- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 46(2); 2022 > Article
-
Original ArticleCOVID-19 Impact of COVID-19 Lockdown on the Metabolic Control Parameters in Patients with Diabetes Mellitus: A Systematic Review and Meta-Analysis
-
Ifan Ali Wafa1
 , Nando Reza Pratama1
, Nando Reza Pratama1 , Nurizzah Farahiyah Sofia1
, Nurizzah Farahiyah Sofia1 , Elsha Stephanie Anastasia1, Tiffany Konstantin1, Maharani Ayuputeri Wijaya1, M. Rifqi Wiyono1, Lilik Djuari2, Hermina Novida3
, Elsha Stephanie Anastasia1, Tiffany Konstantin1, Maharani Ayuputeri Wijaya1, M. Rifqi Wiyono1, Lilik Djuari2, Hermina Novida3
-
Diabetes & Metabolism Journal 2022;46(2):260-272.
DOI: https://doi.org/10.4093/dmj.2021.0125
Published online: March 8, 2022
1Faculty of Medicine, Airlangga University, Surabaya, Indonesia
2Department of Public Health and Preventive Medicine, Airlangga University, Surabaya, Indonesia
3Department of Internal Medicine, Airlangga University, Surabaya, Indonesia
-
Corresponding author: Hermina Novida
 Department of Internal Medicine, Airlangga University, Jalan Mayjen Prof. Dr. Moestopo No.47, Surabaya 60131, Indonesia, E-mail: hermina-n@fk.unair.ac.id
Department of Internal Medicine, Airlangga University, Jalan Mayjen Prof. Dr. Moestopo No.47, Surabaya 60131, Indonesia, E-mail: hermina-n@fk.unair.ac.id - * Ifan Ali Wafa, Nando Reza Pratama, and Nurizzah Farahiyah Sofia contributed equally to this study as first authors.
Copyright © 2022 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Abrupt implementation of lockdowns during the coronavirus disease 2019 (COVID-19) pandemic affected the management of diabetes mellitus in patients worldwide. Limited access to health facilities and lifestyle changes potentially affected metabolic parameters in patients at risk. We conducted a meta-analysis to determine any differences in the control of metabolic parameters in patients with diabetes, before and during lockdown.
-
Methods
- We performed searches of five databases. Meta-analyses were carried out using random- or fixed-effect approaches to glycaemic control parameters as the primary outcome: glycosylated hemoglobin (HbA1c), random blood glucose (RBG), fasting blood glucose (FBG), time-in-range (TIR), time-above-range (TAR), time-below-range (TBR). Mean difference (MD), confidence interval (CI), and P value were calculated. Lipid profile was a secondary outcome and is presented as a descriptive analysis.
-
Results
- Twenty-one studies enrolling a total of 3,992 patients with type 1 or type 2 diabetes mellitus (T1DM or T2DM) were included in the study. Patients with T1DM showed a significant improvement of TIR and TAR (MD=3.52% [95% CI, 0.29 to 6.74], I2=76%, P=0.03; MD=−3.36% [95% CI, −6.48 to −0.25], I2=75%, P=0.03), while FBG among patients with T2DM significantly worsened (MD=3.47 mg/dL [95% CI, 1.22 to 5.73], I2=0%, P<0.01). No significant difference was found in HbA1c, RBG, and TBR. Use of continuous glucose monitoring in T1DM facilitated good glycaemic control. Significant deterioration of lipid parameters during lockdown, particularly triglyceride, was observed.
-
Conclusion
- Implementation of lockdowns during the COVID-19 pandemic did not worsen glycaemic control in patients with diabetes. Other metabolic parameters improved during lockdown, though lipid parameters, particularly triglyceride, worsened.
- Ever since coronavirus disease 2019 (COVID-19) first emerged in China, lockdowns have been implemented in numerous countries as an attempt to reduce the spread of disease [1]. Lockdowns resulted in significant changes to daily life, from decreased physical activity, dietary changes, alterations in psychological and emotional states, and appetite dysregulation in individuals [2–4]. During lockdown, vulnerable populations, such as patients with diabetes mellitus, encountered restrictions in healthcare access that resulted in substandard care for patients [4]. Moreover, routine monitoring of metabolic control parameters, including glycaemic and lipid control, decreased significantly, resulting in an overall worsening of the assessment of patients with diabetes [4,5].
- In an attempt to tackle the limitations on face-to-face meetings, several solutions using advanced technology were proposed as monitoring alternatives for maintaining good glycaemic control [6]. Before the COVID-19 pandemic, technological solutions for continuous glucose monitoring (CGM) and telemedicine were used to a limited extent [7]. CGM usage was associated with improved clinical outcomes of overall care [7,8]. With social restrictions due to COVID-19, CGM is a critical tool for remote glucose monitoring in patients with diabetes. Therefore, the benefits of CGM in monitoring diabetes during the COVID-19 pandemic needs to be further evaluated.
- With this systematic review and meta-analysis, we aim to (1) provide an overview of the correlation between lockdowns and metabolic control and (2) evaluate the association between current medical practices during the lockdown and proper glycaemic control in patients with diabetes.
INTRODUCTION
- This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 reporting guideline [9]. The study protocol has been registered in the international prospective register of systematic reviews database (PROSPERO) (CRD42021252321).
- Search strategy
- A comprehensive electronic-based literature search was conducted in PubMed, ScienceDirect, Cochrane Library, Journal Storage (JSTOR), and Directory of Open Access Journals (DOAJ) databases from 19 to 26 April 2021, for studies published in English. Medical Subject Heading (MeSH) terms were used in combination with the Boolean operator (AND/OR) and the following keywords: (COVID) AND [(pandemic) OR (lockdown) OR (quarantine)] AND (diabetes mellitus [MeSH Terms]) AND [(glycaemic control) OR (glucose level) OR (lipid profile)]. The authors also performed manual searches to retrieve potentially relevant articles from the reference list of retrieved articles.
- Study selection
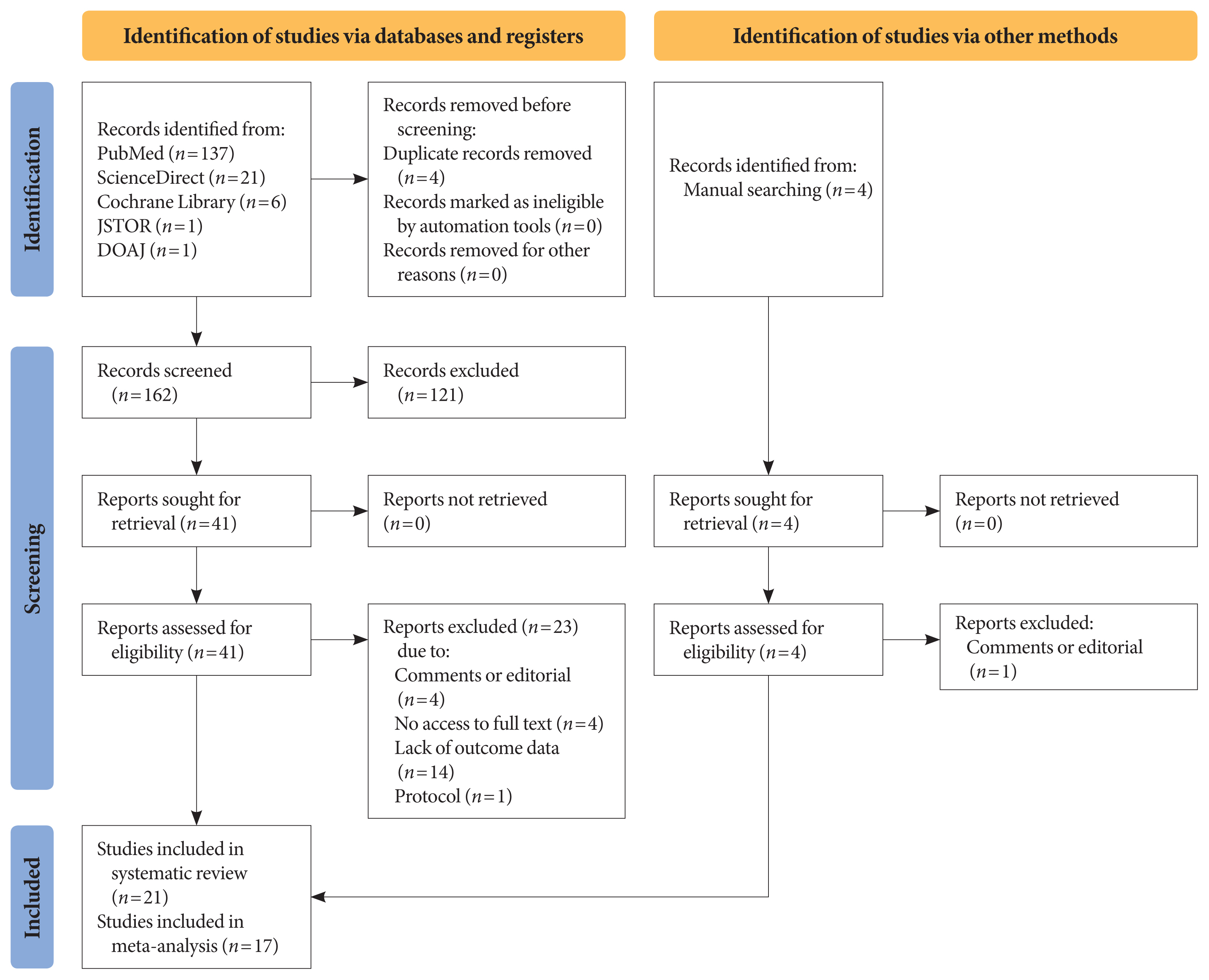
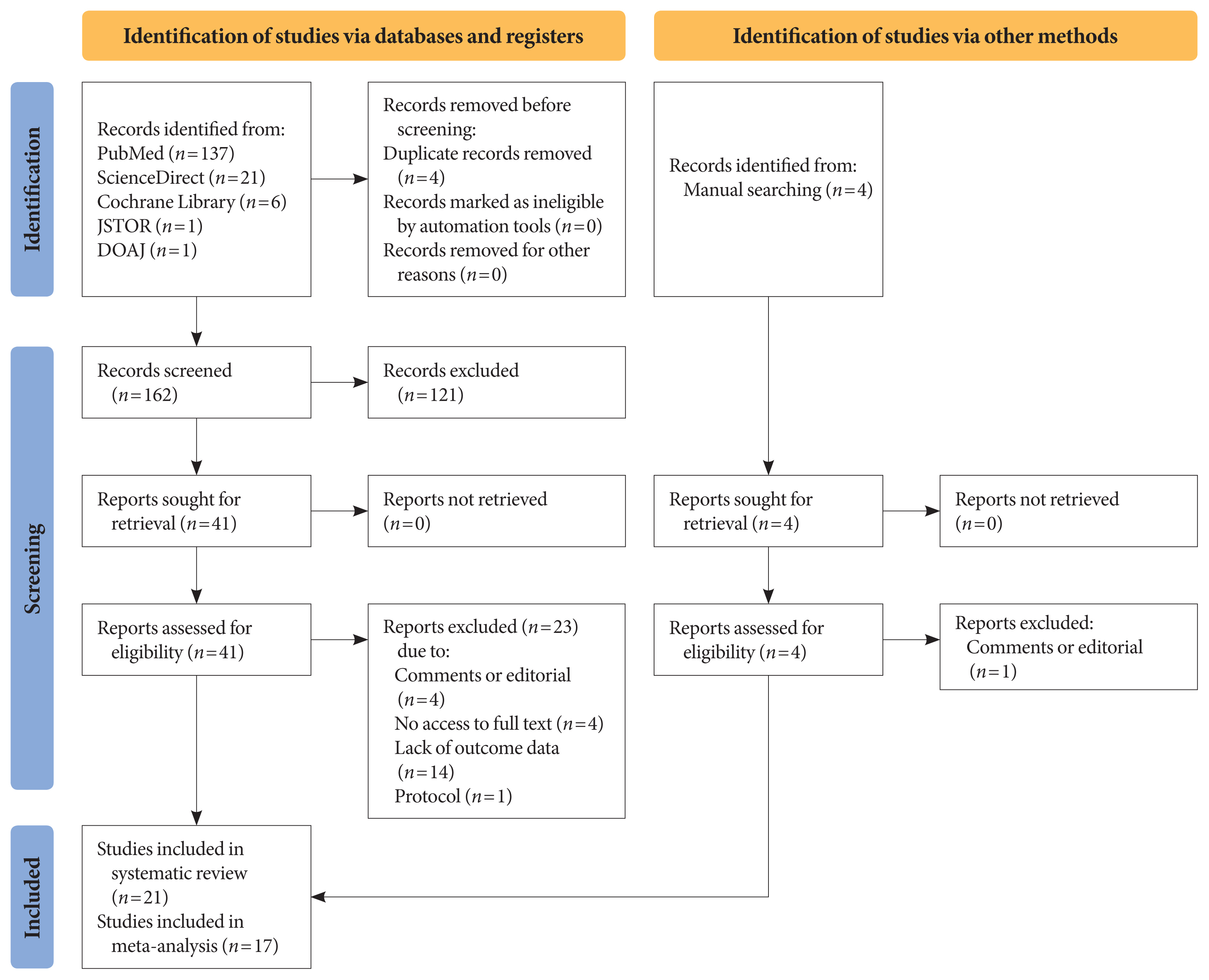
- Title and abstract screening were carried out on each article, after removing duplicates using the Mendeley reference manager. Two authors (I.A.W. and N.R.P.) independently conducted the screening based on the following eligibility criteria: observational studies (cross-sectional, case-control, or cohort), published between 2020 and 2021, including patients of any age with type 1 or type 2 diabetes mellitus (T1DM or T2DM), that reported glycaemic control parameters and/or lipid profile as primary or secondary outcomes, and assessed comparisons of metabolic control among diabetic patients during and before lockdown. We excluded studies with the following criteria: experimental studies, non-English language articles, studies that included other types of diabetes or involved patients with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) comorbid infections, and those studies that did not reported the glycaemic control and/or lipid profile during lockdown. The search, selection, and screening processes were recorded and are documented in the PRISMA flow diagram (Fig. 1).
- Data extraction
- Three independent authors (I.A.W., N.R.P., and N.F.S.) used a standardised form to extract the data. Any discrepancies were resolved by discussion among the authors until a consensus was reached. The following data were extracted: first author name, year of publication, the country setting, study design, sample size, study period, age, and types of diabetes. Glycaemic control parameters (glycosylated hemoglobin [HbA1c] or estimated A1c [eA1c], random blood glucose [RBG], fasting blood glucose [FBG], time-in-range [TIR], time-above-range [TAR], and time-below-range [TBR]), lipid control parameters (total cholesterol, low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglyceride), measurement methods, and comparison of metabolic control before and during COVID-19 lockdowns were also extracted. HbA1c data obtained from laboratory assessment, and eA1c, data from CGM, are defined as HbA1c.
- Quality assessment
- Quality assessment was performed independently by two authors (I.A.W. and N.F.S.). Any discrepancies were resolved by discussion among authors until a consensus was reached. Quality assessment of the studies included in the meta-analysis was performed with the Effective Public Health Practice Project (EPHPP) tool, consisting of six components: (1) selection bias, (2) study design, (3) confounders, (4) blinding, (5) data collection method, and (6) withdrawals and dropouts. Each component was rated as 1 (strong), 2 (moderate), or 3 (weak). Based on the number of components with a weak rating, the global rating of the quality of each study was assigned as either strong, moderate, or weak [10].
- Operational definitions
- TIR, TAR, and TBR are defined as the percentage of time per day an individual spent at various blood glucose levels, respectively, in the target range (70 to 180 mg/dL [3.9 to 10.0 mmol/L]), above range (>180 mg/dL), and below range (<70 mg/dL) [11].
- Data synthesis and analysis
- The primary outcomes of this study were the differences identified in the following glycaemic control parameters, before and during lockdown: HbA1c, RBG, and FBG; along with other glycaemic control parameters obtained from CGM or flash glucose monitoring (FGM) device: TIR, TAR, and TBR. Secondary outcomes were the differences in lipid control parameters during and before the lockdown of the following: total cholesterol, LDL-C, HDL-C, and triglyceride. The mean and standard deviation of the glycaemic control parameters and lipid profile data from each study were extracted. Any outcomes presented as a median and interquartile range were not included in the meta-analysis. The comparison of glycaemic control parameters and lipid profile during and before the lockdown were analysed as continuous variables using the mean difference (MD) and summarised with their corresponding 95% confidence interval (CI). The overall effect (Z test) was considered significant if the P value was <0.05. Heterogeneity was assessed using the Q statistic and I2 test. The I2 test presents the variability percentage of the analysis due to clinical or methodological heterogeneity, rather than sampling error. The random-effects model was used when the I2 was >50% or the P value was <0.10; otherwise, the fixed-effect model was preferred. Potential publication bias was observed visually using Begg’s funnel plots. We pre-planned to perform subgroup analyses for all outcomes based on the study design. Sensitivity analyses were also performed based on the quality of the studies. All statistical analyses were performed using Review Manager version 5.4 (The Cochrane Collaboration, London, UK).
METHODS
- The search strategy yielded 166 articles from primary databases and manual searching after removing four duplications using Mendeley reference manager. After titles and abstracts were screened, 45 articles were selected for full-text evaluation. Of those, 21 studies with a total of 3,992 participants met the eligibility criteria and were included in this study (Fig. 1). Quality assessment resulted in 12 “strong” quality articles, eight “moderate” quality articles, and one “weak” quality article based on the EPHPP tool (Supplementary Table 1). Funnel plot asymmetry was obtained in HbA1c, RBG, TIR, and TAR outcomes (Supplementary Figs. 1–4) but not in FBG and TBR (Supplementary Figs. 5 and 6).
- All studies included were observational studies and consisted of 13 cohort studies, five case-control studies, and three cross-sectional studies. Most studies were conducted in Italy, with others conducted in Spain, France, Greece, the Netherlands, Israel, Saudi Arabia, Turkey, and India. The characteristics of the included studies are presented in Table 1.
- According to the findings in these 21 studies, seven studies reported negative impacts and a deterioration in glycaemic control during lockdown, while others reported no significant differences. However, some studies showed significant improvements in glycaemic control among patients with diabetes during lockdown. Due to insufficient outcome data, only 17 studies were included in the meta-analysis. The lipid control parameters were analysed as secondary outcomes and are presented as descriptive analyses since only two available studies reported this outcome.
- Primary outcomes
- Eleven studies [12–22] with a total of 1,357 pre-lockdown and 1,325 lockdown participants, comprised of patients with either T1DM or T2DM, reported mean levels of HbA1c and eA1c which was obtained either from CGM or FGM (Supplementary Fig. 7). A pooled estimate showed that lockdown did not significantly result in changes to HbA1c (MD=0.06% [95% CI, −0.10 to 0.23], I2=77%, random-effects). All HbA1c subgroup analyses in cohort studies. found no significant differences of HbA1c between pre-lockdown and lockdown (MD=−0.01% [95% CI, −0.17 to 0.14], I2=65%, random-effects), case-control (MD=0.00% [95% CI, −0.32 to 0.32], I2=75%, random-effects), and cross-sectional studies (MD=0.56% [95% CI, −0.67 to 1.78], I2=95%, random-effects).
- Twelve studies [13,15,17,19–27] in patients with T1DM reported MDs of RBG with a total of 2,162 pre-lockdown and 2,210 lockdown participants (Supplementary Fig. 8). Pooled estimates showed no significant differences in RBG level between pre-lockdown and during lockdown periods (MD=0.91 mg/dL [95% CI, −4.52 to 6.34], I2=88%, random-effects). RBG subgroup analysis showed no significant differences in cohort studies (MD=−0.71 mg/dL [95% CI, −7.64 to 6.22], I2=0.64%, random-effects), case-control studies (MD=−2.47 mg/dL [95% CI, −8.91 to 3.98], I2=75%, random-effects), and cross-sectional studies (MD=23.61 mg/dL [95% CI, −15.32 to 62.54], I2=93%, random-effects).
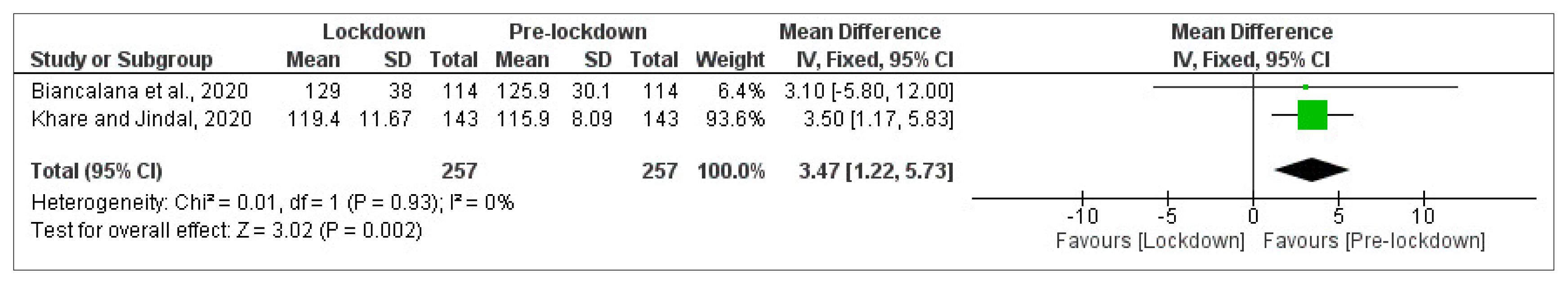
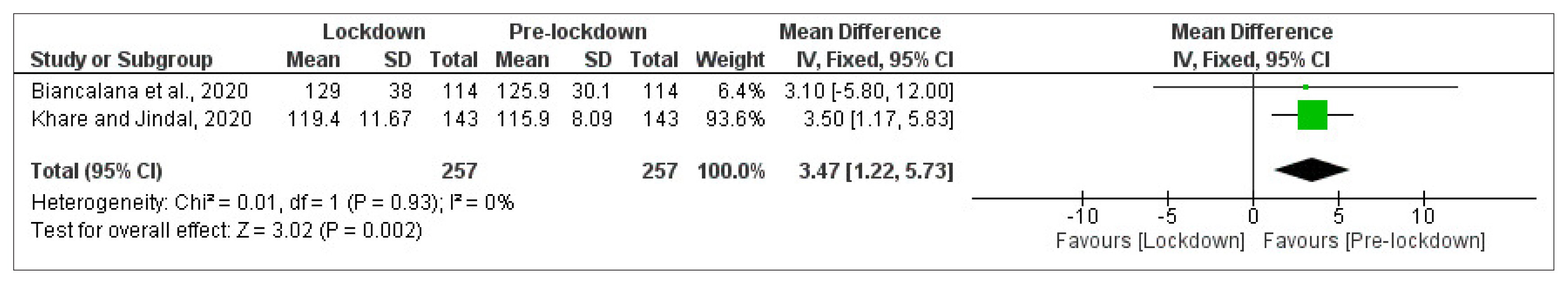
- Mean FBG level was reported in two cohort studies [14,28] of patients with T2DM with a total of 257 participants in each arm (Fig. 2). Meta-analysis showed a significant deterioration in FBG control from pre-lockdown to the lockdown period (MD=3.47 mg/dL [95% CI, 1.22 to 5.73], I2=0%, fixed-effect).
- Data on the mean level of TIR was reported in eight studies [13,15,17,19,20,24–26] among patients with T1DM, with a total of 580 participants in each arm (Fig. 3). A significant improvement of TIR from pre-lockdown to during the lockdown period was observed (MD=3.52% [95% CI, 0.29 to 6.74], I2=76%, random-effects). However, pooled estimates of subgroup analysis did not show significant improvement in TIR during lockdown in both cohort studies (MD=4.61% [95% CI, −0.55 to 9.78], I2=87%, random-effects) and case-control studies (MD=2.24% [95% CI, −0.43 to 4.90], I2=0%, fixed-effect).
- Data on the mean level of TAR was reported in eight studies [13,15,17,19,20,24–26] among patients with T1DM, with a total of 580 participants in each arm (Fig. 4). The pooled effect showed that TAR significantly improved from the pre-lockdown to the lockdown period (MD=−3.36% [95% CI, −6.48 to −0.25], I2=75%, random-effects). However, when subgroup analysis was performed, the pooled effects failed to reach statistical significance in both cohorts (MD=−4.44% [95% CI, −9.83 to 0.94], I2=86%, random-effects) and case-control studies (MD=−2.20% [95% CI, −4.66 to 0.27], I2=0%, fixed-effect).
- Data on the mean level of TBR was reported in eight studies [13,15,17,19,20,24–26] among patients with T1DM, with a total of 580 participants in each arm (Supplementary Fig. 9). TBR was not significantly affected during the lockdown period (MD=−0.05% [95% CI, −0.38 to 0.28], I2=0%, fixed-effect). Subgroup analysis was performed and insignificant changes in TBR were found in both cohort studies (MD=−0.11% [95% CI, −0.52 to 0.29], I2=0%, fixed-effect) and case-control studies (MD=0.08% [95% CI, −0.49 to 0.64], fixed-effect).
- Sensitivity analysis
- Heterogeneity of variance was found in HbA1c, RBG, TIR, and TAR analysis; therefore, a sensitivity analysis was carried out to identify the source of heterogeneity using the leave-one-out method on moderate-quality studies. Bonora et al. [24] was found to be the source of heterogeneity in both TAR and TIR. Neither the direction of effect nor the statistical significance was changed when this study was excluded from each outcome. Sensitivity analysis on each subgroup analysis of HbA1c and RBG was unable to provide significant results.
- Secondary outcomes
- Meta-analysis on lipid profiles could not be performed due to the limited data available. An Italian cohort study [14] of 114 Caucasian patients with T2DM reported significant improvements in total cholesterol and LDL-C levels during the lockdown (P<0.05), despite consistent doses and types of medication administered before and during lockdown. In contrast, a Turkish case-control study [18] of 85 patients with T2DM reported a significant increase in triglyceride and body weight (P<0.05). Multinomial logistic regression in Biancalana et al. [14], and multivariate analysis in the Karatas et al. [18] study also revealed disparate outcomes, where one study reported pre-lockdown triglyceride as a predictor of glycaemic control during the lockdown period, and the other reported that duration of diabetes was the independent predictor of both glycaemic control (HbA1c) and lipid profile (triglyceride) (odds ratio, 1.2; 95% CI, 1.1 to 1.8; P=0.032). Karatas et al. [18] reported that increases in body weight, blood glucose, HbA1c, and plasma lipids simultaneously served as factors in the increased risk of cardiovascular diseases.
RESULTS
HbA1c
Random blood glucose
Fasting blood glucose
Time-in-range
Time-above-range
Time-below-range
Lipid control
- We obtained a total of 21 observational studies with 3,992 participants. The pooled estimates of TIR and TAR among patients with T1DM showed improvements during lockdown, while FBG among patients with T2DM deteriorated. Total cholesterol and LDL-C were improved [14], but triglyceride worsened [18]. The majority of the studies reported that diet, physical activity, and stress management played a primary role in affecting the control of metabolic parameters. However, other factors were found to have an equal effect on worsening control. Social factors including emotional instability, socioeconomic vulnerability, uncertainty in employment status, and difficulty in accessing healthcare and medication aggravated the deterioration of metabolic parameters. In contrast, all arrangements of telemedicine, whether in the form of tele-assistance or use of automated glucose monitoring systems were found to improve the control of diabetic parameters [6–8,29].
- Excessive consumption of high carbohydrate foods, including several types of fruit and vegetables, is a well-known cause of increased triglyceride and blood glucose levels in patients with diabetes [30]. A reduction in physical activity during lockdown may have further contributed to an increase in triglyceride and blood glucose [31]. Refractory glucose fluctuations, shown by unchanged TBR in patients with T1DM and worsening FBG in patients with T2DM, despite improvements in other parameters, could indeed be heightened by several risk factors such as gender, younger age, higher body mass index, higher HbA1c, and current treatment strategies; in particular, triple combination or insulin therapy [32]. Nevertheless, poor dietary practices and physical inactivity were still the main contributors to adverse outcomes in the control of diabetes. Despite the downsides, we found that glycaemic parameters were still well-controlled, possibly due to the use of appropriate insulin treatments and medications which maintain normal blood glucose levels and prevent hyperglycaemia. Therefore, HbA1c did not deteriorate overall, and TIR and TAR were even found to show improvement. In addition to dietary factors, physical activity, and stress management, adherence to medication was essential in maintaining satisfactory glycaemic control during lockdown.
- We observed that individuals who lacked exercise and neglected healthy habits during lockdown were at higher risk of having poor glycaemic control [2,3]. Socioeconomic issues and psychological burden during lockdown had also worsened glycaemic control parameters. This deterioration was evident even among patients with T1DM who previously had appropriate metabolic control [23,33]. These factors led to changes in diet, sleep, and physical activity [2,3,34,35].
- Precisely how daily habit changes affected metabolic control were conflicting, however. For example, Ruissen et al. [16] reported an increase in stress, weight gain, and a reduction in exercise, but this study showed improvements in glycaemic control, in contrast to other studies [21,23,28]. Indeed, the population in this study [16] was previously well-controlled and received insulin treatments during lockdown. Meanwhile, another study reported that some patients with previously well-controlled diabetes had worsened parameters in lockdown [14]. Based on these findings, it is possible to infer that other factors, including adherence to medications or insulin treatment, might also have played a pivotal role in the maintenance of metabolic control [36]. In addition, adequate control of diabetes among patients with gestational diabetes was found to be unfavourable during the COVID-19 pandemic lockdown, even when follow-up via telemedicine was facilitated [37]. Without ignoring confounders, the net effect on metabolic control seems to be the result of all factors combined, rather than the effect of a single factor. It seems apparent that adequate diabetic control requires active participation and care from patients themselves.
- Most of the studies we included [13,15–17,19,20,23,25,26,35, 38,39] used either standalone CGM or CGM with an automated insulin delivery system such as hybrid closed loop for patients monitoring during lockdown. Use of CGM and an automated insulin delivery system as an artificial pancreas that maintains basal insulin levels have recently increased for the management of patients with T1DM [7,8]. Features on some versions of CGM enable the automatic scanning of blood glucose levels and are equipped with an active alarm in real time CGM, while other versions require patients to actively scan blood glucose, as in intermittently scanning CGM [40,41]. CGM can be very helpful in remotely managing diabetes and improving quality of life [42]. In addition to the psychosocial benefits, CGM use for patients with diabetes can improve poor diabetic control, as demonstrated by improvements in HbA1c, TAR, TIR, and TBR [43], and reductions in hypoglycaemia risk [43,44]. The use of auto-mode CGM is associated with improved glycaemic variability (GV) [35].
- Improving GV in patients with diabetes is considered crucial in reducing adverse outcomes. Several studies have shown that poor GV is associated with oxidative stress, which could induce endothelial dysfunction, atherosclerosis, and other cardiovascular complications. Glycaemic fluctuations have been shown to promote inflammatory cytokine release, monocyte adhesion to endothelial cells, endothelial cells apoptosis, and epigenetic changes in endothelial and mononuclear cells. These alterations could foster the downregulation of superoxide dismutase, detoxification genes, and free radical scavengers [45]. The most disadvantageous outcome of poor GV is that it has cytotoxic effects on the pancreas, which significantly reduces glucose-mediated insulin secretion, β-cells’ apoptosis, and mitochondrial alterations. Thus, poor GV promotes the vicious cycle of poor glycaemic control and diabetic complications [46].
- We noted that most of the worsening events in diabetic parameter control were reported from non-European countries, particularly countries in the Middle East, where cultural influences were highly correlated. According to 2019 Global Burden of Diseases (GBD) data, individuals living in Middle Eastern countries have one of the highest daily mean energy intakes and the lowest level of physical activity worldwide [47]. We propose that both of these factors, in conjunction with changes in habits during lockdown, led to a substantial worsening in metabolic control in patient with diabetes who reside in the Middle East.
- Regarding lipid profile findings, a previous observational study observed that a high carbohydrate diet increases fasting triglycerides and is associated with higher mortality rates [30]. Triglycerides are closely related to non-esterified fatty acids and could have hazardous effects on glucose homeostasis due to lipotoxicity [48]. Serum triglycerides could also independently represent a risk factor for cardiovascular and kidney diseases [49].
- In summary, the implementation of lockdowns to limit the spread of COVID-19 resulted in lifestyle modifications for many people worldwide. A sedentary lifestyle, unhealthy dietary intake, and poor stress management could be a factor in the poor management of diabetes. However, the application of CGM for some people had a positive effect on diabetic management, such as improving medication adherence. Despite many factors that can directly influence metabolic control parameters, these parameters tended to improve during COVID-19 lockdowns.
- As a meta-analysis, this study has some limitations. We included retrospective and prospective studies in this review due to the limited number of studies available. In particular, controlled studies were difficult to conduct during the lockdown period. Diversities in the duration and technical implementation of lockdowns between countries worldwide resulted in a heterogeneity of methods and results in this review, which along with the brief study period, had given rise to limitations in data availability. Thus, some outcomes could not be thoroughly analysed. Further study over an extended time period, larger population, and observation of post-lockdown findings could advance the significance of reviews on similar topics. Analysis of lipid profile should be further deepened in a more focused setting. To the best of our knowledge, until submission of this manuscript, this is the first study that compares glycaemic control parameters and lipid profiles of patients with diabetes before and during COVID-19 lockdown. The inclusion of patients with either T1DM or T2DM and with various types of study designs, including cohort, case-control, and cross-sectional studies from various countries worldwide supports the robust findings in this review.
- In conclusion, COVID-19 lockdowns did not have a negative effect on metabolic parameters in patients with diabetes, especially in those individuals who were able to maintain healthy daily habits. A nutritionally balanced diet, regular physical activity, and proper stress management, alongside the use of GCM as a remote diabetic monitoring strategy are recommended for good control of metabolic parameters in patients with diabetes who were unable to access in-person consultations with physicians during lockdown.
DISCUSSION
SUPPLEMENTARY MATERIALS
Supplementary Table 1.
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
Supplementary Fig. 4.
Supplementary Fig. 5.
Supplementary Fig. 6.
Supplementary Fig. 7.
Supplementary Fig. 8.
Supplementary Fig. 9.
-
Acknowledgements
- Authors would like to thank the Department of Public Health and Preventive Medicine at the Medical Faculty of Universitas Airlangga for their support throughout the writing process.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: I.A.W., N.R.P., N.F.S., E.S.A., T.K., M.A.W., M.R.W., L.D., H.N.
Acquisition, analysis, or interpretation of data: I.A.W., N.R.P., N.F.S., E.S.A., T.K., M.A.W., M.R.W.
Drafting the work or revising: I.A.W., N.R.P., N.F.S., E.S.A., T.K., M.A.W., M.R.W.
Final approval of the manuscript: I.A.W., N.R.P., N.F.S., E.S.A., T.K., M.A.W., M.R.W., L.D., H.N.
-
FUNDING
None
NOTES


| Study | Study design | Sample size | Study period | Diabetes type | Age, yr | Quality assessment | Measurement method | Outcomes | Findings |
|---|---|---|---|---|---|---|---|---|---|
|
Al Agha et al. (2021) Saudi Arabia [21] |
CS | 150 | Apr–Jun 2020 | T1DM | 12.45±4.62 | Strong | SMBG | RBG, HbA1c | Worsen due to decreased physical activity, increased carbohydrates and fast food consumption, and worsening mood. |
|
Alaqeel et al. (2021) Saudi Arabia [12] |
RC | 154, 106 | 1 Mar–30 Jun 2020 | T1DM | 9.8±0.2 | Strong | SMBG | HbA1c | Worsen with increased frequency of DKA. |
|
Aragona et al. (2020) Italy [13] |
RC | 63 | 21 Feb–25 Apr 2020 | T1DM | 44.0±12.0 | Strong | FGM, CGM | RBG, eA1c, TIR, TAR, TBR | Improved due to increased daily routine activities and decreased work-related stress. |
|
Barchetta et al. (2020) Italy [23] |
RC | 50 | 20 Jan–11 Apr 2020 | T1DM | 40.7±13.5 | Moderate | FGM, CGM | RBG, eA1c, TIR, TAR, TBR | Worsen due to work instability. |
|
Biancalana et al. (2021) Italy [14] |
PC | 114 | 9 Mar–4 May 2020 | T2DM | 69.4±10.3 | Strong | SMBG | HbA1c, FBG, TC, LDL-C, TG | Worsen. Pre-lockdown TG is the sole predictor of lockdown glycemic control. |
|
Bonora et al. (2020) Italy [24] |
RC | 20 | Feb–June 2020 | T1DM | 36.9±13.4 | Moderate | FGM | RBG, TIR, TAR, TBR | Improved due to slow down in daily routine activities, particularly in stay-at-home patients. |
|
Brener et al. (2020) Israel [25] |
RC | 102 | 23 Feb–7 Apr 2020 | T1DM | 10.9 ± 3.9 | Strong | CGM | RBG, TIR, TAR, TBR | Unchanged. |
|
Christoforidis et al. (2020) Greece [26] |
CC | 34 | 18 Feb–1 Apr 2020 | T1DM | 11.37±4.45 | Moderate | HCL system | RBG, TIR, TAR, TBR | Unchanged due to insulin pumps and glucose monitoring sensors usage. |
|
Cotovad-Bellas et al. (2020) Spain [17] |
CC | 44 | 1 Mar–19 Apr 2020 | T1DM | 37.0±18.0 | Moderate | FGM, CGM | RBG, eA1c, TIR, TAR, TBR | Unchanged due to increased time for exercise and home-made cooking. |
|
Di Dalmazi et al. (2020) Italy [38] |
PC | 130 | 20 Feb–30 Mar 2020 | T1DM |
8.8 (7.7–10.6) 15.6 (14.2–16.8) 45 (29–58.1) |
Moderate | CGM | RBG, eA1c, TIR, TAR, TBR | Unchanged due to the use of CGM, physical activity, and stress reduction. |
|
Dover et al. (2021) United Kingdom [33] |
PC | 572 | 11–21 May 2020 | T1DM | 39 (31–50) | Weak | FGM | RBG, eA1c, TIR, TAR, TBR | Unchanged. Socioeconomic status served as risk factors. |
|
Karatas et al. (2021) Turkey [18] |
CC | 85 | Mar 2019–Oct 2020 | T2DM | 54.81±10.53 | Strong | SMBG | HbA1c, FBG, LDL-C, HDL-C, TG | Worsen. No association with weight gain. |
|
Khare et al. (2020) India [28] |
PC | 143 | The first 3 weeks of lockdown | T2DM | 54.68±9.22 | Strong | SMBG | FBG, PPBG | Worsen due to lifestyle changes, psychological stress, and difficulties in access to treatments and health care facilities. |
|
Longo et al. (2020) Italy [35] |
RC | 30 | 23 Feb–3 May 2020 | T1DM | 31.5 (25–42) | Moderate | HCL system | RBG, eA1c, TIR, TAR, TBR | Improved due to HCL usage. |
| Study | Study design | Sample size | Study period | Diabetes type | Age, yr | Quality assessment | Measurement method | Outcomes | Findings |
|
Mesa et al. (2020) Spain [19] |
CC | 92 | 23 Feb–14 Apr 2020 | T1DM | 42.8±13.9 | Strong | CGM | RBG, eA1c, TIR, TAR, TBR | Improved due to strict daily routines at home. |
|
Parise et al. (2021) Italy [15] |
PC | 166 | 10 Mar–3 June 2020 | T1DM | 40.0±14.0 | Strong | CGM | RBG, eA1c, TIR, TAR, TBR | Improved due to structured virtual visits (tele-assistance). |
|
Potier et al. (2021) France [27] |
CS | 1,378 | 23–28 Apr 2020 | T1DM | 45.6±13.6 | Strong | FGM | RBG | Improved due to improved diet, increased physical activity, and increased FGM scans. |
|
Ruissen et al. (2021) Netherlands [16] |
PC | 435 | 24 Feb–7 May 2020 |
T1DM T2DM |
50.1±14.9 62.5±11.6 |
Strong | FGM, CGM | HbA1c, TIR, TAR, TBR | Improved due to good control. |
|
Tornese et al. (2020) France [39] |
RC | 13 | 10 Feb–22 Mar 2020 | T1DM | 14.2 (11.4–15.5) | Moderate | HCL system | RBG, eA1c, TIR, TAR, TBR | Improved due to physical activity. |
|
Verma et al. (2020) India [22] |
CS | 52 | 25 Mar–31 May 2020 | T1DM | 11.9 | Moderate | SMBG | RBG, HbA1c | Worsen due to the unavailability of insulin/glucostrips. |
|
Vinals et al. (2021) Spain [20] |
CC | 59 | 23 Feb–14 Apr 2021 | T1DM | 46.18±13.02 | Strong | HCL system | RBG, eA1c, TIR, TAR, TBR | Unchanged due to strict daily routines at home. |
Values are presented as mean±standard deviation or median (interquartile range).
CS, cross-sectional; T1DM, type 1 diabetes mellitus; SMBG, self-monitoring blood glucose; RBG, random blood glucose; HbA1c, glycosylated hemoglobin; RC, retrospective cohort; DKA, diabetic ketoacidosis; FGM, flash glucose monitoring; CGM, continuous glucose monitoring; eA1c, estimated A1c; TIR, time-in-range; TAR, time-above-range; TBR, time-below-range; PC, prospective cohort; T2DM, type 2 diabetes mellitus; FBG, fasting blood glucose; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglyceride; CC, case-control; HCL, hybrid closed loop; HDL-C, high-density lipoprotein cholesterol; PPBG, postprandial blood glucose.
- 1. Alshareef R, Al Zahrani A, Alzahrani A, Ghandoura L. Impact of the COVID-19 lockdown on diabetes patients in Jeddah, Saudi Arabia. Diabetes Metab Syndr 2020;14:1583-7.ArticlePubMedPMC
- 2. Cheikh Ismail L, Osaili TM, Mohamad MN, Al Marzouqi A, Jarrar AH, Abu Jamous DO, et al. Eating habits and lifestyle during COVID-19 lockdown in the United Arab Emirates: a cross-sectional study. Nutrients 2020;12:3314.ArticlePubMedPMC
- 3. Di Renzo L, Gualtieri P, Pivari F, Soldati L, Attina A, Cinelli G, et al. Eating habits and lifestyle changes during COVID-19 lockdown: an Italian survey. J Transl Med 2020;18:229.ArticlePubMedPMCPDF
- 4. Ruiz-Roso MB, Knott-Torcal C, Matilla-Escalante DC, Garcimartin A, Sampedro-Nunez MA, Davalos A, et al. COVID-19 lockdown and changes of the dietary pattern and physical activity habits in a cohort of patients with type 2 diabetes mellitus. Nutrients 2020;12:2327.ArticlePubMedPMC
- 5. Ghosh A, Arora B, Gupta R, Anoop S, Misra A. Effects of nationwide lockdown during COVID-19 epidemic on lifestyle and other medical issues of patients with type 2 diabetes in north India. Diabetes Metab Syndr 2020;14:917-20.ArticlePubMedPMC
- 6. van der Linden J, Welsh JB, Hirsch IB, Garg SK. Real-time continuous glucose monitoring during the coronavirus disease 2019 pandemic and its impact on time in range. Diabetes Technol Ther 2021;23:S1-7.
- 7. Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther 2017;19:S25-37.ArticlePubMed
- 8. Vettoretti M, Cappon G, Acciaroli G, Facchinetti A, Sparacino G. Continuous glucose monitoring: current use in diabetes management and possible future applications. J Diabetes Sci Technol 2018;12:1064-71.ArticlePubMedPMCPDF
- 9. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.ArticlePubMedPMC
- 10. Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool. Methodological research. J Eval Clin Pract 2012;18:12-8.ArticlePubMed
- 11. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593-603.PubMedPMC
- 12. Alaqeel A, Aljuraibah F, Alsuhaibani M, Huneif M, Alsaheel A, Dubayee MA, et al. The impact of COVID-19 pandemic lockdown on the incidence of new-onset type 1 diabetes and ketoacidosis among Saudi children. Front Endocrinol (Lausanne) 2021;12:669302.ArticlePubMedPMC
- 13. Aragona M, Rodia C, Bertolotto A, Campi F, Coppelli A, Giannarelli R, et al. Type 1 diabetes and COVID-19: the “lockdown effect”. Diabetes Res Clin Pract 2020;170:108468.ArticlePubMedPMC
- 14. Biancalana E, Parolini F, Mengozzi A, Solini A. Short-term impact of COVID-19 lockdown on metabolic control of patients with well-controlled type 2 diabetes: a single-centre observational study. Acta Diabetol 2021;58:431-6.ArticlePubMedPDF
- 15. Parise M, Tartaglione L, Cutruzzola A, Maiorino MI, Esposito K, Pitocco D, et al. Teleassistance for patients with type 1 diabetes during the COVID-19 pandemic: results of a pilot study. J Med Internet Res 2021;23:e24552.ArticlePubMedPMC
- 16. Ruissen MM, Regeer H, Landstra CP, Schroijen M, Jazet I, Nijhoff MF, et al. Increased stress, weight gain and less exercise in relation to glycemic control in people with type 1 and type 2 diabetes during the COVID-19 pandemic. BMJ Open Diabetes Res Care 2021;9:e002035.ArticlePubMed
- 17. Cotovad-Bellas L, Tejera-Perez C, Prieto-Tenreiro A, Sanchez-Bao A, Bellido-Guerrero D. The challenge of diabetes home control in COVID-19 times: proof is in the pudding. Diabetes Res Clin Pract 2020;168:108379.ArticlePubMedPMC
- 18. Karatas S, Yesim T, Beysel S. Impact of lockdown COVID-19 on metabolic control in type 2 diabetes mellitus and healthy people. Prim Care Diabetes 2021;15:424-7.ArticlePubMedPMC
- 19. Mesa A, Vinals C, Pueyo I 1st, Roca D, Vidal M, Gimenez M, et al. The impact of strict COVID-19 lockdown in Spain on glycemic profiles in patients with type 1 diabetes prone to hypoglycemia using standalone continuous glucose monitoring. Diabetes Res Clin Pract 2020;167:108354.ArticlePubMedPMC
- 20. Vinals C, Mesa A, Roca D, Vidal M, Pueyo I, Conget I, et al. Management of glucose profile throughout strict COVID-19 lockdown by patients with type 1 diabetes prone to hypoglycaemia using sensor-augmented pump. Acta Diabetol 2021;58:383-8.ArticlePubMedPDF
- 21. Al Agha AE, Alharbi RS, Almohammadi OA, Yousef SY, Sulimani AE, Alaama RA. Impact of COVID-19 lockdown on glycemic control in children and adolescents. Saudi Med J 2021;42:44-8.ArticlePubMedPMC
- 22. Verma A, Rajput R, Verma S, Balania V, Jangra B. Impact of lockdown in COVID 19 on glycemic control in patients with type 1 diabetes mellitus. Diabetes Metab Syndr 2020;14:1213-6.ArticlePubMedPMC
- 23. Barchetta I, Cimini FA, Bertoccini L, Ceccarelli V, Spaccarotella M, Baroni MG, et al. Effects of work status changes and perceived stress onglycaemiccontrol in individuals with type 1 diabetes during COVID-19 lockdown in Italy. Diabetes Res Clin Pract 2020;170:108513.ArticlePubMedPMC
- 24. Bonora BM, Boscari F, Avogaro A, Bruttomesso D, Fadini GP. Glycaemic control among people with type 1 diabetes during lockdown for the SARS-CoV-2 outbreak in Italy. Diabetes Ther 2020;11:1-11.ArticlePMCPDF
- 25. Brener A, Mazor-Aronovitch K, Rachmiel M, Levek N, Barash G, Pinhas-Hamiel O, et al. Lessons learned from the continuous glucose monitoring metrics in pediatric patients with type 1 diabetes under COVID-19 lockdown. Acta Diabetol 2020;57:1511-7.ArticlePubMedPMCPDF
- 26. Christoforidis A, Kavoura E, Nemtsa A, Pappa K, Dimitriadou M. Coronavirus lockdown effect on type 1 diabetes management on children wearing insulin pump equipped with continuous glucose monitoring system. Diabetes Res Clin Pract 2020;166:108307.PubMedPMC
- 27. Potier L, Hansel B, Larger E, Gautier JF, Carreira D, Assemien R, et al. Stay-at-home orders during the COVID-19 pandemic, an opportunity to improve glucose control through behavioral changes in type 1 diabetes. Diabetes Care 2021;44:839-43.ArticlePubMedPDF
- 28. Khare J, Jindal S. Observational study on effect of lock down due to COVID 19 on glycemic control in patients with diabetes: experience from Central India. Diabetes Metab Syndr 2020;14:1571-4.ArticlePMC
- 29. Martens T, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA 2021;325:2262-72.PubMed
- 30. Jung CH, Choi KM. Impact of high-carbohydrate diet on metabolic parameters in patients with type 2 diabetes. Nutrients 2017;9:322.ArticlePubMedPMC
- 31. Figueiro TH, Arins G, Santos C, Cembranel F, Medeiros PA, d’Orsi E, et al. Association of objectively measured sedentary behavior and physical activity with cardiometabolic risk markers in older adults. PLoS One 2019;14:e0210861.ArticlePubMedPMC
- 32. Umpierrez GE, P Kovatchev B. Glycemic variability: how to measure and its clinical implication for type 2 diabetes. Am J Med Sci 2018;356:518-27.ArticlePubMedPMC
- 33. Dover AR, Ritchie SA, McKnight JA, Strachan M, Zammitt NN, Wake DJ, et al. Assessment of the effect of the COVID-19 lockdown on glycaemic control in people with type 1 diabetes using flash glucose monitoring. Diabet Med 2021;38:e14374.ArticlePubMedPDF
- 34. Zachary Z, Brianna F, Brianna L, Garrett P, Jade W, Alyssa D, et al. Self-quarantine and weight gain related risk factors during the COVID-19 pandemic. Obes Res Clin Pract 2020;14:210-6.ArticlePubMedPMC
- 35. Longo M, Caruso P, Petrizzo M, Castaldo F, Sarnataro A, Gicchino M, et al. Glycemic control in people with type 1 diabetes using a hybrid closed loop system and followed by telemedicine during the COVID-19 pandemic in Italy. Diabetes Res Clin Pract 2020;169:108440.ArticlePubMedPMC
- 36. Neto JCGL, da Silva AP, de Araujo MFM, Damasceno MMC, Landim MBP, de Freitas RWJF. Metabolic control and medication adherence in people with diabetes mellitus. Acta Paul Enferm 2017;30:152-8.Article
- 37. Ghesquiere L, Garabedian C, Drumez E, Lemaitre M, Cazaubiel M, Bengler C, et al. Effects of COVID-19 pandemic lockdown on gestational diabetes mellitus: a retrospective study. Diabetes Metab 2021;47:101201.ArticlePubMed
- 38. Di Dalmazi G, Maltoni G, Bongiorno C, Tucci L, Di Natale V, Moscatiello S, et al. Comparison of the effects of lockdown due to COVID-19 on glucose patterns among children, adolescents, and adults with type 1 diabetes: CGM study. BMJ Open Diabetes Res Care 2020;8:e001664.ArticlePubMedPMC
- 39. Tornese G, Ceconi V, Monasta L, Carletti C, Faleschini E, Barbi E. Glycemic control in type 1 diabetes mellitus during COVID-19 quarantine and the role of in-home physical activity. Diabetes Technol Ther 2020;22:462-7.ArticlePubMed
- 40. Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabetes Metab J 2019;43:383-97.ArticlePubMedPMCPDF
- 41. Edelman SV, Argento NB, Pettus J, Hirsch IB. Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care 2018;41:2265-74.ArticlePubMedPDF
- 42. Polonsky WH, Hessler D, Ruedy KJ, Beck RW. DIAMOND Study Group. The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 2017;40:736-41.ArticlePubMedPDF
- 43. Maiorino MI, Signoriello S, Maio A, Chiodini P, Bellastella G, Scappaticcio L, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care 2020;43:1146-56.ArticlePubMedPDF
- 44. Langendam M, Luijf YM, Hooft L, Devries JH, Mudde AH, Scholten RJ. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev 2012;1:CD008101.ArticlePubMed
- 45. Costantino S, Paneni F, Battista R, Castello L, Capretti G, Chiandotto S, et al. Impact of glycemic variability on chromatin remodeling, oxidative stress, and endothelial dysfunction in patients with type 2 diabetes and with target HbA1c levels. Diabetes 2017;66:2472-82.ArticlePubMedPDF
- 46. Kaze AD, Santhanam P, Erqou S, Ahima RS, Echouffo-Tcheugui JB. Long-term variability of glycemic markers and risk of all-cause mortality in type 2 diabetes: the Look AHEAD study. BMJ Open Diabetes Res Care 2020;8:e001753.ArticlePubMedPMC
- 47. Azizi F, Hadaegh F, Hosseinpanah F, Mirmiran P, Amouzegar A, Abdi H, et al. Metabolic health in the Middle East and North Africa. Lancet Diabetes Endocrinol 2019;7:866-79.ArticlePubMed
- 48. Seghieri M, Trico D, Natali A. The impact of triglycerides on glucose tolerance: lipotoxicity revisited. Diabetes Metab 2017;43:314-22.ArticlePubMed
- 49. Marston NA, Giugliano RP, Im K, Silverman MG, O’Donoghue ML, Wiviott SD, et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation 2019;140:1308-17.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Disruption of diabetes and hypertension care during the COVID-19 pandemic and recovery approaches in the Latin America and Caribbean region: a scoping review protocol
Samira Barbara Jabakhanji, Oluwabunmi Ogungbe, Sonia Y Angell, Lawrence Appel, David Byrne, Roopa Mehta, John McCaffrey, Lori Rosman, Edward W Gregg, Kunihiro Matsushita
BMJ Open.2024; 14(1): e074443. CrossRef - Influence of the COVID-19 pandemic on the achievement of guideline targets for HbA1c, blood pressure, and LDL cholesterol in people with diabetes in Japan
Shingo Kuwajima, Takahito Itoh, Tatsuya Sato, Shoya Ino, Satoru Shibata, Kouhei Ohno, Hiroyuki Hotta, Tomoaki Matsumoto, Hitoshi Ooiwa, Hirofumi Kubo, Takayuki Miki
Diabetology International.2024;[Epub] CrossRef - Glycemic Control in Type 1 Diabetes Mellitus and COVID-19: What We Learned From the Lockdown Experience
Catarina Almeida, André Ferreira, Daniela Duarte, Ana Filipa Viegas, André Santos, Alexandra Vaz, Edite Nascimento
Cureus.2023;[Epub] CrossRef - Changes in body weight and glycemic control in association with COVID-19 Shutdown among 23,000 adults with type 2 diabetes
Emily Panza, Kevin E. Kip, Kripa Venkatakrishnan, Oscar C. Marroquin, Rena R. Wing
Acta Diabetologica.2023; 60(6): 787. CrossRef - The Impact of a Lockdown for the COVID-19 Pandemic on Seasonal HbA1c Variation in Patients with Type 2 Diabetes
Yu-Cheng Cheng, Yu-Hsuan Li, Hsiu-Chen Liu, Chiann-Yi Hsu, Wan-Jen Chang, I-Te Lee, Chin-Li Lu
Life.2023; 13(3): 763. CrossRef - The Impact of Partial Lockdown During COVID-19 Pandemic on Metabolic Control in People with Type 2 Diabetes Mellitus
Ayşe Zülal TOKAÇ, Tuğde Buse UĞUR, Buse Ecem KURUGÖL, Sevilay ALİGÜLÜ, Osman HAYRAN
Journal of Biotechnology and Strategic Health Research.2023; 7(1): 67. CrossRef - Retrospective Study on the Impact of COVID-19 Lockdown on Patients with Type 2 Diabetes in Northern Taiwan
Hsuan Huang, Hsiao-Ling Su, Chih-Hsung Huang, Yi-Hsin Lin
Diabetes, Metabolic Syndrome and Obesity.2023; Volume 16: 2539. CrossRef - RIPK1 and RIPK3 inhibitors: potential weapons against inflammation to treat diabetic complications
Dan Ke, Zhen Zhang, Jieting Liu, Peijian Chen, Yucen Dai, Xinhai Sun, Yanhui Chu, Luxin Li
Frontiers in Immunology.2023;[Epub] CrossRef - “Does Physical Exercise Promote Health Benefits for Diabetic Patients during the COVID-19 Pandemic?”: A Systematic Review
Erivaldo de Souza, Daniela Meneses-Santos, Josué Cruz Santos, Felipe J. Aidar, Carla Roberta de Oliveira Carvalho, Jymmys Lopes dos Santos, Anderson Carlos Marçal
Sports.2023; 11(10): 192. CrossRef - Impact of National Lockdown From COVID-19 Pandemic in Patients With Type 2 Diabetes: An Observational Study
Nuntakorn Thongtang, Niracha Chanwimol, Lukana Preechasuk, Varisara Boonyuang, Pinyo Rattanaumpawan, Supawadee Likitmaskul, Apiradee Sriwijitkamol
Asia Pacific Journal of Public Health.2022; 34(6-7): 708. CrossRef
- Figure
- Related articles
-
- Comparative Effect of Glucose-Lowering Drugs for Type 2 Diabetes Mellitus on Stroke Prevention: A Systematic Review and Network Meta-Analysis
- The Beneficial Effect of Glycemic Control against Adverse Outcomes in Patients with Type 2 Diabetes Mellitus and Chronic Kidney Disease
- Clinical Effects of a Home Care Pilot Program for Patients with Type 1 Diabetes Mellitus: A Retrospective Cohort Study
- Safety of COVID-19 Vaccines among Patients with Type 2 Diabetes Mellitus: Real-World Data Analysis
- Effectiveness of Resistance Exercise on Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Systematic Review with Meta-Analysis

 KDA
KDA PubReader
PubReader ePub Link
ePub Link Cite
Cite