- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 37(4); 2013 > Article
-
Sulwon Lecture 2012Others Intestinal and Hepatic Niemann-Pick C1-Like 1
- Sung-Woo Park
-
Diabetes & Metabolism Journal 2013;37(4):240-248.
DOI: https://doi.org/10.4093/dmj.2013.37.4.240
Published online: August 14, 2013
Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding author: Sung-Woo Park. Division of Endocrinology and Metabolism, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 110-746, Korea. sungwoo0913.park@samsung.com
Copyright © 2013 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- Polytopic transmembrane protein, Niemann-Pick C1-Like 1 (NPC1L1) is localized at the apical membrane of enterocytes and the canalicular membrane of hepatocytes. It mediates intestinal cholesterol absorption and prevents extensive loss of cholesterol by transporting biliary cholesterol into hepatocytes. NPC1L1 is a molecular target of ezetimibe, an agent for hypercholesterolemia. Recently, NPC1L1 inhibition has been shown to prevent metabolic disorders such as fatty liver disease, obesity, diabetes, and atherosclerosis. In this review, the identification and characterization of NPC1L1, NPC1L1-dependent cholesterol transport, the relationship with pathogenesis of metabolic disease and its newly introduced function for virus entry are discussed.
- Cholesterol is an essential structural component for cell membrane integrity, permeability and fluidity. In mammals, it also serves the precursor for the synthesis of bile acids, vitamin D and steroid hormones. Furthermore, cholesterol plays as a critical signaling molecule. Therefore, dysfunctional regulation of cholesterol and its related molecules affect human health. Elevated blood cholesterol level is a major risk factor for atherosclerotic coronary heart disease, leading cause of death in developed countries [1,2].
- Cholesterol homeostasis in the body is tightly balanced by de novo biosynthesis, intestinal absorption, and biliary and fecal excretion. Compared to well-defined process of cholesterol biosynthesis, the mechanisms for intestinal cholesterol absorption and excretion were poorly defined [3,4]. In 2004, Altman et al. [5] find that Niemann-Pick C1-Like 1 (NPC1L1) plays an important role in intestinal cholesterol absorption. Ezetimibe, first pharmacological inhibitor of cholesterol absorption has been shown to target NPC1L1 [5,6]. Recently, NPC1L1 has been implicated in hepatitis C virus (HCV) entry [7]. From clinical trials and animal studies, there are accumulated data showing that NPC1L1 and NPC1L1 associated cholesterol metabolism influence metabolic syndrome such as nonalcoholic fatty liver disease (NAFLD), diabetes, obesity, and atherosclerotic coronary heart disease. Here, I discuss NPC1L1, NPC1L1-dependent intestinal and hepatic cholesterol uptake and its associated metabolic disease.
INTRODUCTION
- NPC1L1 was first identified as a homolog of Niemann-Pick C1 (NPC1), a gene which defection causes inherited lipid storage disorder Niemann-Pick disease type C1 [8]. Like its homologue, NPC1L1 is a polytopic transmembrane protein consisting of 13 transmembrane domains, N-terminal domain (NTD) and N-linked glycosylation sites [9]. Five of 13 membrane domains consist of sterol sensing domain (SSD). Conserved SSD is also found in several other transmembrane proteins, all of which are involved in cholesterol metabolism. These proteins include NPC1, 3-hydroxy-3-methylglutaryl CoA reductase (HMG-CoA reductase), the rate-limiting enzyme in cholesterol biosynthesis, sterol regulatory element binding protein (SREBP)-cleavage activating protein, a protein that regulates transport and proteolytical activation of SREBPs which controls sterol and other lipid biosynthesis, and patched, 12-pass transmembrane protein receptor for cholesterol linked signaling peptide hedgehog [10,11]. Sterol binding pocket is localized in crystal structure of NTD of NPC1L1. NTD of NPC1L1 directly binds to cholesterol [12], which leads to confirmation change and cholesterol entry [13]. Extensive N-glycosylation sites consist of three extracellular/luminal loops of NPC1L1. As posttranslational modification, N-glycosylation affects maturation and function of NPC1L1 by folding, secretion and endoplasmic reticulum (ER) retention [14].
- It has been demonstrated in several studies that NPC1L1-dependent cholesterol transport may be regulated by clathrin-mediated endocytosis [15-17]. At steady state, NPC1L1 proteins are mainly found in endocytic recycling compartment (ERC). When cholesterol is depleted, NPC1L1 proteins move from ERC to plasma membrane (PM) [15]. On cholesterol repletion, cholesterol is sensed by PM transported NPC1L1 [15] and incorporated into PM by the formation of NPC1L1-flotillin-cholesterol membrane microdomains [16]. Subsequently, this formation is internalized by clathrin/AP2 mediated endocytosis. The vesicles are then moved to ERC [16]. Excessive cholesterol could be transported into cells in this NPC1L1 dependent manner.
- NPC1L1 is widely expressed in many human tissues but highly expressed in the liver and small intestine [5,18,19]. According to species, distribution and pattern of NPC1L1 expression are different. Mouse and rat NPC1L1 are more abundant in small intestine than liver [5,19]. The reasons for different patterns of NPC1L1 expression among species remain elusive.
DISCOVERY AND CHARACTERIZATION
- Cholesterol transporter, NPC1L1 is reduced by cholesterol feeding and increased by NPC1L1 inhibitor, ezetimibe in animal models [20,21]. Several transcription factors involved in cholesterol metabolism are suggested as regulatory factor for NPC1L1 expression. SREBP2, a transcription factor for cholesterol biosynthesis shows positive relationship with mRNA expression of NPC1L1 in human hepatoma HepG2 cells and intestinal Caco2 cells [22-24]. SREBP2 together with hepatocyte nuclear factor 4α synergistically activates human NPC1L1 promoter [24]. In vivo and in vitro studies demonstrate the regulatory effects of nuclear receptors including liver X receptor (LXR), retinoid X receptor, and peroxisome proliferator-activated receptors (PPARs) on NPC1L1 transcription. PPARα agonist, fenofibrate administered mice remarkably decrease intestinal cholesterol absorption accompanied with the reduction in NPC1L1 mRNA expression [25]. PPARδ agonist also decreases mRNA level of NPC1L1 in small intestine and increases fecal sterol excretion. A single dose of LXR agonist mice and treatment of LXR activators, GW3965 and T0901317 in the human enterocyte cell line reduce mRNA expression of NPC1L1 [26]. However, the effects of nuclear receptors on NPC1L1 transcription are discrepant according to tissue and species.
TRANSCRIPTIONAL REGULATION OF NPC1L1
- NPC1L1 is abundantly expressed in the jejunum and proximal ileum and specifically located to the brush border membrane of intestinal enterocytes where cholesterol absorption takes place [5,27]. NPC1L1 deficient mice remarkably decrease intestinal cholesterol absorption by 70% [5] and attenuate diet induced hypercholesterolemia [28]. However, the genetic ablation of NPC1L1 doesn't change triglyceride uptake by intestine [5].
- As mentioned above, NPC1L1 is tightly regulated by intracellular itinerary. Cholesterol is transported into cells with NPC1L1 through clathrin/AP2-mediated endocytosis [15,16]. Delivered cholesterol to ER is packaged into cholesterol ester by acyl coenzyme A: cholesterol acyltransferase 2 (ACAT2), which in turn incorporated into chylomicrons for lymphatic secretion. Several studies demonstrate that NPC1L1 has substrate specificity on sterol absorption. In NPC1L1 deficient mice [5,19,20], ezetimibe treated mice [29] and patients with cholesterolemia or sitosterolemia [30,31], sterols are significantly decreased. In cell culture system, phytosterols show the decrease of NPC1L1 internalization and uptake compared to cholesterol [16,17]. This illustrates that phytosterol uptake is NPC1L1 dependent but NPC1L1 has high affinity with cholesterol compared to phytosterols.
INTESTINAL NPC1L1
- Hepatic NPC1L1 expression differs among mammalian species. Human and rat NPC1L1 are abundantly expressed in the liver, but hepatic NPC1L1 expression is undetectable in mouse liver [5,19,32]. Still the reasons for different tissue patterns of NPC1L1 expression and abundant hepatic NPC1L1 expression in humans are not fully determined.
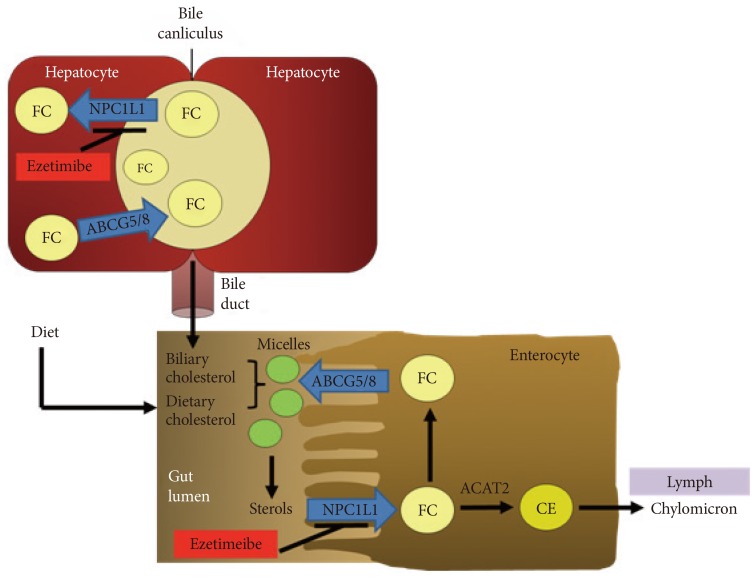
- Liver is the main site for cholesterol homeostasis in the body. In the liver, cholesterol de novo biosynthesis by HMG-CoA reductase, taking up lipoprotein cholesterol via low density lipoprotein receptors (LDLr) for regulating blood cholesterol levels, lipoprotein release, storage by esterification, degradation, and conversion into bile acid for cholesterol disposal and regulation of intestinal lipid absorption occur [3,4]. Compared to intestinal NPC1L1, still the functions of hepatic NPC1L1 in human remain largely unknown. To investigate the function of hepatic NPC1L1, mice expressing human NPC1L1 in the liver were generated. In these transgenic mice, human NPC1L1 localizes to the canalicular membrane of the hepatocytes as found in humans [15,32]. There are significant reduction of cholesterol in bile acid and elevation of liver cholesterol without changes of hepatic expression in canalicular cholesterol efflux transporter, ATP-binding cassette transporter G5 and G8 (ABCG5/8) [32]. In addition, ezetimibe treatment reduces biliary cholesterol in patients with gallstones and in mice [33,34]. These observations suggest that hepatic NPC1L1 may counterbalance ABCG5/8-meidated biliary secretion of cholesterol, thereby prevent extensive loss of cholesterol by transporting biliary cholesterol to hepatocytes, as illustrated in Fig. 1.
HEPATIC NPC1L1
- Ezetimibe was discovered as an active and potent metabolite of Schering-Plough's SCH48461 substance and introduced as the first of a new class of selective cholesterol absorption inhibitor. Ezetimibe is absorbed and metabolized by intestine and liver to its glucuronide [35]. Both ezetimibe and its metabolite inhibit intestinal cholesterol absorption by preventing cholesterol transport from intestinal lumen to small intestinal enterocytes [36], resulting in lowering plasma cholesterol by 15% to 20% [6]. In the liver, ezetimibe reduces cholesterol delivery, which consequently increases cholesterol clearance from the circulation to hepatocytes via LDLr upregulation [21,32]. Thus, clinically proven ezetmibe has been used to treat hypercholesterolemia.
- For a long time, the molecular target of ezetimibe has not been identified. In animal studies, NPC1L1 has a critical role in absorption of cholesterol and phytosterol, all of which is inhibited by ezetimibe [5,6]. Ezetimibe has no inhibitory effect on cholesterol transport in NPC1L1 knockout mice [5]. These findings suggest that NPC1L1 and ezetimibe act in the same pathway. In radioligand binding assay, ezetimibe binds to brush border membranes from various species and human NPC1L1 expressing cells but not those of NPC1L1 knockout mice [6]. Ezetimibe prevents the specific binding of NPC1L1 to clathrin/AP2 coated vesicles, internalization and enocytosis of cholesterol and thereby inhibits NPC1L1 mediated cholesterol uptake [16,17,37]. Taken together, NPC1L1 is a direct molecular target of ezetimibe.
NPC1L1 INHIBITOR: EZETIMIBE
- There is a strong positive correlation between high level of blood cholesterol and the incidence of atherosclerosis. Ezetimibe treatment and NPC1L1 deletion significantly reduce cholesterol absorption, decrease plasma cholesterol, and thereby inhibit the development of atherosclerosis in apolipoprotein E knockout mice, a model of atherosclerosis [28,38]. In subjects with hypercholesterolemia, ezetimibe monotheraphy or ezetimibe-statin coadministration significantly decreases blood LDL-cholesterol (LDL-C) [39,40]. However, a 2-year clinical trial, Ezetimibe and Simvastatin in Hypercholesterolemia Enhances Atherosclerosis Regression (ENHANCE) shows no distinct change of carotid intima-media thickness between coadministration of ezetimibe and simvastatin and simvastatin alone in 720 heterozygous familial hypercholesterolemic patients [41]. This ENHANCE study has limitations such as subject number, subject selection, primary endpoints, treatment duration, and methodology. A long-term large clinical trial, Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) considers the problems of ENHANCE study and has been ongoing to demonstrate the cholesterol lowering effects of ezetimibe and its health outcomes. Although IMPROVE-IT is not completed, recent reported study of heart and renal protection demonstrates that the combination of simvastatin and ezetimibe is safe and effective to decrease cholesterol in 9,270 chronic kidney disease (CKD) patients. Combination medication also helps to reduce atherosclerotic events [42]. However, simvastatin monotheraphy shows the same extent of lowering cholesterol and major cardiovascular event, compared to simvastatin plus ezetimibe in CKD patients. Ongoing IMPROVE-IT trial will answer the contribution of ezetimibe to the reduction of cholesterol and the improvement of health outcomes.
- Besides the effects of ezetimibe treatment or NPC1L1 deficiency on hypercholesterolemia, there are accumulated data showing beneficial outcomes of ezetimibe on health. Ezetimibe improves hepatic steatosis in wild type mice fed a high fat, high cholesterol diet [43], and a high fat diet [44,45]. And it also shows similar effects on leptin receptor deficient db/db mice [46] and Zucker obese fatty rats [47,48] resulting in the improvement of fatty liver and metabolic syndrome. In a rodent model of NAFLD induced by a methionine choline-deficient diet, ezetimibe administration ameliorates NAFLD, accompanied with the decrease of hepatic triglyceride [49]. Similar to ezetimibe treatment, NPC1L1 ablation prevents the development of fatty liver in animals [50,51]. In humans, ezetimibe shows favorable effects on fatty liver in obese subjects on a weight loss diet [52], patients with nonalcoholic steatohepatitis and dyslipidemia [53] and nonobese participants with NAFLD [54]. Given that strong correlation between NAFLD and insulin sensitivity, ezetimibe improves insulin sensitivity in animals with NAFLD [48-50]. High fat diet induced glucose intolerance and insulin resistance are also ameliorated in NPC1L1 knockout mice [50,51]. This demonstrates that ezetimibe or NPC1L1 deletion has beneficial impacts on NAFLD by insulin sensitivity.
- One of proposed mechanisms is that ezetimibe treatment or NPC1L1 deletion prevents NAFLD by reducing reactive oxygen species, activating C-Jun-N-terminal kinase, and deceasing ER stress [47,48]. All of these alterations explain how NPC1L1 ablation and ezetimibe act cholesterol uptake in the liver, rather than improve steatosis. Jia et al. [51] demonstrate inhibitory effects of ezetimibe on lipogenesis in the liver. Genetic dysfunction of NPC1L1 or ezetimibe treatment reduces intestinal fat absorption and decreases cholesterol dependent LXR activation. Inactivation of LXR subsequently suppresses hepatic lipogenesis by modulating gene expression of SREBP-1c thereby prevents high fat induced hepatic steatosis. Focusing on favorable regulation of ezetimibe in glycemic control, our team investigated the mechanism how ezetimibe administration improves glycemic control. Chronic ezetimibe administration in male hyperphagic Otsuka Long-Evans Tokushima Fatty (OLETF) rats improves hyperglycemia compared to OLETF rats with vehicle. We also find that ezetimibe increases pancreatic size and beta cell mass. In addition, 20-week chronic ezetimibe administration increases serum glucagon-like peptide-1 (GLP-1) and decreases serum dipeptidyl peptidase-4 (DPP4) activity [55]. GLP-1 is released from β-cells in response to meal and affects metabolic syndrome by increasing insulin secretion and reducing glucagon production. DPP4 plays a critical role in glucose homeostasis by its action to GLP-1 degradation [56]. From these data, we suggest that NPC1L1 inhibition by ezetimibe may improve glucose homeostasis by mediating GLP-1.
NPC1L1 AND DISEASE
- As HCV leads to liver disease, it is very critical to understand the mechanisms of viral entry, virus-host cell interactions and escape for drug development. In 2011, Sainz et al. [7] find that cholesterol transporter, NPC1L1 is involved in HCV entry. Silencing antibody specifically against NPC1L1 suppresses HCV infection by regulating replication or secretion. They also discover that NPC1L1 inhibitor, ezetimibe blocks HCV uptake into cells in a cholesterol dependent manner and delays the establishment of HCV infection in mice pretreated for 2 weeks before infection, confirming the ability of this drug to inhibit HCV infection in vivo. Despite new interesting finding that NPC1L1 affects HCV entry, clinical practice studies are needed to evaluate efficacy of NPC1L1 as antiviral target.
NPC1L1 MEDIATED HCV ENTRY
- Ezetimibe, NPC1L1 inhibitor, effectively reduces cholesterol absorption but it alone has not shown enough improvement in atherosclerotic or vascular events in patients with cardiovascular disease. Therefore, ezetimibe has been used with other cholesterol lowering agents such as statins. The inhibitory effects of dual combination on cholesterol is more effective, even small dose combination, than monotherapy [57]. U.S. Food and Drug Administration has approved a new fixed dose combination agent including atorvastatin and ezetimibe for the treatment of hyperlipidemia next to simvastatin/ezetimibe combination.
- Table 1 summarizes the development of other NPC1L1 inhibitors. Spiroimidazolidinone was identified by similarity-based virtual screening and has low binding affinities of brush border membranes. Spiroimidazolidinone significantly inhibits mouse cholesterol absorption by 67% [58]. MD-0727 is introduced as an analog of ezetimibe and effectively decreases LDL-C in clinical trials [59]. It might be a next novel cholesterol absorption inhibitor. Other compounds such as AZD4121, canosimibe, KT6-971, and MK-6213 were developed and have been discontinued due to several issues including target product profile, bioavailability and efficacy [60].
DEVELOPMENT OF OTHER CHOLESTEROL ABSORPTION INHIBITORS
- As a homolog of NPC1 protein, NPC1L1 was first introduced. NPC1L1 contains multiple conserved transmembrane domains, involved in cholesterol metabolism. NPC1L1 is expressed in not only intestinal brush border but also hepatocanalicular membrane. It mediates intestinal cholesterol absorption and hepatic biliary cholesterol secretion. NPC1L1 is a molecular target of ezetimbe, the first hypolipidomic agent. Ezetimibe inhibits NPC1L1 dependent cholesterol uptake by preventing its intracellular trafficking and clathrin mediated endocytosis. Besides NPC1L1 actions to cholesterol transport, NPC1L1 is recently found to be responsible for HCV virus infection. Still, the molecular mechanisms for NPC1L1 dependent cholesterol uptake in small intestine and liver in vivo and sequential cholesterol transport to ER remain elusive. It is also undetermined how NPC1L1 proteins are responsible for the incidence of metabolic disorders such as NAFLD, obesity diabetes, and virus entry. Future studies are required to investigate molecular mechanisms of NPC1L1.
CONCLUSIONS
-
Acknowledgements
- I thank the following individuals at the Diabetes Mellitus Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine: Drs. Se Eun Park, Eun Jung Rhee, Won Young Lee, Ki Won Oh, Cheol-Young Park, and all staff for their contribution, assistance, discussion and support and also Dr. Eugene Chang and all researchers at Diabetes Research Institute, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine for scientific discussion and technical support.
ACKNOWLEDGMENTS
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics: 2013 update: a report from the American Heart Association. Circulation 2013;127:e6-e245. PubMed
- 2. Kreisberg RA, Oberman A. Clinical review 141: lipids and atherosclerosis: lessons learned from randomized controlled trials of lipid lowering and other relevant studies. J Clin Endocrinol Metab 2002;87:423-437. PubMed
- 3. Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol 2006;22:129-157. ArticlePubMed
- 4. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature 1990;343:425-430. ArticlePubMedPDF
- 5. Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004;303:1201-1204. ArticlePubMed
- 6. Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O'Neill KA, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc Natl Acad Sci U S A 2005;102:8132-8137. ArticlePubMedPMC
- 7. Sainz B Jr, Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Marsh KA, Yu X, Chayama K, Alrefai WA, Uprichard SL. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med 2012;18:281-285. ArticlePubMedPMCPDF
- 8. Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O'Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 1997;277:228-231. ArticlePubMed
- 9. Wang J, Chu BB, Ge L, Li BL, Yan Y, Song BL. Membrane topology of human NPC1L1, a key protein in enterohepatic cholesterol absorption. J Lipid Res 2009;50:1653-1662. ArticlePubMedPMC
- 10. Davies JP, Ioannou YA. Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J Biol Chem 2000;275:24367-24374. ArticlePubMed
- 11. Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet 2003;33:508-513. ArticlePubMedPDF
- 12. Infante RE, Radhakrishnan A, Abi-Mosleh L, Kinch LN, Wang ML, Grishin NV, Goldstein JL, Brown MS. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem 2008;283:1064-1075. PubMed
- 13. Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 2009;137:1213-1224. ArticlePubMedPMC
- 14. Wang LJ, Wang J, Li N, Ge L, Li BL, Song BL. Molecular characterization of the NPC1L1 variants identified from cholesterol low absorbers. J Biol Chem 2011;286:7397-7408. ArticlePubMed
- 15. Yu L, Bharadwaj S, Brown JM, Ma Y, Du W, Davis MA, Michaely P, Liu P, Willingham MC, Rudel LL. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. J Biol Chem 2006;281:6616-6624. ArticlePubMed
- 16. Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL, Song BL. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab 2008;7:508-519. ArticlePubMed
- 17. Brown JM, Rudel LL, Yu L. NPC1L1 (Niemann-Pick C1-like 1) mediates sterol-specific unidirectional transport of non-esterified cholesterol in McArdle-RH7777 hepatoma cells. Biochem J 2007;406:273-283. ArticlePubMedPMCPDF
- 18. Davies JP, Levy B, Ioannou YA. Evidence for a Niemann-Pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics 2000;65:137-145. ArticlePubMed
- 19. Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem 2005;280:12710-12720. ArticlePubMed
- 20. Davis HR Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem 2004;279:33586-33592. ArticlePubMed
- 21. Telford DE, Sutherland BG, Edwards JY, Andrews JD, Barrett PH, Huff MW. The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. J Lipid Res 2007;48:699-708. ArticlePubMed
- 22. Alrefai WA, Annaba F, Sarwar Z, Dwivedi A, Saksena S, Singla A, Dudeja PK, Gill RK. Modulation of human Niemann-Pick C1-like 1 gene expression by sterol: role of sterol regulatory element binding protein 2. Am J Physiol Gastrointest Liver Physiol 2007;292:G369-G376. ArticlePubMed
- 23. Pramfalk C, Jiang ZY, Cai Q, Hu H, Zhang SD, Han TQ, Eriksson M, Parini P. HNF1alpha and SREBP2 are important regulators of NPC1L1 in human liver. J Lipid Res 2010;51:1354-1362. PubMedPMC
- 24. Iwayanagi Y, Takada T, Suzuki H. HNF4alpha is a crucial modulator of the cholesterol-dependent regulation of NPC1L1. Pharm Res 2008;25:1134-1141. ArticlePubMedPDF
- 25. Valasek MA, Clarke SL, Repa JJ. Fenofibrate reduces intestinal cholesterol absorption via PPARalpha-dependent modulation of NPC1L1 expression in mouse. J Lipid Res 2007;48:2725-2735. PubMed
- 26. Duval C, Touche V, Tailleux A, Fruchart JC, Fievet C, Clavey V, Staels B, Lestavel S. Niemann-Pick C1 like 1 gene expression is down-regulated by LXR activators in the intestine. Biochem Biophys Res Commun 2006;340:1259-1263. ArticlePubMed
- 27. Sane AT, Sinnett D, Delvin E, Bendayan M, Marcil V, Menard D, Beaulieu JF, Levy E. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J Lipid Res 2006;47:2112-2120. ArticlePubMed
- 28. Davis HR Jr, Hoos LM, Tetzloff G, Maguire M, Zhu LJ, Graziano MP, Altmann SW. Deficiency of Niemann-Pick C1 Like 1 prevents atherosclerosis in ApoE-/- mice. Arterioscler Thromb Vasc Biol 2007;27:841-849. ArticlePubMed
- 29. Yu L, von Bergmann K, Lutjohann D, Hobbs HH, Cohen JC. Ezetimibe normalizes metabolic defects in mice lacking ABCG5 and ABCG8. J Lipid Res 2005;46:1739-1744. ArticlePubMed
- 30. Salen G, von Bergmann K, Lutjohann D, Kwiterovich P, Kane J, Patel SB, Musliner T, Stein P, Musser B. Multicenter Sitosterolemia Study Group. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation 2004;109:966-971. ArticlePubMedPMC
- 31. Salen G, Starc T, Sisk CM, Patel SB. Intestinal cholesterol absorption inhibitor ezetimibe added to cholestyramine for sitosterolemia and xanthomatosis. Gastroenterology 2006;130:1853-1857. ArticlePubMed
- 32. Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest 2007;117:1968-1978. ArticlePubMedPMC
- 33. Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology 2008;134:2101-2110. ArticlePubMed
- 34. Zuniga S, Molina H, Azocar L, Amigo L, Nervi F, Pimentel F, Jarufe N, Arrese M, Lammert F, Miquel JF. Ezetimibe prevents cholesterol gallstone formation in mice. Liver Int 2008;28:935-947. ArticlePubMed
- 35. Kosoglou T, Statkevich P, Johnson-Levonas AO, Paolini JF, Bergman AJ, Alton KB. Ezetimibe: a review of its metabolism, pharmacokinetics and drug interactions. Clin Pharmacokinet 2005;44:467-494. PubMed
- 36. Toth PP, Davidson MH. Cholesterol absorption blockade with ezetimibe. Curr Drug Targets Cardiovasc Haematol Disord 2005;5:455-462. ArticlePubMed
- 37. Petersen NH, Faergeman NJ, Yu L, Wustner D. Kinetic imaging of NPC1L1 and sterol trafficking between plasma membrane and recycling endosomes in hepatoma cells. J Lipid Res 2008;49:2023-2037. ArticlePubMed
- 38. Davis HR Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler Thromb Vasc Biol 2001;21:2032-2038. ArticlePubMed
- 39. Assmann G, Kannenberg F, Ramey DR, Musliner TA, Gutkin SW, Veltri EP. Effects of ezetimibe, simvastatin, atorvastatin, and ezetimibe-statin therapies on non-cholesterol sterols in patients with primary hypercholesterolemia. Curr Med Res Opin 2008;24:249-259. ArticlePubMed
- 40. Davidson MH, Ballantyne CM, Kerzner B, Melani L, Sager PT, Lipka L, Strony J, Suresh R, Veltri E. Ezetimibe Study Group. Efficacy and safety of ezetimibe coadministered with statins: randomised, placebo-controlled, blinded experience in 2382 patients with primary hypercholesterolemia. Int J Clin Pract 2004;58:746-755. ArticlePubMed
- 41. Kastelein JJ, Akdim F, Stroes ES, Zwinderman AH, Bots ML, Stalenhoef AF, Visseren FL, Sijbrands EJ, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E. ENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med 2008;358:1431-1443. ArticlePubMed
- 42. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R. SHARP Investigators. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 2011;377:2181-2192. ArticlePubMedPMC
- 43. Zheng S, Hoos L, Cook J, Tetzloff G, Davis H Jr, van Heek M, Hwa JJ. Ezetimibe improves high fat and cholesterol diet-induced non-alcoholic fatty liver disease in mice. Eur J Pharmacol 2008;584:118-124. ArticlePubMed
- 44. Nozaki Y, Fujita K, Yoneda M, Wada K, Shinohara Y, Takahashi H, Kirikoshi H, Inamori M, Kubota K, Saito S, Mizoue T, Masaki N, Nagashima Y, Terauchi Y, Nakajima A. Long-term combination therapy of ezetimibe and acarbose for non-alcoholic fatty liver disease. J Hepatol 2009;51:548-556. ArticlePubMed
- 45. Muraoka T, Aoki K, Iwasaki T, Shinoda K, Nakamura A, Aburatani H, Mori S, Tokuyama K, Kubota N, Kadowaki T, Terauchi Y. Ezetimibe decreases SREBP-1c expression in liver and reverses hepatic insulin resistance in mice fed a high-fat diet. Metabolism 2011;60:617-628. ArticlePubMed
- 46. Fukuda M, Nakamura T, Kataoka K, Nako H, Tokutomi Y, Dong YF, Yasuda O, Ogawa H, Kim-Mitsuyama S. Ezetimibe ameliorates cardiovascular complications and hepatic steatosis in obese and type 2 diabetic db/db mice. J Pharmacol Exp Ther 2010;335:70-75. ArticlePubMed
- 47. Nomura M, Ishii H, Kawakami A, Yoshida M. Inhibition of hepatic Niemann-Pick C1-like 1 improves hepatic insulin resistance. Am J Physiol Endocrinol Metab 2009;297:E1030-E1038. ArticlePubMed
- 48. Deushi M, Nomura M, Kawakami A, Haraguchi M, Ito M, Okazaki M, Ishii H, Yoshida M. Ezetimibe improves liver steatosis and insulin resistance in obese rat model of metabolic syndrome. FEBS Lett 2007;581:5664-5670. ArticlePubMed
- 49. Assy N, Grozovski M, Bersudsky I, Szvalb S, Hussein O. Effect of insulin-sensitizing agents in combination with ezetimibe, and valsartan in rats with non-alcoholic fatty liver disease. World J Gastroenterol 2006;12:4369-4376. ArticlePubMedPMC
- 50. Labonte ED, Camarota LM, Rojas JC, Jandacek RJ, Gilham DE, Davies JP, Ioannou YA, Tso P, Hui DY, Howles PN. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1-/- mice. Am J Physiol Gastrointest Liver Physiol 2008;295:G776-G783. ArticlePubMedPMC
- 51. Jia L, Ma Y, Rong S, Betters JL, Xie P, Chung S, Wang N, Tang W, Yu L. Niemann-Pick C1-Like 1 deletion in mice prevents high-fat diet-induced fatty liver by reducing lipogenesis. J Lipid Res 2010;51:3135-3144. ArticlePubMedPMC
- 52. Chan DC, Watts GF, Gan SK, Ooi EM, Barrett PH. Effect of ezetimibe on hepatic fat, inflammatory markers, and apolipoprotein B-100 kinetics in insulin-resistant obese subjects on a weight loss diet. Diabetes Care 2010;33:1134-1139. ArticlePubMedPMCPDF
- 53. Yoneda M, Fujita K, Nozaki Y, Endo H, Takahashi H, Hosono K, Suzuki K, Mawatari H, Kirikoshi H, Inamori M, Saito S, Iwasaki T, Terauchi Y, Kubota K, Maeyama S, Nakajima A. Efficacy of ezetimibe for the treatment of non-alcoholic steatohepatitis: an open-label, pilot study. Hepatol Res 2010;40:613-621. ArticlePubMed
- 54. Enjoji M, Machida K, Kohjima M, Kato M, Kotoh K, Matsunaga K, Nakashima M, Nakamuta M. NPC1L1 inhibitor ezetimibe is a reliable therapeutic agent for non-obese patients with nonalcoholic fatty liver disease. Lipids Health Dis 2010;9:29ArticlePubMedPMC
- 55. Yang SJ, Choi JM, Kim L, Kim BJ, Sohn JH, Kim WJ, Park SE, Rhee EJ, Lee WY, Oh KW, Park SW, Kim SW, Park CY. Chronic administration of ezetimibe increases active glucagon-like peptide-1 and improves glycemic control and pancreatic beta cell mass in a rat model of type 2 diabetes. Biochem Biophys Res Commun 2011;407:153-157. ArticlePubMed
- 56. Rask E, Olsson T, Soderberg S, Johnson O, Seckl J, Holst JJ, Ahren B. Northern Sweden Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA). Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care 2001;24:1640-1645. ArticlePubMedPDF
- 57. Prospective Studies Collaboration. Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, Qizilbash N, Peto R, Collins R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007;370:1829-1839. ArticlePubMed
- 58. Howell KL, DeVita RJ, Garcia-Calvo M, Meurer RD, Lisnock J, Bull HG, McMasters DR, McCann ME, Mills SG. Spiroimidazolidinone NPC1L1 inhibitors. Part 2: structure-activity studies and in vivo efficacy. Bioorg Med Chem Lett 2010;20:6929-6932. ArticlePubMed
- 59. Bayes M, Rabasseda X, Prous JR. Gateways to clinical trials. Methods Find Exp Clin Pharmacol 2007;29:53-71. PubMed
- 60. Davis HR Jr, Tershakovec AM, Tomassini JE, Musliner T. Intestinal sterol transporters and cholesterol absorption inhibition. Curr Opin Lipidol 2011;22:467-478. ArticlePubMed
REFERENCES
Fig. 1The role of Niemann-Pick C1-Like 1 (NPC1L1) in cholesterol transport in the intestine and liver. NPC1L1 is located at intestinal brush border and hepatocanicular membrane. In the lumen of small intestine, biliary and dietary cholesterol are mixed and solubilized to form micelles. NPC1L1 transports sterols from brush border membrane to intracellular compartments. Most free cholesterol (FC) is esterified by acyl coenzyme A: cholesterol acyltransferase 2 (ACAT2) to form cholesterol esters (CE), packed into chylomicrons and then secreted into mesenteric lymph. Liver NPC1L1 facilitates the transfer of secreted biliary cholesterol back into hepatocytes and prevents cholesterol loss. The cholesterol efflux transporter, ATP-binding cassette transporter G5 and G8 (ABCG5/ABCG8) also localizes at apical membrane of hepatocytes. Ezetimibe inhibits NPC1L1-meidated cholesterol transport in intestine and liver.
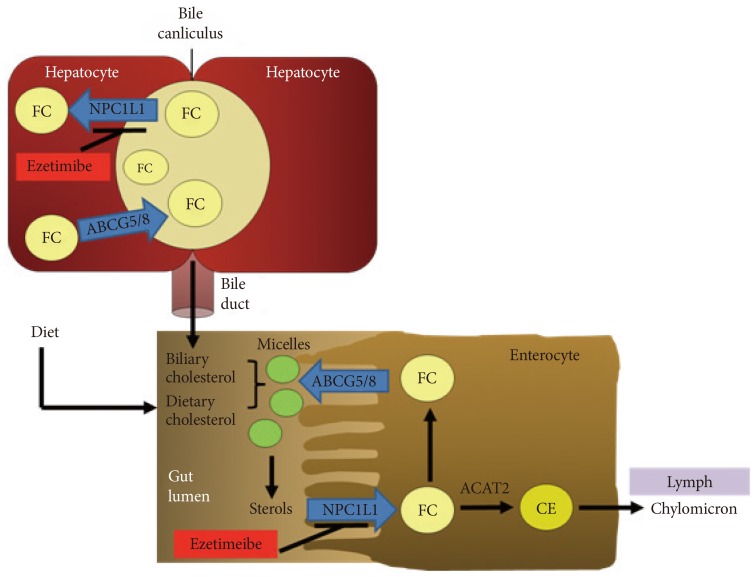

Figure & Data
References
Citations
Citations to this article as recorded by 

- Multifunctional nanoparticle-mediated combining therapy for human diseases
Xiaotong Li, Xiuju Peng, Makhloufi Zoulikha, George Frimpong Boafo, Kosheli Thapa Magar, Yanmin Ju, Wei He
Signal Transduction and Targeted Therapy.2024;[Epub] CrossRef - Ezetimibe Induces Paraptosis through Niemann–Pick C1-like 1 Inhibition of Mammalian-Target-of-Rapamycin Signaling in Hepatocellular Carcinoma Cells
Yuting Yin, Chun Wu, Yufeng Zhou, Meiyin Zhang, Shijuan Mai, Minshan Chen, Hui-Yun Wang
Genes.2023; 15(1): 4. CrossRef - Ezetimibe and Cancer: Is There a Connection?
Jia Gu, Neng Zhu, Hong-Fang Li, Chan-Juan Zhang, Yong-Zhen Gong, Duan-Fang Liao, Li Qin
Frontiers in Pharmacology.2022;[Epub] CrossRef - The role of NPC1L1 in cancer
Renshuai Zhang, Jun Zeng, Wenjing Liu, Jingsen Meng, Chao Wang, Lingyu Shi, Shanbo Yang, Jing Chang, Dongming Xing
Frontiers in Pharmacology.2022;[Epub] CrossRef - Development of a Diabetic Foot Ulceration Prediction Model and Nomogram
Eun Joo Lee, Ihn Sook Jeong, Seung Hun Woo, Hyuk Jae Jung, Eun Jin Han, Chang Wan Kang, Sookyung Hyun
Journal of Korean Academy of Nursing.2021; 51(3): 280. CrossRef - Structural insights into the mechanism of human NPC1L1-mediated cholesterol uptake
Miaoqing Hu, Fan Yang, Yawen Huang, Xin You, Desheng Liu, Shan Sun, Sen-Fang Sui
Science Advances.2021;[Epub] CrossRef - Comparison of the Efficacy and Safety of Rosuvastatin/Ezetimibe Combination Therapy and Rosuvastatin Monotherapy on Lipoprotein in Patients With Type 2 Diabetes: Multicenter Randomized Controlled Study
Jiwoo Lee, You-Cheol Hwang, Woo Je Lee, Jong Chul Won, Kee-Ho Song, Cheol-Young Park, Kyu Jeung Ahn, Joong-Yeol Park
Diabetes Therapy.2020; 11(4): 859. CrossRef - Serum, liver and bile sitosterol and sitostanol in obese patients with and without NAFLD
Milla-Maria Tauriainen, Ville Männistö, Dorota Kaminska, Maija Vaittinen, Vesa Kärjä, Pirjo Käkelä, Sari Venesmaa, Helena Gylling, Jussi Pihlajamäki
Bioscience Reports.2018;[Epub] CrossRef - Comparison of the Effects of Ezetimibe-Statin Combination Therapy on Major Adverse Cardiovascular Events in Patients with and without Diabetes: A Meta-Analysis
Namki Hong, Yong-ho Lee, Kenichi Tsujita, Jorge A. Gonzalez, Christopher M. Kramer, Tomas Kovarnik, George N. Kouvelos, Hiromichi Suzuki, Kyungdo Han, Chan Joo Lee, Sung Ha Park, Byung-Wan Lee, Bong-Soo Cha, Eun Seok Kang
Endocrinology and Metabolism.2018; 33(2): 219. CrossRef - Ezetimibe ameliorates steatohepatitis via AMP activated protein kinase-TFEB-mediated activation of autophagy and NLRP3 inflammasome inhibition
Soo Hyun Kim, Gyuri Kim, Dai Hoon Han, Milim Lee, Irene Kim, Bohkyung Kim, Kook Hwan Kim, Young-Mi Song, Jeong Eun Yoo, Hye Jin Wang, Soo Han Bae, Yong-Ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Myung-Shik Lee
Autophagy.2017; 13(10): 1767. CrossRef - The Interpretation of Cholesterol Balance Derived Synthesis Data and Surrogate Noncholesterol Plasma Markers for Cholesterol Synthesis under Lipid Lowering Therapies
Frans Stellaard, Dieter Lütjohann
Cholesterol.2017; 2017: 1. CrossRef -
Antileishmanial Activity of Ezetimibe: Inhibition of Sterol Biosynthesis,
In Vitro
Synergy with Azoles, and Efficacy in Experimental Cutaneous Leishmaniasis
Valter Viana Andrade-Neto, Edézio Ferreira Cunha-Júnior, Marilene Marcuzzo do Canto-Cavalheiro, Geórgia Correa Atella, Talita de Almeida Fernandes, Paulo Roberto Ribeiro Costa, Eduardo Caio Torres-Santos
Antimicrobial Agents and Chemotherapy.2016; 60(11): 6844. CrossRef - The P72R Polymorphism of p53 Predisposes to Obesity and Metabolic Dysfunction
Che-Pei Kung, Julia I-Ju Leu, Subhasree Basu, Sakina Khaku, Frederick Anokye-Danso, Qin Liu, Donna L. George, Rexford S. Ahima, Maureen E. Murphy
Cell Reports.2016; 14(10): 2413. CrossRef - Increased postprandial apolipoprotein B-48 level after a test meal in diabetic patients: A multicenter, cross-sectional study
Cheol-Young Park, Joong-Yeol Park, Jongwon Choi, Dae Jung Kim, Kyong Soo Park, Kun-Ho Yoon, Moon-Kyu Lee, Sung-Woo Park
Metabolism.2016; 65(6): 843. CrossRef - Relationship between fruit and fish intakes and cardiovascular disease risk factors in Korean women with type 2 diabetes mellitus: Based on the 4th and 5th Korea National Health and Nutrition Examination Surveys
Ji Soo Oh, Hyesook Kim, Ki Nam Kim, Namsoo Chang
Journal of Nutrition and Health.2016; 49(5): 304. CrossRef - Plant sterols: Friend or foe in CNS disorders?
Tim Vanmierlo, Jeroen F.J. Bogie, Jo Mailleux, Jasmine Vanmol, Dieter Lütjohann, Monique Mulder, Jerome J.A. Hendriks
Progress in Lipid Research.2015; 58: 26. CrossRef - Ezetimibe Stimulates Intestinal Glucagon-Like Peptide 1 Secretion Via the MEK/ERK Pathway Rather Than Dipeptidyl Peptidase 4 Inhibition
Eugene Chang, Lisa Kim, Jung Mook Choi, Se Eun Park, Eun-Jung Rhee, Won-Young Lee, Ki-Won Oh, Sung-Woo Park, Dong Il Park, Cheol-Young Park
Metabolism.2015; 64(5): 633. CrossRef - Intestinal absorption characteristics of imperialine: in vitro and in situ assessments
Qing Lin, Li-qin Ling, Ling Guo, Tao Gong, Xun Sun, Zhi-rong Zhang
Acta Pharmacologica Sinica.2015; 36(7): 863. CrossRef - Ezetimibe improves hepatic steatosis in relation to autophagy in obese and diabetic rats
Eugene Chang
World Journal of Gastroenterology.2015; 21(25): 7754. CrossRef - Vitamin E Transporters in Cancer Therapy
Saeed Alqahtani, Amal Kaddoumi
The AAPS Journal.2015; 17(2): 313. CrossRef - Future therapeutic targets for the treatment and prevention of cholesterol gallstones
Ibrahim Guillermo Castro-Torres, René de Jesús Cárdenas-Vázquez, Claudia Velázquez-González, Rosa Ventura-Martínez, Minarda De la O-Arciniega, Elia Brosla Naranjo-Rodríguez, Mariano Martínez-Vázquez
European Journal of Pharmacology.2015; 765: 366. CrossRef - Membrane composition and dynamics: A target of bioactive virgin olive oil constituents
Sergio Lopez, Beatriz Bermudez, Sergio Montserrat-de la Paz, Sara Jaramillo, Lourdes M. Varela, Almudena Ortega-Gomez, Rocio Abia, Francisco J.G. Muriana
Biochimica et Biophysica Acta (BBA) - Biomembranes.2014; 1838(6): 1638. CrossRef

 KDA
KDA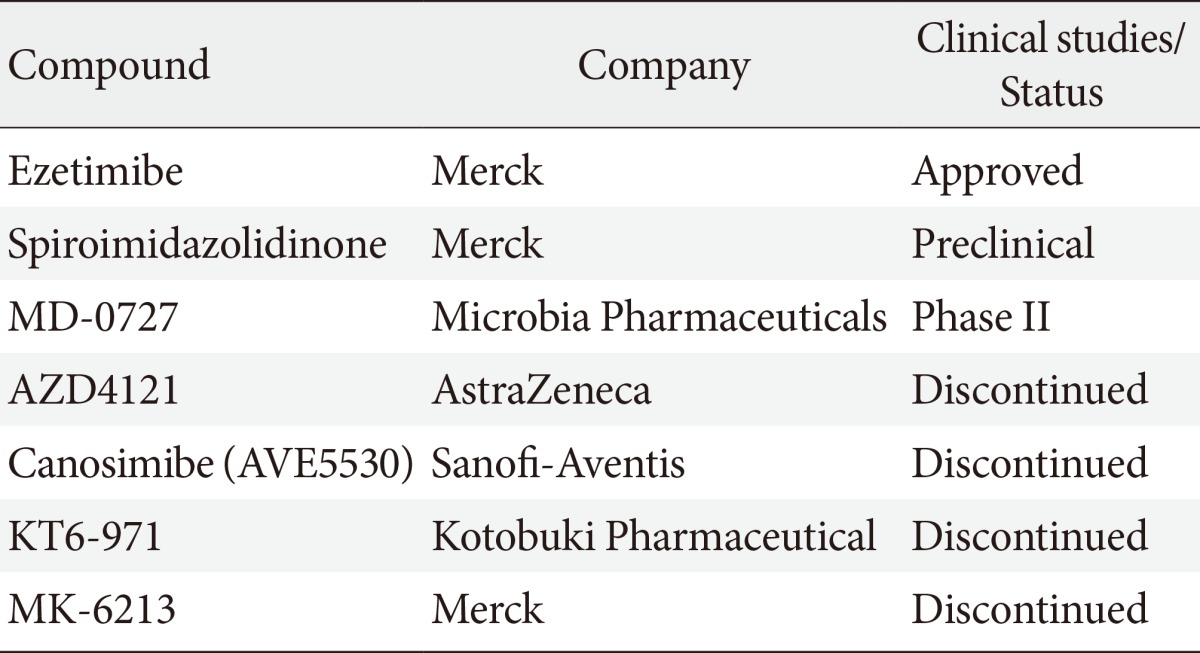
 PubReader
PubReader Cite
Cite