The Hijacking of Cellular Signaling and the Diabetes Epidemic: Mechanisms of Environmental Disruption of Insulin Action and Glucose Homeostasis
Article information
Abstract
The burgeoning epidemic of metabolic disease causes significant societal and individual morbidity and threatens the stability of health care systems around the globe. Efforts to understand the factors that contribute to metabolic derangements are critical for reversing these troubling trends. While excess caloric consumption and physical inactivity superimposed on a susceptible genetic background are central drivers of this crisis, these factors alone fail to fully account for the magnitude and rapidity with which metabolic diseases have increased in prevalence worldwide. Recent epidemiological evidence implicates endocrine disrupting chemicals in the pathogenesis of metabolic diseases. These compounds represent a diverse array of chemicals to which humans are exposed via multiple routes in adulthood and during development. Furthermore, a growing ensemble of animal- and cell-based studies provides preclinical evidence supporting the hypothesis that environmental contaminants contribute to the development of metabolic diseases, including diabetes. Herein are reviewed studies linking specific endocrine disruptors to impairments in glucose homeostasis as well as tying these compounds to disturbances in insulin secretion and impairments in insulin signal transduction. While the data remains somewhat incomplete, the current body of evidence supports the hypothesis that our chemically polluted environment may play a contributing role in the current metabolic crisis.
INTRODUCTION
The global burden of diabetes and associated metabolic disorders has reached catastrophic proportions and continues to rise at an alarming rate. Currently, 382 million individuals worldwide are estimated to have diabetes, with this number projected to increase to 592 million by 2035, the vast majority of which is type 2 diabetes mellitus (T2DM) [1]. This comes at staggering and unsustainable costs to both the individual and society. In the United States alone, diabetes is estimated to cost $245 billion annually, while contributing to approximately 246,000 deaths per year [2]. Moreover, diabetes remains the leading cause of kidney failure, blindness, and nontraumatic amputations, thus contributing to significant individual morbidity and mortality. Consequently, efforts to understand the pathogenesis of this debilitating disease are critical for reversing these trends.
Consumption of a calorically dense diet coupled with physical inactivity are clear risk factors for the development of T2DM; moreover, in certain individuals a susceptible genetic background predisposes to the development of disease. These factors alone, however, fail to fully account for both the rapidity and magnitude with which diabetes rates have increased across the globe. As such attention has turned to other factors that contribute to the development of metabolic disruption, including the contribution of endocrine disrupting chemicals (EDCs). EDCs are exogenous compounds that modulate endogenous hormonal action through a variety of pathways. These compounds come from diverse chemical classes, including pesticides, industrial chemicals and waste products, plasticizers, flame retardants, phytochemicals, and pharmaceutical agents. Human exposure occurs through a variety of routes, including ingestion, inhalation, injection, dermal contact, as well as transplacental and lactational conveyance.
Classically, EDCs have been characterized by their ability to disrupt estrogen, androgen, and thyroid signaling; however, increasing evidence suggests that they have the capacity to modulate a host of signaling cascades, including those critical to maintaining energy homeostasis. Evidence supporting a role for EDCs in the pathogenesis of diabetes include correlations between the rise in synthetic chemical production and the prevalence of diabetes in the United States [3], an enlarging library of epidemiological studies [4], and basic science studies at the animal and cellular level [3,5]. Collectively, these findings provide strong support for environmental contaminants as a mediator of metabolic disruption, and taken as a whole, mandate efforts to not only understand the precise chemical threat but also to develop programs and policies to reduce that risk to the public.
ANIMAL MODELS OF DYSGLYCEMIA
An increasing body of epidemiological studies have been published linking various organic and inorganic compounds with multiple measures of dysglycemia, insulin resistance, the metabolic syndrome, and frank diabetes [4]. While these studies are highly suggestive of connections between various exposures and diabetes risk, they fall short of establishing causality. As such, animal models provide an important link in the chain of evidence connecting environmental contaminants with alterations in glucose homeostasis.
In support of epidemiological studies, several compounds have been shown to alter glucose homeostasis in animal models, including the induction of hyperglycemia and glucose intolerance. These effects have been observed with inorganic compounds such as arsenic [6] as well as organic toxins such as triphenyltin [7,8], diethylhexylphthalate (DEHP) [9], and polychlorinated biphenyls (PCBs) [10]. In addition to frank disturbances in glucose handling, additional studies have demonstrated hyperinsulinism and impairments in insulin sensitivity. This has been observed with such structurally diverse chemicals as arsenic [11], organic contaminants such as bisphenol A (BPA) [12], polybrominated diphenyl ethers (PBDE) [13], and persistent organic pollutants (POPs) [14] as well as the particulate matter found in air pollution [15].
While most studies have demonstrated perturbations in insulin action and glucose handling that would be consistent with diabetogenic effects, some studies have shown improved glucose tolerance or even hypoglycemia. These include studies examining the metabolic effects of BPA [16] and DEHP [17,18]. Whether these studies actually reflect improvements in overall energy metabolism, however, is not immediately evident as hypoglycemia can be a sign of metabolic toxicity arising from states of induced starvation. Classic studies examining the effects of dioxin have demonstrated a wasting syndrome associated with exposure [19]. Hypoglycemia and improved glucose tolerance may reflect similar states of severe metabolic disruption; however, more work is needed to determine whether this is the case or whether some environmental contaminants may exert beneficial metabolic effects. Furthermore, because BPA and DEHP show discrepant effects when all studies are considered, the ultimate metabolic phenotype may vary depending on such factors as dose and duration of exposure, route of EDC delivery, and animal model.
β-CELL DYSFUNCTION AND IMPAIRED INSULIN SECRETION
In healthy, nondiabetic individuals, circulating glucose levels are held in a narrow range, which is maintained during states of increased insulin resistance through a compensatory increase in β-cell insulin secretion; however, as insulin resistance persists β-cells lose their capacity to meet secretory demand, and the affected individual transitions from normoglycemia to impaired glucose tolerance and ultimately to frank T2DM [20]. Thus, T2DM results from both increased insulin resistance and β-cell failure to overcome the increased resistance to insulin action. As such, environmental toxicants that can either decrease insulin sensitivity or impair β-cell insulin production can contribute to the pathogenesis of T2DM.
Because of their relatively small mass, high burden of protein synthesis, reduced capacity to handle oxidative stress, and lack of detoxification mechanisms, β-cells are a likely target of diabetogenic EDCs. Furthermore, in addition to their potential role in T2DM, EDCs that module β-cell function may also play a role in type 1 diabetes mellitus (T1DM), which pathophysiologically results from β-cell destruction/dysfunction. The first synthetic chemical to demonstrate diabetogenic effects was the rodenticide, pyrinuron (Vacor) [21]. Accidental or intentional exposure was found to result in β-cell death and the development of T1DM [22], an effect similar to that observed with the nitrosourea alkylating agent streptozotocin that selectively destroys β-cells. Other compounds have also been shown to disrupt β-cell structure and function as well as to promote β-cell death, although these effects may be less β-cell-specific and more broadly toxic to other cell types as well. These β-cell disruptors include organic compounds such as 2,3,7,8-tetrachlorodibenzodioxin (TCDD) [23-26] and PCBs [27] as well as the inorganic pollutants arsenic [28-30], cadmium [31,32], and mercury [33]. Conversely, some EDCs have also been shown to augment β-cell insulin secretion, including BPA [34] and PCBs [35]. Whether these latter compounds improve glucose homeostasis in some physiological contexts is not clear, and the studies examining the effects of BPA have suggested that augmentations of insulin release may actually contribute to insulin-induced downregulation of its receptor and consequential insulin resistance [12,34].
Several disruptions in cellular signaling have been shown to occur in β-cells as a consequence of EDC exposure. BPA has been shown to augment the phosphorylation of the transcription factor cyclic adenosine monophosphate-response element binding protein [36], while exposure to triphenyltin results in an impairment in protein kinase A activity [37]. PCB treatment of β-cells increases the activity of mitogen-activated protein kinase 1 and 2 (MAPK 1 and 2) [38]. Because glucose-stimulated insulin secretion is a calcium-dependent process, disruption of this signaling pathway is a likely target for EDC-mediated β-cell disruption. PCB treatment results in an increase in intracellular calcium levels and an activation of Ca2+/calmodulin-dependent kinase II (CaMK2), a pathway that appears to be critical for PCB-induced insulin release [38]. In addition, multiple other compounds have been shown to modulate calcium signaling in β-cells, including reductions mediated by arsenic [30] and triphenyltin [37]. In contrast, TCDD exposure resulted in an increase in intracellular calcium levels in INS-1 cells, a β-cell model cell line [25,39]. Environmentally relevant doses of BPA increase intracellular Ca2+ oscillations through a decrease in the activity of the KATP channel, an effect that appears to be mediated by the estrogen receptor-β [40]. Interestingly, BPA has been shown to suppress Ca2+ oscillations induced by low glucose levels in glucagon producing α-cells as well [41]. Whether other β-cell disrupting compounds, particularly inorganic ions such as cadmium and mercury, affect calcium signaling pathways has not been resolved, but such a mechanism seems likely. Collectively, these studies suggest that disruption of β-cell function is a biologically plausible mechanism by which environmental contaminants can contribute to diabetes pathogenesis. Moreover, given the need for β-cells to increase insulin secretion to compensate for increased insulin resistance, EDCs that modulate insulin release may synergize with other diabetes risk factors that augment insulin resistance, including increased calorie consumption and obesity as well as physical inactivity.
DISRUPTION OF CELLULAR DEVELOPMENT AND FUNCTION
The Environmental Obesogen Hypothesis postulates that EDCs have the capacity to promote the development of obesity through their action on adipocyte development [42]. In support of this theory, a number of compounds have been shown to promote adipocyte development from either preadipocytes, mesenchymal stem cells, or both; these include tributyltin [43,44]; BPA [45,46]; PCB-77 and TCDD [47]; tolylfluanid, endrin, and dicyclohexylphthalate [46]; as well as triflumizole [48].
The promotion of adipocyte development, however, may not be deleterious with regard to glucose homeostasis and could actually improve energy metabolism through the generation of more numerous, metabolically active adipocytes capable of safely storing free fatty acids in the form of triglycerides in their lipid droplets. In support of this are states of lipodystrophy in which a failure of adipose development leads to marked insulin resistance and diabetes [49]. As such, compounds that have the capacity to impair adipocyte development may also play a role in the development of metabolic dysfunction. Several EDCs have been shown to antagonize adipocyte development, including endrin [50], PCB-77 [47], TCDD [47,51,52], and arsenic [53]. Moreover, arsenic has also been shown to inhibit myocyte development [54,55]. Given the importance of muscle in the disposal of glucose, particularly in the postprandial state, inhibition of either myocyte or adipocyte development could provide one plausible mechanism by which EDCs disrupt global energy homeostasis and contribute to the development of diabetes.
In addition to the expansion of adipose mass through adipocyte hyperplasia and hypertrophy, adipose tissue contributes to global energy homeostasis through the secretion of a panel of secreted factors, i.e. adipokines, that are released into the circulation. Adiponectin is an insulin-sensitizing adipokine with anti-inflammatory properties that also exerts beneficial effects on β-cell function [56]. In contrast, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), resistin, and monocyte chemoattractant protein-1 (MCP-1) are adipocyte-secreted molecules that promote inflammation both locally and systemically [57]. The overall contribution of adipose tissue to global insulin sensitivity is, in part, dictated by the pattern of secreted adipokines.
A number of environmental toxins have been shown to modulate this balance of metabolically beneficial and harmful adipokines. Several compounds have been shown to reduce expression and secretion of the insulin-sensitizing adipokine adiponectin whose levels are most tightly correlated with systemic insulin sensitivity, including cadmium [58], tributyltin [59], BPA [60,61], and particulate matter [62]. IL-10 is an anti-inflammatory adipokine that augments insulin sensitivity [63] and may protect β-cells from destruction [64], and levels have been shown to be reduced by particulate matter [65]. Expression of TNF-α is increased by exposure to TCDD [66,67], POPs [68], PCB-77 [10,47], and particulate matter [65]. PCB-77 [69] and particulate matter [65] have also been shown to increase IL-6 levels, while the dichlorodiphenyltrichloroethane (DDT) metabolite dichlorodiphenyldichloroethylene (DDE) increases resistin levels [70]. In addition to its effects on TNF-α and IL-6, PCB-77 also increases expression of MCP-1, a key mediator of macrophage infiltration into fat and the augmentation of an inflammatory fat phenotype [47]. Of particular note in these studies, are experiments demonstrating that environmentally relevant doses of the nearly ubiquitous EDC BPA have the capacity to inhibit adiponectin release and increase TNF-α and IL-6 release from human adipose tissue explants [71], suggesting that the animal- and cell-based assays may be recapitulated in exposed humans as well.
In general these findings support a transformation in the adipose secretome away from an anti-inflammatory, insulin sensitizing phenotype, and toward a proinflammatory, insulin desensitizing profile. The results are not, however, uniform across all EDCs. For example, DDE has been shown to increase adiponectin levels [70]. Whether these findings reflect differences in the model system or in the specificity of the compounds to modulate particular adipokine axes needs to be determined.
IMPAIRED CELLULAR INSULIN ACTION
Insulin mediates its action on sensitive tissues through binding to its receptor, resulting in the autotransphosphorylation of the receptor and the activation of its intrinsic tyrosine kinase activity. The activated receptor then activates insulin receptor substrate (IRS) proteins through the phosphorylation of tyrosine residues, and this in turn recruits phosphatidylinositol 3-kinase (PI3-kinase) to the cell membrane resulting in the generation of higher order phosphotidylinositides. This facilitates movement of Akt (protein kinase B) to the cell membrane where it undergoes activating phosphorylation by phosphoinositide-dependent kinase (PDK). Subsequent phosphorylation by mammalian target of rapamycin complex 2 (mTORC2) further activates Akt resulting in its downstream effects, including those on gene transcription as well as glucose uptake through the translocation of facilitative glucose transporter 4 (GLUT4) to the cell membrane. Each of the steps in this signaling cascade is a potential target for metabolic disruption via environmental contaminants.
The effects of various EDCs on insulin action in metabolic tissue have been examined (Fig. 1). The phenylsulfamide fungicide tolylfluanid was shown to impair insulin signal transduction in primary murine and human adipose tissue through a specific downregulation of IRS-1, an effect mediated both transcriptionally and posttranslationally [72]. IRS-1 and its other isoforms may represent an important point of convergence in endocrine disruption of insulin signaling as multiple distinct signal transduction cascades impinge on IRS proteins to modulate their function [73]. In general, tyrosine phosphorylation activates IRS-1 activity, while serine/threonine phosphorylation deactivates the protein and signals toward its ubiquitination and proteasomal degradation. Multiple EDCs have been shown to modulate signaling pathways that have the capacity to alter IRS-1 phosphorylation, including serine/threonine phosphorylation (particulate matter via increased c-Jun N-terminal kinase [JNK] [74] and increased protein kinase C [65]; TCDD via increased JNK and MAPK [67]; arsenic via decreased p70-S6-kinase [55]; and PCBs via increase CaMK2 and MAPK [38]). Conversely, tyrosine phosphorylation could be increased via BPA modulation of PI3-kinase [45]. In addition to tolylfluanid, several compounds have been shown to attenuate signaling through IRS-1, including TCDD [67], DEHP [75], and particulate matter [74].

Insulin signaling targets of endocrine disrupting chemicals (EDCs). Multiple studies have examined the effects of EDCs on various aspects of insulin synthesis, release, and cellular action. The molecular targets identified from these various studies are summarized. Of note, this figure synthesizes data from various model systems, including multiple different targets of insulin action (i.e., adipose tissue, liver, and muscle). The data has been combined for clarity but should not be understood to mean that the EDCs shown exert similar effects in all biological tissues or in all species. TCDD, 2,3,7,8-tetrachlorodibenzodioxin; BPA, bisphenol A; DEHP, diethylhexylphthalate; PCB, polychlorinated biphenyl; PDK, phosphoinositide-dependent kinase; mTORC2, mammalian target of rapamycin complex 2; POP, persistent organic pollutant; GLUT4, glucose transporter 4.
Other compounds have been shown to attenuate insulin-stimulated Akt phosphorylation on serine 473 (arsenic [76,77], particulate matter [65], PCB-77 [69], and tolylfluanid [72]) and/or threonine 308 (arsenic [77] and BPA [12]), while additional studies have demonstrated modulation of the insulin signaling cascade at the level of the insulin receptor (TCDD [67], BPA [78,79], and DEHP [75,80]) or downstream from Akt (e.g., arsenic [55]). Finally, TCDD [67], BPA [78], DEHP [75], and cadmium [81] have also been shown to antagonize insulin action via effects on GLUT4. These mechanisms may be able to explain the impairment of insulin-stimulated glucose uptake seen in other studies of TCDD [66], arsenic [6], and DEHP [75,80]; however, the molecular mechanisms by which other POPs attenuate glucose uptake remain to be resolved [14]. Interestingly, despite inhibition of insulin signal transduction by BPA, one study demonstrated an augmentation of insulin-stimulated glucose uptake [82], once again underscoring the fact that the ultimate effect on insulin action may be dependent on the nuances of the experimental system.
One of the central challenges in EDC research is the estimation of effects mediated by chemical mixtures that may have additive, synergistic, or antagonistic effects on any given biological readout. There are approximately 150,000 unique chemicals registered with the European Chemicals Agency [83]; and outside of the context of specific accidental, intentional, or occupational exposures, humans are exposed to mixtures of compounds with near infinite combinations of toxins and concentrations of exposure. This complexity of exposure complicates our understanding of the biological effects in any given individual. Analysis of the insulin signaling pathways does, however, provide some insights into how combinations of chemicals might modulate insulin action. For example, compounds that inhibit signaling through the pathway at different points are likely to have additive or synergistic effects that promote the development of insulin resistance and diabetes. Moreover, points of pathway convergence (e.g., IRS proteins) may provide sites of intervention at which therapeutics might be directed to treat environmentally-mediated diabetes.
PERTURBATIONS IN INTERMEDIARY METABOLISM
In addition to direct effects on cellular signaling pathways, a host of compounds have been shown to alter the expression and function of enzymes regulating intermediary metabolism. In classic studies, TCDD was shown to reduce expression of phosphoenolpyruvate carboxykinase (PEPCK), a central regulator of gluconeogenesis [84]. Similarly, dioxin-like PCBs reduced primary hepatocyte glycogen levels and impaired gluconeogenesis due to a specific downregulation of PEPCK expression that was proportional to activation of the AhR [85]. Similar reductions in PEPCK have also been observed with the flame retardant PBDE [13]. In general, these studies suggest that several compounds have the capacity to impair gluconeogenesis. In isolation, this would be predicted to promote the development of hypoglycemia, especially during periods of fasting; however, whether these changes result in compensatory changes (e.g., upregulation of counterregulatory hormones or stimulation of appetite and subsequent weight gain) that may promote insulin resistance at the organismal level is worthy of further investigation.
PATHWAYS OF METABOLIC DISRUPTION
The potential mechanisms by which EDCs modulate insulin production and action are myriad. Given the fact that approximately 150,000 chemicals are registered [83], the complexity of environment-metabolism interactions is nearly infinite. However, some common pathways may link multiple EDCs with diabetes. The current epidemic includes key features of the metabolic syndrome (e.g., abdominal obesity, insulin resistance, dyslipidemia, and hypertension) that are shared with Cushing's syndrome, the physiological consequences of glucocorticoid excess. As such, EDCs that enhance or mimic glucocorticoid action may play a special role in toxin-mediated metabolic disruption.
Glucocorticoids signal through the glucocorticoid receptor, a nuclear receptor. Binding of ligand to the receptor induces the cytosolic to nuclear translocation and dimerization of the receptor, which in conjunction with co-regulators, binds glucocorticoid response elements on DNA and thereby alters gene expression. Moreover, glucocorticoid activity is regulated by the interconversion between active and inactive states in vivo mediated by 11β-hydroxysteroid dehydrogenase-1 and -2 (11β-HSD-1/2). EDCs that modulate this signaling pathway may be of particular interest with regard to metabolic disruption (Fig. 2). The phenylsulfamide fungicide tolylfluanid was shown to mimic the murine glucocorticoid corticosterone by inducing receptor nuclear translocation, binding to glucocorticoid response elements, and altering expression of glucocorticoid-responsive genes [86]. In addition to tolylfluanid, other compounds that have been shown to mimic or modulate glucocorticoid action at the glucocorticoid receptor include the dithiocarbamate fungicide thiram [87]; methylsulfonyl-PCBs [88,89]; dicyclohexylphthalate and endrin [46]; as well as BPA [46,90]. Thiram [91] as well as cadmium and tributyltin [92] have been shown to modulate the activity of 11β-HSD-2, while BPA has been shown to increase levels of 11β-HSD-1 [93]. Binding of the glucocorticoid receptor to glucocorticoid response elements has been shown to be altered by arsenic in a concentration-dependent fashion [94,95]. Finally, developmental exposure to nicotine [96] or nonylphenol [97] has been shown to raise circulating glucocorticoid levels in the offspring of treated mothers. As with the insulin signaling cascade, mixtures of compounds that modify different aspects of the glucocorticoid signaling cascade may be predicted to act additively or synergistically to disrupt glucose homeostasis and promote a diabetic state.
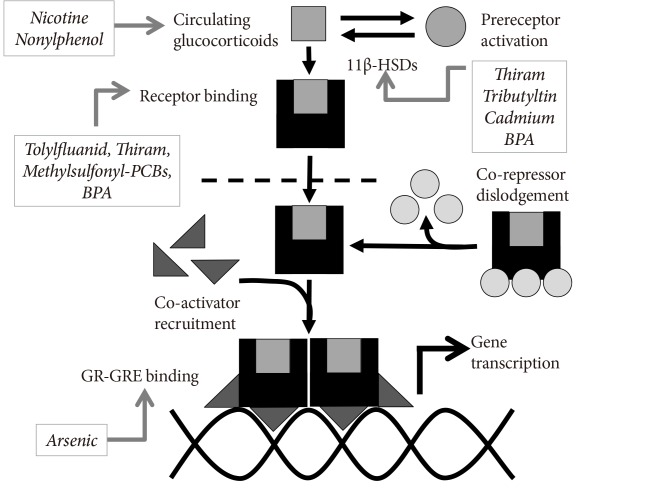
Endocrine disrupting chemical (EDC) modulation of glucocorticoid activity. The effects of multiple studies examining the effects of EDCs on the glucocorticoid signaling pathway are summarized, including effects at various concentrations and in different model systems. As such, these studies should not be interpreted to mean that each EDCs operates similarly in all tissues; however, pooling of the data suggests potential mechanisms of synergy among various EDCs that modulate activity of this signaling pathway. 11β-HSD, 11β-hydroxysteroid dehydrogenase; BPA, bisphenol A; PCB, polychlorinated biphenyl; GR-GRE, glucocorticoid receptor-glucocorticoid response element.
FUTURE DIRECTIONS
The diabetes crisis necessitates coordinated action to address its causes, treat those affected, and prevent its complications. In order to do so, a comprehensive understanding of the factors that contribute to diabetes pathogenesis is required to direct those interventions. Increasing evidence implicates exposure to environmental pollutants as a plausible contributing factor in addition to more classical risk factors such as a poor diet and physical inactivity. The data reviewed herein demonstrate the capacity of several compounds to modulate both insulin production and insulin action, with several compounds exhibiting multiple sites of action. Elimination of these compounds from use, environmental remediation of contaminated sites, or development of targeted therapies to antagonize their deleterious actions may offer opportunities to curb the burden of diabetes in exposed populations.
While the evidence discussed supports a role for EDCs in the pathogenesis of diabetes and is in line with epidemiological studies linking EDCs with alterations in glucose metabolism, there remain several important challenges to establish causality in human populations. First, the exciting studies discussed examined EDC-mediated disruptions on insulin production and action at various concentrations; however, further work will be required to ensure that these effects occur at environmentally and physiologically relevant levels. Thus, additional work is required to characterize human exposure to metabolic disruptors, with particular attention paid to EDC levels in metabolically active tissues that bioaccumulate lipophilic EDCs, e.g., adipose tissue. Furthermore, given the likely diversity of exposure across the population and the increasing recognition that EDCs exhibit nonmonotonic dose-response relationships [98], studies examining effects over the range of human exposures is prudent. Second, while most studies have examined the effects of individual metabolic disruptors, human exposure is characterized by contact with multiple compounds. Examination of the insulin and glucocorticoid signaling pathways suggest ways in which combinations of exposures might work additively or synergistically to disrupt glucose homeostasis, e.g., coordinate exposure to toxins that disrupt β-cell function and insulin signaling in target tissues or multiple chemicals disrupting insulin signaling at successive points in the signal transduction cascade. Studies specifically examining the physiological impact of simultaneous chemical insults may provide particularly useful information regarding the true threat of environmental contaminants on human metabolic health. Third, the present discussion pools together data from multiple model systems to conceptualize how various EDCs impact glucose homeostasis. It is important to recognize, however, that some metabolic effects may be dependent on the specific experimental system used, including species-specific differences as has been shown for endocrine disruption of peroxisome proliferator-activated receptor-α (PPAR-α) activity [18] and 11β-HSD-2 [92], as well as potential tissue-specific responses. Finally, the impact of society-wide dietary changes (e.g., increased consumption of a carbohydrate-rich Western diet) and reductions in physical activity are central drivers of the metabolic disease epidemic. As such, model systems that examine the coordinate insult of these lifestyle factors coupled with EDC exposure are critical for understanding the role of metabolic disruptors in diabetes pathogenesis.
The current body of evidence links various EDCs with multiple mechanisms of action in the β-cell as well as in insulin-responsive tissues. There remain, however, exciting areas of metabolism that are understudied or entirely unexamined. Of particular interest are pathways that serve as the targets of antidiabetic agents, including the sulfonylurea receptor, adenosine monophosphate (AMP)-activated protein kinase, incretins, glucagon, and the sodium-glucose cotransporter-2. While PPAR-γ has received a significant amount of attention with regard to its role in obesogen action [44], how EDC modulation of PPAR-γ activity affects glucose homeostasis warrants further investigation. In addition to current mechanisms of pharmacological action, emerging metabolic pathways may also provide novel targets for diabetogenic EDCs, including fibroblast growth factors [99] and enzymatic targets such as Per-Arnt-Sim (PAS) kinase [100] to name just a few. Likewise, our burgeoning understanding of the genetics of diabetes, particularly with regard to monogenic forms of diabetes, offer multiple novel genetic targets that could be modulated by EDC action and thereby promote the development of diabetes [101].
CONCLUSIONS
The current burden of diabetes and other metabolic diseases threatens individual health as well as the stability of healthcare systems across the globe. Reversing this epidemic will require a rapid expansion in our knowledge of the complex set of factors that promote metabolic dysfunction, including the influence of environmental contaminants. The last decade has seen a dramatic increase in the number of studies linking various pollutants with disruptions in energy handling; however, many questions remain. Improved understanding of the molecular mechanisms responsible for EDC-mediated disruptions in energy homeostasis will provide further biological support to the theory that these compounds play a significant role in the metabolic disease epidemic; offer insights into potentially additive, antagonistic, and synergistic actions among various EDCs; and potentially identify nodal points of action that might serve as novel therapeutic targets to treat environmentally-mediated diabetes. Coupled with the expanding body of epidemiological evidence linking environmental contaminants to metabolic disease, it is hoped that this knowledge will also provide a scientifically justified impetus for a transformation in public policy that seeks to limit human exposure to metabolically disruptive pollutants in order to protect future generations from this novel health threat.
ACKNOWLEDGMENTS
Due to reference restraints the author was unable to include all the important work performed in the field of endocrine disruption of metabolism. The current manuscript was meant to emphasize important aspects of endocrine disruption of insulin action and energy homeostasis. Any omissions were not meant to exclude important work contributing to the hypothesis that environmental contaminants play an important pathogenic role in the global epidemic of metabolic disease.
This work was supported by grants from the National Institutes of Health (K08-ES019176, R21-ES021354, and the Diabetes Research and Training Center [P60-DK020595]); an Early Career Development Award from the Central Society for Clinical and Translational Research; and a Junior Investigator Award from the Brinson Foundation.
Notes
No potential conflict of interest relevant to this article was reported.