size (odds ratio [OR]) and 95% confidence intervals (CIs) for; pooled estimates of effect size are indicated by vertical points of diamonds and 95% CI are represented by horizontal points.

Forest plot of neonatal intensive care unit obtained from Mantel–Haenszel method. Effect size (odds ratio [OR]) and 95% confidence intervals (CIs) for; pooled estimates of effect size are indicated by vertical points of diamonds and 95% CI are represented by horizontal points.
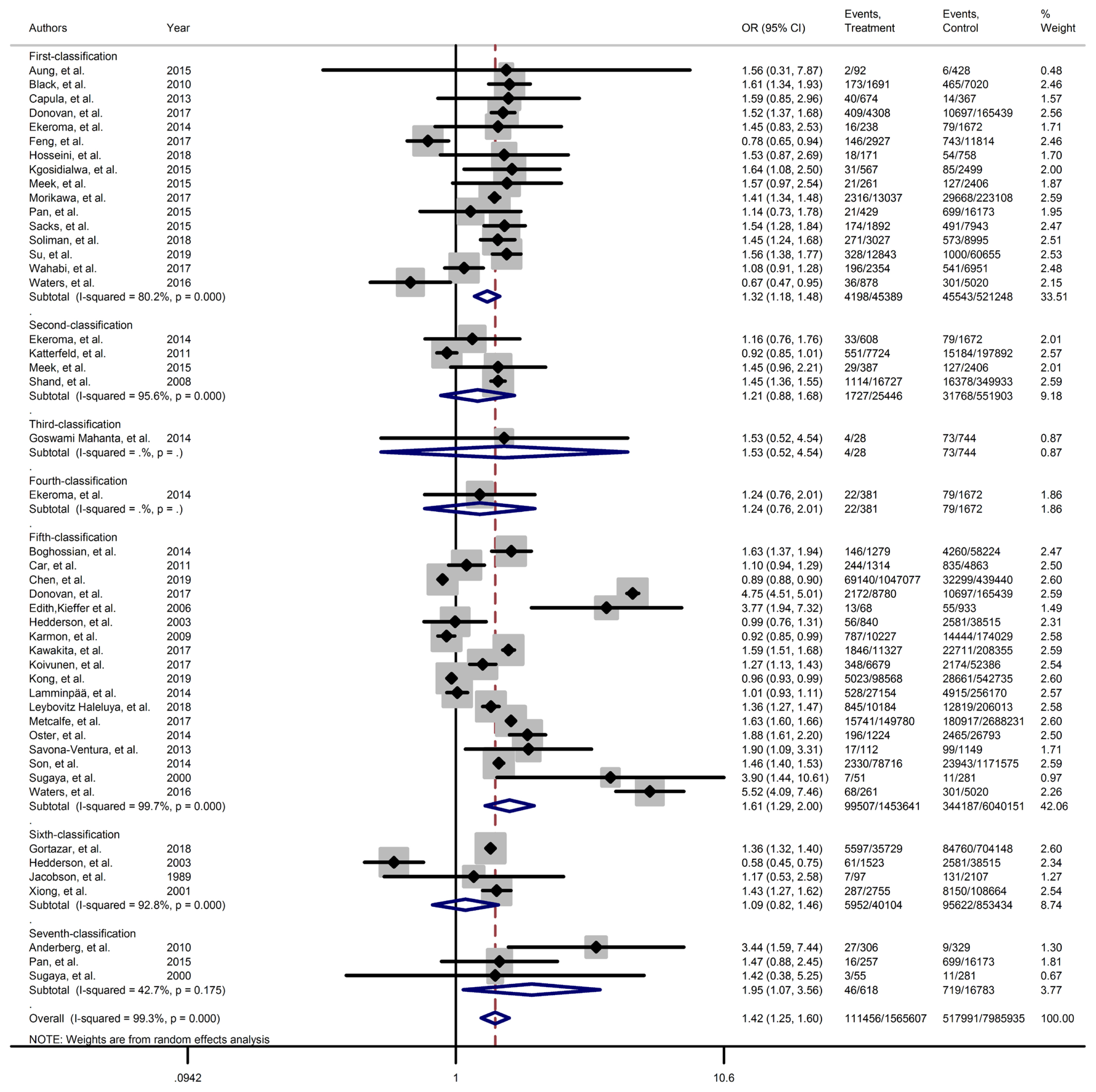
Forest plot of preterm obtained from Mantel–Haenszel method. Effect size (odds ratio [OR]) and 95% confidence intervals (CIs) for; pooled estimates of effect size are indicated by vertical points of diamonds and 95% CI are represented by horizontal points.

Results of heterogeneity and publication bias estimation and subgroup meta-analysis for risk adverse neonatal outcome among women with gestational diabetes based on various GDM screening strategy group
Results of meta-analyses showed that, regardless of GDM screening criteria, the risk of adverse neonatal outcomes including LGA (pooled overall OR, 2.02; 95% CI, 1.67 to 2.43), NICU admission (pooled overall OR, 1.68; 95% CI, 1.53 to 1.85), preterm birth (pooled overall OR, 1.41; 95% CI, 1.25 to 1.60), neonatal hypoglycemia (pooled overall OR, 4.84; 95% CI, 3.24 to 7.25), birth trauma (pooled overall OR, 1.51; 95% CI, 1.24 to 1.82), macrosomia (pooled overall OR, 1.61; 95% CI, 1.43 to 1.82), hyperbilirubinemia (pooled overall OR, 1.50; 95% CI, 1.22 to 1.86), and RDS (pooled overall OR, 1.51; 95% CI, 1.23 to 1.85) significantly increased in women with GDM as compared with the non-GDM group. However, the adverse events of stillbirth was not significantly different between the groups (pooled overall OR, 1.06; 95% CI, 0.78 to 1.44) and the risk of SGA in women with GDM was 0.2 fold lower than in non-GDM (pooled overall OR, 0.80; 95% CI, 0.69 to 0.92).
However, the same results were found for subgroup of GDM diagnostic classification analyses. In this respect, the subgroups analyses demonstrated that the risk of adverse neonatal events including LGA, macrosomia, hyperbilirubinemia, NICU admission, neonatal hypoglycemia, preterm birth, and birth trauma in women with GDM in all of the GDM diagnostic classification were significantly higher than non-GDM counterparts (Table 2). For example subgroup analyses in IADPSG classification showed that the risk of LGA (pooled OR, 1.90; 95% CI, 1.64 to 2.21), macrosomia (pooled OR, 1.59; 95% CI, 1.35 to 1.89), hyperbilirubinemia (pooled OR, 1.17; 95% CI, 1.07 to 1.28), RDS (pooled OR, 1.60; 95% CI, 1.18 to 2.15), NICU admission (pooled OR, 1.70; 95% CI, 1.25 to 2.30), neonatal hypoglycemia (pooled OR, 4.16; 95% CI, 2.42 to 7.17), preterm birth (pooled OR, 1.32; 95% CI, 1.18 to 1.48), and birth trauma (pooled OR, 1.48; 95% CI, 1.24 to 1.69) in women with GDM were significantly higher than non-GDM population. As well, no significant results were found in the risk of stillbirth (pooled OR, 0.71; 95% CI, 0.46 to 1.09) and also the risk of SGA (pooled OR, 0.77; 95% CI, 0.66 to 0.91) was significantly lower than in non-GDM counterparts.
However, the results of meta-regression revealed that the magnitude of the risk of those adverse neonatal outcomes in the IADPSG criteria, as the strictest one, was similar to other classification (Supplementary Fig. 7).
Publication bias and risk of bias
The results of Begg’s test showed that there were no substantial publication bias among various outcomes, except for outcome of LGA, which was corrected by trim and fill method of correction (Table 2). Majority of included studies were judged to be at low risk of bias for evaluated domains (Supplementary Figs. 8 and 9). Majority of cross-sectional studies had a low or probably low risk of bias in the development of outcome of interest in case and controls, selection of cases and controls and also in assessment of exposure domains. However, half of them had probably high risk of bias in control of prognostic variables. Moreover, cohort studies were judged to have at low risk of bias for selection of exposed and non-exposed cohorts, presence of outcome of interest at start of study, outcome assessment, assessment of prognostic factors and adequacy of follow-up of cohorts; however, one-fifth of them of them had probable high risk of bias in assessment of exposure and 15% of them had high risk of bias controlling prognostic variables.
DISCUSSION
In this meta-analysis of observational studies, we evaluated the impact of several diagnostic criteria for GDM on the risk of adverse-neonatal-outcomes. Briefly, the results showed that neonates of women with GDM have higher risk of adverse outcomes of LGA macrosomia, hyperbilirubinemia, RDS, neonatal hypoglycemia, NICU admission, preterm birth and birth trauma than the neonates of women without GDM group; however, the magnitude of these adverse neonatal outcomes were not significantly varies by different diagnostic criteria for GDM.
During pregnancy failure to adapt with physiological changes of pregnancy as a result of the dysfunction in pancreatic β-cell, and developing the GDM considered as a threatening factor for maternal and child health [87]. It is well established that the risk of adverse neonatal outcomes are increased among women with GDM [88].
There are many guidelines which provide various recommendations for screening and diagnosis criteria of GDM [89,90], but debate about the screening and diagnosis for GDM still continue in the literature. Different approaches identify different feto-maternal and neonatal risks leading to variation in prevalence and pregnancy related outcomes [3,32–86,91]. However stringent criteria of IADPSG, that is accepted by many organizations, led to increase of GDM cases [3]. However, there are limited evidence to support the IADPSG criteria to prove clinically significant improvements in maternal and neonatal outcomes. The main purpose of these struggles is to find a practical strategy with minimum costs, adverse maternal-fetal outcomes and maximum availability especially in low health resources countries. The results of this systemic review and meta-analysis confirmed the previous findings about increased risk of adverse neonatal outcomes among women with GDM. In addition, it revealed that the magnitude of those increased risk are similar in various GDM diagnostic criteria.
There are extensive discussions regarding the cost-effectiveness of different GDM diagnosis criteria in the literature [92–95]. Considering that those increased cased without any improvement in pregnancy outcomes potentially may lead to over medicalization of pregnant women [96,97] and therefore increased health costs, and decreased physical, psychological, social, and other aspects of quality of life in pregnant women [98].
In line with the findings of current meta-analysis, in another our recent published meta-analysis with the same classification for GDM screening, we found that magnitude of the risks of adverse maternal outcomes including primary cesarean section, induction of labor, maternal hemorrhage, pregnancy related hypertension, and gestational weight gain are similar all GDM screening strategies classifications [99]. In agreement, Wendland et al. [88] (2012), in a systematic review study demonstrated that risk of LGA among participants with GDM was higher than non-GDM counterparts in both WHO and the IADPSG criteria, and also the magnitude of this risk was similar in both criteria. Hartling et al. [100] (2014), in their meta-analysis found higher glucose thresholds did not consistently demonstrate greater risk of adverse pregnancy outcomes. Further, Hosseini and Janghorbani [101] (2018) in a meta-analysis reported that women with GDM diagnosed with either the one-step or the two-step approach were at increased risk for selected adverse pregnancy outcomes. The associations with the two-step method were slightly stronger. However, all of these mentioned studies have not compared the various existing criteria and did not provided the majority of neonatal outcomes.
However, in the present study, the risk of stillbirth was not significantly different between the women with GDM and non-GDM groups. Additionally, compared to non-GDM women, the risk of SGA was significantly lower in women with GDM. It may be due to that all of women diagnosed with GDM have been received glucose lowering therapy in order to decrease the feto-maternal adverse outcomes, particularly some sever outcomes such as stillbirth. It is well known that optimal control of maternal blood glucose could strongly decrease risk of still birth [102]. Also it should be noted that stillbirth rates vary based on the management option (insulin/diet) and gestational week, but due to the limitations of the data we cannot adjust the mentioned factors. In addition it is well documented that intensive therapy for GDM may affect the fetal growth and increase the SGA rate [103,104]. Moreover, vasculopathy plays a role in increased risk of SGA in GDM suffering women [105–107].
This review has certain strengths and limitations. Population-based design of included studies with high quality, large sample size of GDM, and non-GDM participants from different countries, estimation of the pooled risk of several neonatal outcomes in different subgroups of GDM classifications let us to present reliable evidence. In addition, Subgroup analysis and assessed multiple available GDM screening and diagnostic criteria were considered as the strength of our study. However, our meta-analysis has limitations, such as the presence of significant heterogeneity in some subgroup analyses, only studies published in English included, and did not investigate grey literature. In addition, due to lack of data, we could not perform some subgroup analysis. Additionally, different definitions of outcome measures across included studies may impose potential limitations in this meta-analysis. There is a need for future meta-analysis and observational studies about the long term effect of GDM from childhood into adulthood GDM based on the different classifications of GDM diagnosis criteria.
In conclusion, our results showed that the risk of adverse neonatal outcome increased among women with GDM, but the magnitude of risk was not different among those women who were diagnosed through more or less intensive strategies. These findings may help health-care-providers and policy makers to select the most cost-effective approach for the screening of GDM among pregnant women.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2021.0178.
Summary of available studies included in the present meta-analysis
Quality assessment of studies included using the Newcastle–Ottawa Quality Assessment Scale for cohort studies
Quality assessment of included studies using the Newcastle–Ottawa Quality Assessment Scale for cross-sectional study
Forest plot of macrosomia obtained from Mantel–Haenszel method. Effect size (odds ratio [OR]) and 95% confidence intervals (CIs) for; pooled estimates of effect size are indicated by vertical points of diamonds and 95% CI are represented by horizontal points.
Forest plot of hyperbilirubinemia obtained from Mantel–Haenszel method. Effect size (odds ratio [OR]) and 95% confidence intervals (CIs) for; pooled estimates of effect size are indicated by vertical points of diamonds and 95% CI are represented by horizontal points.
Forest plot of stillbirth obtained from Mantel–Haenszel method. Effect size (odds ratio [OR]) and 95% confidence intervals (CIs) for; pooled estimates of effect size are indicated by vertical points of diamonds and 95% CI are represented by horizontal points.
Forest plot of respiratory distress syndrome obtained from Mantel–Haenszel method. Effect size (odds ratio [OR]) and 95% confidence intervals (CIs) for; pooled estimates of effect size are indicated by vertical points of diamonds and 95% CI are represented by horizontal points.
Forest plot of neonatal hypoglycemia obtained from Mantel–Haenszel method. Effect size (odds ratio [OR]) and 95% confidence intervals (CIs) for; pooled estimates of effect size are indicated by vertical points of diamonds and 95% CI are represented by horizontal points.
Forest plot of birth trauma obtained from Mantel–Haenszel method. Effect size (odds ratio [OR]) and 95% confidence intervals (CIs) for; pooled estimates of effect size are indicated by vertical points of diamonds and 95% CI are represented by horizontal points.
Bubble plots fitted meta-regression line for associations neonatal adverse outcomes of gestational diabetes mellitus (GDM) based on GDM diagnosis classification. OR, odds ratio; SGA, small for gestational age; LGA, large for gestational age; RDS, respiratory distress syndrome; NICU, neonatal intensive care unit.
Risk of bias in each cross-sectional study (A) and overall (B).
Risk of bias in each included cohort study (A) and overall (B).
ACKNOWLEDGMENTS
The authors wish to acknowledge Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: F.R.T., S.B.G.
Acquisition, analysis, or interpretation of data: F.R.T., M.S.G.N., R.B.Y., S.B.G.
Drafting the work or revising: F.R.T., M.S.G.N., R.B.Y., S.B.G.
Final approval of the manuscript: F.R.T., M.S.G.N., R.B.Y., S.B.G.
FUNDING
This research project was funded by the Shahid Beheshti University of Medical Sciences. Also, Nord University, Bodø, Norway covered the article processing charges.