Association of Urinary N-Acetyl-β-D-Glucosaminidase with Cardiovascular Autonomic Neuropathy in Type 1 Diabetes Mellitus without Nephropathy
Article information
Abstract
Background
Cardiovascular autonomic neuropathy (CAN) is a common microvascular complication of diabetes and related to albuminuria in diabetic nephropathy (DN). Urinary N-acetyl-β-D-glucosaminidase (uNAG) is a renal tubular injury marker which has been reported as an early marker of DN even in patients with normoalbuminuria. This study evaluated whether uNAG is associated with the presence and severity of CAN in patients with type 1 diabetes mellitus (T1DM) without nephropathy.
Methods
This cross-sectional study comprised 247 subjects with T1DM without chronic kidney disease and albuminuria who had results for both uNAG and autonomic function tests within 3 months. The presence of CAN was assessed by age-dependent reference values for four autonomic function tests. Total CAN score was assessed as the sum of the partial points of five cardiovascular reflex tests and was used to estimatethe severity of CAN. The correlations between uNAG and heart rate variability (HRV) parameters were analyzed.
Results
The association between log-uNAG and presence of CAN was significant in a multivariate logistic regression model (adjusted odds ratio, 2.39; 95% confidence interval [CI], 1.08 to 5.28; <i>P</i>=0.031). Total CAN score was positively associated with loguNAG (β=0.261, <i>P</i>=0.026) in the multivariate linear regression model. Log-uNAG was inversely correlated with frequency-domain and time-domain indices of HRV.
Conclusion
This study verified the association of uNAG with presence and severity of CAN and changes in HRV in T1DM patients without nephropathy. The potential role of uNAG should be further assessed for high-risk patients for CAN in T1DM patients without nephropathy.
Highlights
• Urinary N-acetyl-β-D-glucosaminidase (uNAG), a renal tubular injury marker, has been reported as an early marker of diabetic nephropathy (DN).
• Cardiovascular autonomic neuropathy (CAN), an impairment of heart rate variability (HRV), has been related to microalbuminuria and the progression of DN.
• In this study, increase in uNAG was correlated with a reduction in HRV parameters and significantly associated with both the presence and severity of CAN in type 1 diabetes (T1D) patients without albuminuria.
• Increase in uNAG might be an indicator of CAN in T1D patients with normoalbumiuria.
INTRODUCTION
Diabetes has become the most common single cause of end-stage renal disease [1]. Renal tubular damage occurs early in diabetic nephropathy (DN) and may play a pivotal role in the pathogenesis of DN [2]. Therefore, numerous studies have focused on different tubular damage markers that are clinically used as potential biomarkers for the early detection of DN. Urinary N-acetyl-β-D-glucosaminidase (uNAG) exists in the lysosomes of proximal tubule epithelial cells, and it has a molecular weight of 130,000 to 140,000 daltons which cannot pass through the basal membrane of the renal glomerulus [3]. Because uNAG excretion is increased by proximal tubular cell injury, uNAG has been suggested as a highly sensitive screening tool to detect early DN [4,5]. An increase in uNAG level already occurs in patients with diabetes without albuminuria [6].
Cardiovascular autonomic neuropathy (CAN) is another common microvascular complication of diabetes that affects autonomic nerve fibers innervating the heart and blood vessels, causing impairment of heart rate variability (HRV) and other vasodynamic changes [7]. All diabetic complications share the similar mechanisms of vascular damage and are strongly interconnected [8], thus CAN is also related to microalbuminuria which is an early clinical manifestation of DN [1,9], and is suggested to be an independent predictor for progression of DN [10,11]. However, it is unknown whether uNAG, a marker for early DN, is related to CAN in diabetic subjects without albuminuria and chronic kidney disease (CKD).
Therefore, the aim of this study was to evaluate whether uNAG level is positively associated with the presence and severity of CAN in patients with type 1 diabetes mellitus (T1DM), particularly those without CKD and albuminuria. We further investigated the relationship between uNAG level and each of the parameters achieved by HRV test.
METHODS
Inclusion and exclusion criteria
We sequentially retrieved medical records from 353 subjects with T1DM aged at least 19 years and with results for urinary markers and diabetic autonomic function tests at the same time or within 3 months between February 2016 and June 2018 at the Diabetes Clinic of Samsung Medical Center, Seoul, Korea. T1DM was defined as β-cell destruction, which was identified by clinical characteristics, fasting C-peptide level lower than 0.6 ng/mL, glucagon-stimulated C-peptide lower than 1.8 ng/mL, or positive results for anti-glutamic acid decarboxylase antibody [12].
We excluded subjects with a previous history of medical comorbidities, those with CKD stage 3 to 5 based on estimated glomerular filtration rate (eGFR) calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, and those with at least two sequential results of microalbuminuria or one result of macroalbuminuria. Considering the effect of the drug on the results of autonomic function tests, subjects on β-blockers or diuretics were excluded.
The following clinical data was obtained from individual review of the medical records: age, sex, body mass index, duration of diabetes, comorbidities, systolic and diastolic blood pressure, current smoking status, type of insulin therapy, use of anti-hypertensive agents including angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB), use of lipid-lowering agents, results of autonomic function tests, and levels of glycosylated hemoglobin (HbA1c), glycoalbumin (GA), fasting C-peptide, total cholesterol, high and low density lipoprotein cholesterol, and triglycerides. This study protocol was approved by the Institutional Review Board (IRB) of Samsung Medical Center (IRB-No 2019-12-087-001) and was performed according to the Declaration of Helsinki. Written informed consent by the patients was waived due to a retrospective nature of our study.
Assessment of urinary markers
The uNAG level was measured along with urinary excretion of albumin and creatinine. The 6-methyl-2-phylidil-N-acetyl-1-thio-β-D-glucosaminide (MPT-NAG) substrate method (Nittobo Medical, Tokyo, Japan) was used to quantify uNAG. The level of uNAG was divided by urinary creatinine excretion and expressed as U/g Cr, which is a standard international unit. The coefficient of variation for uNAG was 5%. The level of uNAG was measured from 0.8 to 300 IU/L.
The immunoturbidimetric method was used to measure urinary albumin level, and the kinetic Jaffe method was used to measure urinary creatinine level. The urine albumin-to-creatinine ratio (uACR) was used to standardize the results. According to the National Kidney Foundation and the American Diabetes Association, normal uACR level is lower than 30 μg/mg, albuminuria is uACR greater than or equal to 30 μg/mg, and macroalbuminuria is greater than or equal to 300 μg/mg [13,14].
Assessment of cardiovascular autonomic neuropathy
As suggested by Ewing et al. [15], the presence and severity of CAN were evaluated based on the result of five cardiovascular autonomic function tests. In the outpatient clinic, patients received a protocol to reduce the effect of other drugs or behaviors. Patients were asked to avoid smoking, alcohol, and intense physical exercise for 24 hours before the tests. Intake of food or coffee was also banned for 3 hours before the tests. Taking medications such as antihistamines, antidepressants, acetaminophen, β-blockers, and diuretics were prohibited for 12 hours before the tests.
The presence of CAN was defined as two or more abnormal results from three parasympathetic function tests according to an age-specific reference, regardless of the presence of orthostatic hypotension. Parasympathetic function tests include heart rate responses during positional change from supine to standing (30:15 ratio), during the Valsalva maneuver (Valsalva ratio), and during deep breathing (exhalation-to-inhalation ratio) [16]. Heart rate responses were measured automatically from electrocardiography recordings using the DICAN evaluation system (Medicore Co. Ltd., Seoul, Korea).
To estimate the severity of CAN, total CAN score was assessed as the sum of the partial points obtained from five standard cardiovascular reflex tests. Each of the three parasympathetic function tests was graded as 0 for normal and 1 for abnormal results. The parasympathetic function test results were graded using age-specific reference ranges [17]. Two sympathetic function tests comprise blood pressure responses during sustained handgrip and while standing. Each of the two sympathetic function tests was graded as 0 for normal, 0.5 for borderline, and 1 for abnormal result.
To analyze the change in HRV, beat-to-beat heart rate was measured for 5 minutes at rest. Frequency-domain analysis was performed using low frequency (LF; 0.04 to 0.15 Hz) and high frequency (HF; 0.15 to 0.40 Hz) spectral components. Time-domain analysis was performed based on the standard deviation of NN intervals (SDNN) and the root mean square of successive RR interval differences (RMSSD).
Assessment of other clinical variables
HbA1c level was measured by high-performance liquid chromatography using a VARIANT II TURBO analyzer (Bio-Rad Laboratories, Hercules, CA, USA). Serum GA level was measured by an enzymatic method using a Lucica GA-L kit (Asahi Kasei Pharma Corporation, Tokyo, Japan). The reference interval for HbA1c was 4.0% to 6.0%, and that for GA was 11.0% to 16.0%. Hypertension was defined as a systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg. Subjects using antihypertensive medications were also counted as having hypertension.
Statistical analyses
Data are shown as mean±standard deviation, median (interquartile range: 25th to 75th percentile), or number (percentage). To compare baseline characteristics, the Student’s t-test and Mann-Whitney U test were used for continuous variables according to data distribution, and Pearson’s chi-square test was used for categorical variables. Log-transformation was used to achieve a normal distribution in cases of skewed data. Due to the non-normality distribution of the uNAG level that exhibited a right-skewed pattern (Kolmogorov-Smirnov P< 0.01), natural log-transformed uNAG (log-uNAG, Kolmogorov-Smirnov P=0.200) was used in the correlation analyses and regression models. Correlation between parameters was assessed by Pearson’s correlation coefficient.
Univariate and multivariate logistic regression analysis models were established to represent the independent association between uNAG and presence of CAN. Among the risk factors and potential confounders, age, log-transformed HbA1c, and eGFR were factors with P<0.10 on univariate logistic regression and were included in the multivariate logistic regression analysis.
The association between uNAG and severity of CAN, defined using CAN stage, was analyzed using linear regression analyses. The factors with P<0.10 in a univariate model were selected and further adjusted in multivariate linear regression analysis.
The collinearity of independent variables was assessed using the variance inflation factor (VIF) test. A VIF ≥5 was considered to indicate collinearity. There was no collinearity in the analyses that included the above variables.
Correlation analyses were performed to investigate the relationship between log-uNAG level and each of the natural log-transformed HRV parameters (i.e., LF, HF, SDNN, and RMSSD [log-LF, log-HF, log-SDNN, and log-RMSSD]). All statistical analyses were performed using SPSS statistics software version 25.0 (IBM Corp., Armonk, NY, USA). Two-tailed P values less than 0.05 were considered statistically significant.
RESULTS
Study subjects
Among the 353 subjects, those with a previous history of medical comorbidities such as cardiovascular disease (n=10), stroke (n=3), liver cirrhosis (n=3), or any malignancy (n=15) were excluded from the analysis. Subjects with an eGFR <60 mL/min/1.73 m2 (n=33) or albuminuria (n=35) were also excluded. Subjects who were taking β-blockers (n=2) or diuretics (n=5) were excluded. Finally, a total of 247 subjects were enrolled (Fig. 1).
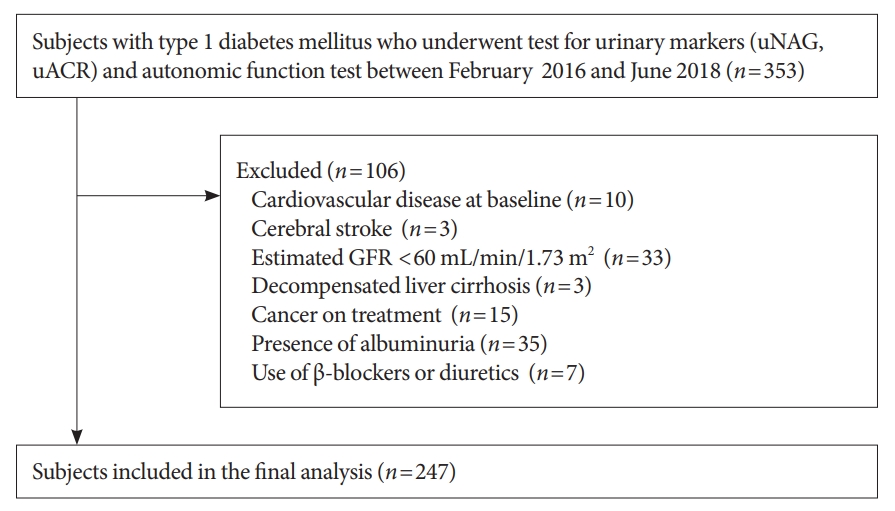
Flowchart for selection of study subjects. uNAG, urinary N-acetyl-β-D-glucosaminidase; uACR, urine albumin-to-creatinine ratio; GFR, glomerular filtration rate.
The baseline clinical characteristics of the study subjects according to presence of CAN are described in Table 1. Patients with CAN were younger, had higher levels of eGFR, and a higher proportion of patients with HbA1c ≥9% than patients without CAN. No significant difference was seen in level of uNAG between the no CAN and CAN groups. The proportion of patients using insulin, ACEI/ARB, calcium channel blockers, β-blockers, lipid lowering drugs, or anti-platelet drugs did not differ between the two groups.
Associations between uNAG and presence of CAN
As presented in Table 2, the multivariate analysis model adjusting for age, log-transformed HbA1c and eGFR, showed log-uNAG was significantly associated with the presence of CAN (odds ratio [OR], 2.28; 95% confidence interval [CI], 1.06 to 4.92; P=0.035). After further adjustment for use of ACEI/ARB, uNAG was significantly associated with the presence of CAN (OR, 2.39; 95% CI, 1.08 to 5.28; P=0.031). The log-uACR level was positively associated with log-uNAG level (Pearson’s correlation R2=0.0857, P<0.001). When log-uACR was adjusted for, log-uNAG was not associated with the presence of CAN, whereas log-uACR was positively associated with the presence of CAN (data not shown).
Association between uNAG and total CAN score
The results of univariate and multivariate linear regression analyses between log-uNAG level and total CAN score are shown in Table 3. We found a significant positive association between log-uNAG and total CAN score in multivariate analysis after correcting for age, log-HbA1c, and eGFR (β=0.278, P=0.023). After further adjustment for DM duration and use of ACEI/ARB, uNAG was significantly associated with total CAN score (β=0.261, P=0.026). When log-uACR was included for adjustment, total CAN score was positively associated with log-uACR and not associated with log-uNAG (data not shown).
Association between uNAG and HRV parameters
Scatter plots of Pearson’s correlation coefficient between natural log-transformed HRV parameters and log-uNAG are shown in Fig. 2. Not only frequency-domain indices such as log-LF (Pearson’s correlation R2=0.0882, P<0.001) and log-HF (R2=0.0802, P<0.001), but also time-domain indices such as log-SDNN (R2=0.0757, P<0.001) and log-RMSSD (R2=0.0659, P<0.001) were inversely correlated with log-uNAG.
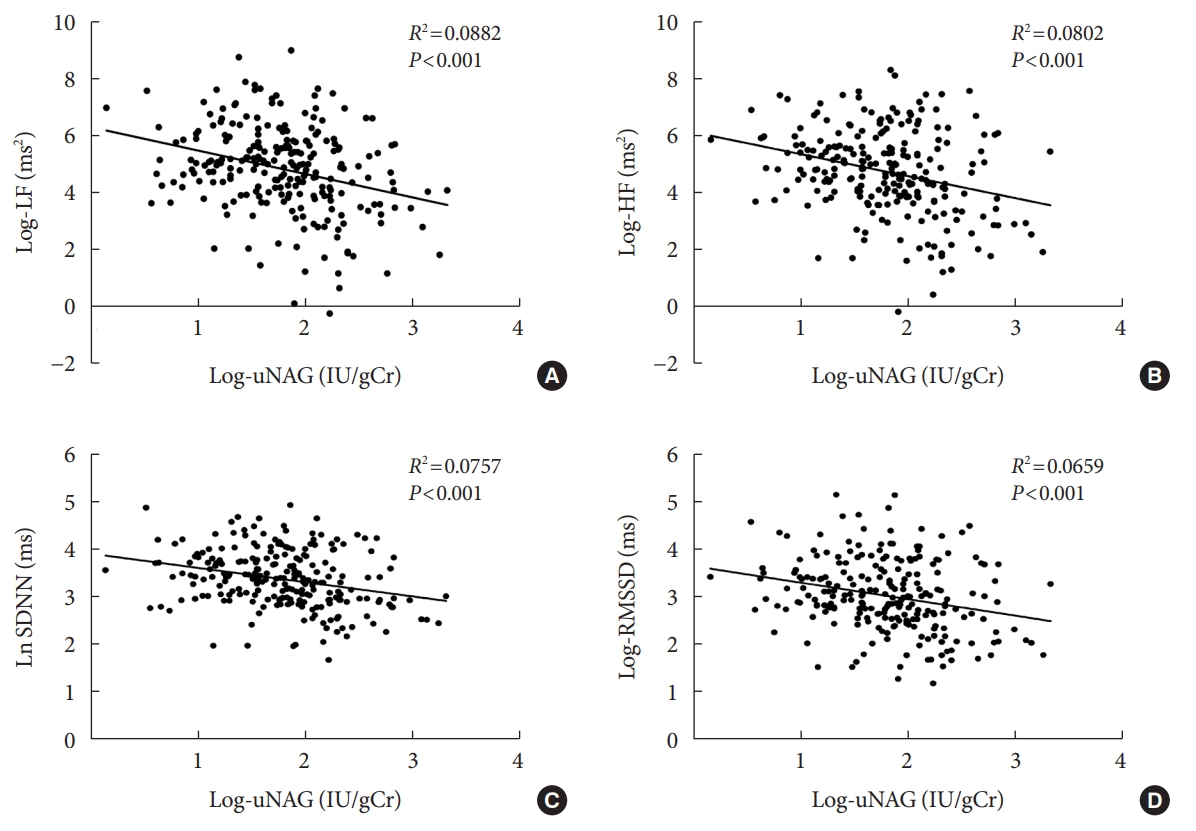
Scatter plots of Pearson’s correlation coefficients between parameters of heart rate variability and urinary N-acetyl-β-Dglucosaminidase (uNAG) in subjects with type 1 diabetes mellitus without albuminuria. (A) Correlation between log-low frequency (LF) and log-uNAG (Pearson’s correlation R2=0.0882, P<0.001). (B) Correlation between log-high frequency (HF) and log-uNAG (Pearson’s correlation R2=0.0802, P<0.001). (C) Correlation between log-standard deviation of all NN intervals (SDNN) and log-uNAG (Pearson’s correlation R2=0.0757, P<0.001). (D) Correlation between log-root mean square of successive RR interval differences (RMSSD) and log-uNAG (Pearson’s correlation R2=0.0659, P<0.001).
DISCUSSION
In this study, we found that uNAG level was independently associated with the presence and severity of CAN in T1DM patients without overt CKD and albuminuria. Furthermore, elevated uNAG level was correlated with a decrease in HRV indices which were the earliest CAN findings.
The relationship between uNAG and diabetes micro/macro-complications was not well identified until now. A previous study showed that uNAG was higher in diabetic patients with at least one microvascular complication including retinopathy, microalbuminuria, or peripheral neuropathy, than in those without any complications, suggesting the possibility of uNAG as an early marker of microvascular complications [18]. Not only microvascular complications but carotid artery atherosclerosis was also associated with uNAG level in T1DM patients [19].
Our study primally presented the significant association between uNAG level and CAN in patients with T1DM. A single previous study suggested the possibility of uNAG as an independent marker for CAN in patients with T2DM by analyzing E:I ratio and 30:15 ratio [20]. We defined CAN more specifically by using five parameters from the Ewing test which represent both sympathetic and parasympathetic function.
Frequency- and time-domain indices of HRV are commonly measured to assess autonomic function and HRV reduction represents early cardiac autonomic dysfunction and increased risk for cardiovascular morbidity [21]. LF and HF mainly reflect the activity of the parasympathetic nerve system (PNS), even in asymptomatic CAN at the early stage [22]. SDNN and RMSSD are influenced by PNS and reflect the vagally mediated changes in HRV [23]. In this study, we confirmed that an increase in uNAG level is correlated with a reduction in HRV indices (LF, HF, SDNN, and RMSSD), the earliest indicator of CAN, suggesting uNAG could be a potential marker for early autonomic dysfunction in patients with T1DM.
Previous studies suggested a few mechanisms by which CAN might play a role in the progression of CKD in T1DM patients [24]. In the early phase of autonomic dysfunction, denervation in the peripheral PNS occurs and cardiac sympathetic nervous system tone increases in compensation [25]. As time passes, sympathetic denervation develops and induces renal hemodynamic changes resulting in albuminuria and decreased renal function [26,27]. However, intrarenal vasoconstriction can cause renal tubular hypoxia and dysfunction which develop earlier than microalbuminuria in patients with diabetes, and can be detected the increased uNAG level [28]. The significant association between CAN and uNAG in T1DM patients without albuminuria, which is supported in the current study, is in line with the previous description.
The severity of CAN was associated with higher uNAG level, suggesting that increase of uNAG might be related to an advanced CAN stage. Because CAN correlates with increased cardiovascular mortality, predicting CAN is crucial to determine high CVD risk patients [29]. Overall, our results suggest that uNAG might be used as a simple and fast screening tool for detecting CAN and high CVD risk patients, even in patients without overt DN.
This study has several limitations. First, the influence of certain medications affecting autonomic function and the level of albuminuria could not be completely removed. However, to address the influence of such medications, we excluded patients on β-blockers or diuretics and adjusted for use of ACEI or ARB (as dichotomized variables) in multivariate analysis models. Second, the study had a cross-sectional design, which is cannot establish causality for the included factors. Although the tests for CAN and uNAG were conducted at different times in some patients, the time interval was limited to 3 months between tests as several previous studies had allowed the same interval between autonomic function tests and other laboratory parameters, including proteinuria or glucose level in patients with diabetes [17,30-32]. Indeed, we confirmed that no change in medication for each patient had occurred during the testing interval. Also, our results are not representative of all T1DM cases in Korea because the data were obtained from one general hospital. The results of the current study suggest the necessity for a longitudinal study in a representative population to investigate the potential role and mechanism of uNAG in the pathophysiology of CAN in T1DM patients without DN.
This study showed that a higher uNAG level was associated with CAN prevalence and severity in T1DM patients without CKD and albuminuria. An increase in uNAG level was also correlated with early CAN findings, presented by changes in HRV in T1DM patients with normoalbuminuria. The results of the current study suggest the potential role for uNAG as a marker for CAN in T1DM patients without albuminuria.
Notes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: M.S.C., J.E.J., J.H.K.
Acquisition, analysis, or interpretation of data: M.S.C., J.E.J.,S.W.P., J.H.Y., J.A., G.K., S.M.J., K.Y.H., M.K.L.
Drafting the work or revising: M.S.C., J.E.J., J.H.K.
Final approval of the manuscript: M.S.C., J.E.J., S.W.P., J.H.Y., J.A., G.K., S.M.J., K.Y.H., M.K.L., J.H.K.
FUNDING
None
ACKNOWLEDGMENTS
None




