Effects of Exercise Alone on Insulin Sensitivity and Glucose Tolerance in Obese Youth
Article information
Abstract
As with the dramatic increases in childhood obesity over the past decades, the incidence of type 2 diabetes has increased among children and adolescents in the United States. Insulin resistance is a common feature of childhood obesity and increases the risk of type 2 diabetes, metabolic syndrome, and atherogenic lipoprotein profile in obese youth. Although cross-sectional studies report beneficial effects of physical activity or cardiorespiratory fitness on insulin sensitivity, the role of regular exercise alone (e.g., no calorie restriction) as a strategy to reduce the risk of type 2 diabetes is unclear in obese children and adolescents. In this mini review, we examined the independent effects of various exercise on glucose tolerance and insulin sensitivity in obese youth.
INTRODUCTION
Insulin resistance is defined as the decreased peripheral tissue response to insulin-mediated cellular actions and the term "insulin resistance" refers to reduced whole-body glucose uptake in response to physiological levels of insulin [1]. Insulin resistance is a common feature of obesity and is strongly associated with the etiology of type 2 diabetes, hypertension and coronary heart disease [2]. Currently, the gold standard method to evaluate insulin resistance is the hyperinsulinemic-euglycemic clamp technique [3].
As with the increasing epidemic of childhood obesity in recent years, the prevalence of type 2 diabetes has increased among obese youth, particularly in a racial/ethnic minority group [4]. Although transient insulin resistance can occur during puberty [5], obesity is the most prevalent pathophysiological cause of insulin resistance in children and adolescents [1]. Sinha et al. [6] reported that impaired glucose tolerance is present in 25% of prepubertal obese children and 21% of obese adolescents based on a 2-hour oral glucose tolerance test (OGTT). A longitudinal study has shown that transition from normal to impaired glucose tolerance and from impaired glucose tolerance to type 2 diabetes is significantly associated with weight gain in children and adolescents [7]. Increased abdominal fat, particularly visceral fat is recognized as a strong predictor of insulin resistance in obese youth independent of total adiposity [8].
In adults, epidemiologic studies suggest that regular physical activity is protective against development of type 2 diabetes [9-11]. Intervention studies report that engaging in regular exercise is associated with significant improvements in glycemic control [12] and insulin sensitivity [13,14] in nondieting men and women. Further, evidence from well-controlled randomized studies suggests that a combination of aerobic (e.g., treadmill walking, jogging) and resistance (e.g., weight lifting) exercise is more effective than either exercise modality alone to improve insulin sensitivity [15,16] and glycemic control [12,17] in men and women. Although cross-sectional studies report the beneficial effects of physical activity [18] or having a high cardiorespiratory fitness [19] on insulin sensitivity in children and adolescents, whether engaging in exercise alone is effective in improving insulin sensitivity is not firmly established.
The purpose of this mini review is to examine the effects of exercise alone (e.g., no calorie restriction) on glucose tolerance and insulin sensitivity in obese children and adolescents. Given that fasting insulin alone or combined with fasting glucose is a poor surrogate measure of insulin resistance [1], this review will focus on the exercise intervention studies, in which insulin resistance was evaluated by the OGTT, intravenous glucose tolerance test (IVGTT) and hyperinsulinemic euglycemic clamp test in nondieting children and adolescents.
EFFECT OF AEROBIC EXERCISE ALONE ON INSULIN SENSITIVITY AND GLUCOSE TOLERANCE IN OBESE YOUTH
Skeletal muscle is the major site of insulin-mediated glucose uptake in the postprandial state as the majority (~85%) of glucose uptake by peripheral tissues occurs in muscle [20]. Exercise has an "insulin-like effect" to facilitate glucose transport from the circulation into the working muscles [21]. It has been suggested that the mechanism by which exercise increases glucose uptake may be via the translocation of glucose transporters (e.g., GLUT-4) from an intracellular pool to the surface of the cell, where glucose uptake takes place [21,22]. Indeed, studies demonstrated increased GLUT-4 concentrations with aerobic training, which is accompanied by increases in insulin-mediated glucose uptake in adults [23-25]. However, the exercise-induced increase in muscle GLUT-4 concentration and the corresponding increase in insulin sensitivity decreases rapidly after the cessation of exercise [24], which suggests that exercise should be performed on a regular basis to maintain enhanced insulin sensitivity.
Currently, we are aware of six intervention studies, wherein the effects of aerobic exercise without calorie restriction on insulin sensitivity and glucose tolerance were examined in obese children and adolescents. Inspection of Table 1 reveals that the majority of studies have employed short duration of aerobic exercise (8 weeks to 3 months) and reported no significant weight loss after the training. For example, Nassis et al. [26] have shown that in overweight and obese girls (n=19), 12 weeks of aerobic exercise (3 days/wk, 40 min/session, running, stair climbing, and jump rope) resulted in a significant reduction (-23.3%) in insulin area under the curve (AUC) and increase in cardiovascular fitness (18.8%) in the absence of changes in body weight and % body fat. A significant increase (6.2%) in lower limb fat free mass was observed after the training, which was significantly correlated with a reduction in insulin AUC (r=-0.68). Similarly, van der Heijden et al. [27] performed a nonrandomized controlled study to examine the effect of 12-week moderate intensity (≥70% VO2peak) aerobic exercise (4 days/wk, 30 min/session, treadmill, elliptical, or a bike) on insulin action in obese Hispanic adolescents. In this study, the stable label IVGTT was employed to distinguish peripheral and hepatic insulin sensitivity. Despite the absence of weight loss and calorie restriction, significant improvements in peripheral (50%) and hepatic insulin sensitivity (23%) were observed, which was also accompanied by reductions in total fat (-1.1%) and increases in lean body mass (1.1 kg). Conversely, Monzavi et al. [28] observed no changes in oral glucose tolerance after a 12-week family-based lifestyle intervention program in overweight youth.
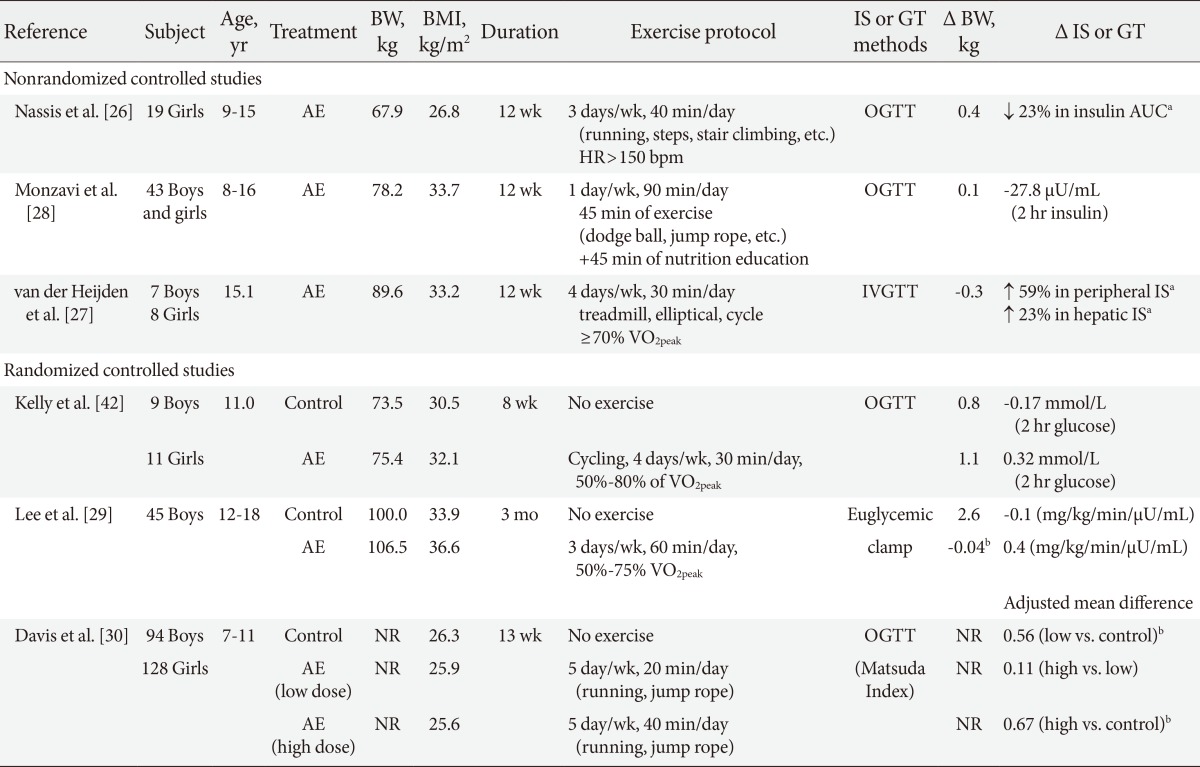
Effect of aerobic exercise without calorie restriction alone on insulin sensitivity and glucose tolerance in obese youth
In adults, some studies suggest that the improvement in insulin sensitivity in response to aerobic exercise training is mediated by reductions in adiposity [13,14]. Gan et al. [13] reported that in obese men, aerobic exercise alone is associated with significant improvements in insulin sensitivity as measured by hyperinsulinemic-euglycemic clamp technique, and that the improvement in insulin sensitivity is associated with concomitant reductions in visceral fat and not by skeletal muscle characteristics such as intramyocellular lipid and long-chain acyl CoA levels. Currently, there has only been one randomized controlled study in obese youth that examined the effects of aerobic exercise on insulin action and secretion, and total and regional fat topography. We [29] have recently demonstrated that in obese adolescent boys, 3 months of aerobic exercise (3 times/wk, 60 min/session, 60% to 75% of VO2peak) without calorie restriction results in significant reductions in total (-2.6%) and visceral fat (-6.7%) and intrahepatic lipid content (-1.9%) by comparison to nonexercising controls. However, despite significant improvement in body composition, there were no improvements in oral glucose tolerance, and insulin sensitivity and secretion as accessed by the 3-hour hyperinsulinemic-euglycemic clamp test and the 2-hour hyperglycemic clamp test, respectively. Further, no changes in skeletal muscle mass and intramyocellular lipid content were observed in response to aerobic training.
At present, we are aware of one randomized controlled trial that examined the effect of different doses of aerobic training on insulin resistance in the pediatric population. Davis et al. [30] examined the effects of low-dose (5 days/wk, 20 min/session) versus high-dose (5 day/wk, 40 min/session) of aerobic exercise program (without dietary restrictions) on oral glucose tolerance in a large sample of overweight and obese children (n=222, 7 to 11 years). After 3 months of aerobic exercise, they observed a dose-response trend, such that the reductions in insulin AUC was larger in the high-dose exercise (adjusted mean difference, -3.56×103 µU/mL) and the low-dose exercise groups (adjusted mean difference, -2.96×103 µU/mL) than the control group. Dose-response associations were also observed for reductions in % body fat and visceral fat, independent of gender and race. Observation that the low- and high-dose of exercise groups showed similar improvements on insulin resistance, and that the increment of benefit between the control and low-dose exercise groups was larger than those observed between the low- and high-dose exercise groups, suggest that greater health benefits can be obtained when obese youth move from being sedentary to becoming moderately active.
EFFECT OF RESISTANCE EXERCISE ALONE OR IN COMBINATION WITH AEROBIC EXERCISE ON INSULIN SENSITIVITY AND GLUCOSE TOLERANCE IN OBESE YOUTH
Although aerobic types of physical activities have been traditionally recommended for children and adolescents, a recent guideline suggests that properly designed and supervised resistance training is beneficial to improve musculoskeletal strength, cardiovascular risk profiles, motor skills and psychosocial well-being of youth [31]. Currently, we are aware of five randomized controlled studies, in which the effects of resistance exercise without calorie restriction on insulin sensitivity and glucose tolerance were examined in obese youth (Table 2). Evidence from randomized controlled trials [29,32] suggests that progressive resistance training is effective in improving insulin sensitivity in previously sedentary obese adolescent boys. Shaibi and colleagues [32] examined the effects of a 16-week resistance training alone (2 days/wk, 60 min/session) on insulin sensitivity in overweight Hispanic adolescent boys. The exercise program was progressive with respect to the number of sets (one to three sets), repetitions (three to 15 repetitions), and resistance used (62% to 97% of baseline 1 repetition maximum) and each exercise session consisted of single and multiple-joint exercises using both free weights and weight stack equipment. After 16 weeks, insulin sensitivity measured by IVGTT significantly increased in the resistance exercise group (45%) compared with nonexercising controls (-0.9%) and the improvement in insulin sensitivity was independent of changes in total fat and lean body mass. Similarly, we conducted a randomized controlled study in obese adolescent boys to examine the effects of resistance versus aerobic exercise training, matched for exercise duration and frequency, on insulin sensitivity by a 3-hour hyperinsulinemic-euglycemic clamp technique [29]. We observed a significant increase in insulin sensitivity (28%) in the resistance exercise group alone, which was accompanied by significant increases in skeletal muscle mass (1.4 kg). We also found that compared with controls, resistance exercise was as effective as aerobic exercise in reducing total (resistance, -2.5 kg vs. aerobic, -3.0 kg) and visceral fat (resistance, -0.2 kg vs. aerobic, -0.1 kg), and liver fat content (resistance, -2.0% vs. aerobic, -1.9%) as measured by whole-body magnetic resonance imaging and proton magnetic resonance spectroscopy. However, unlike the study of Shaibi et al. [32], we observed that the changes in insulin sensitivity were significantly associated with reductions in total and visceral fat as quantified by whole-body MRI technique, which reinforces the importance of adiposity reduction in improving insulin sensitivity in obese adolescent boys.
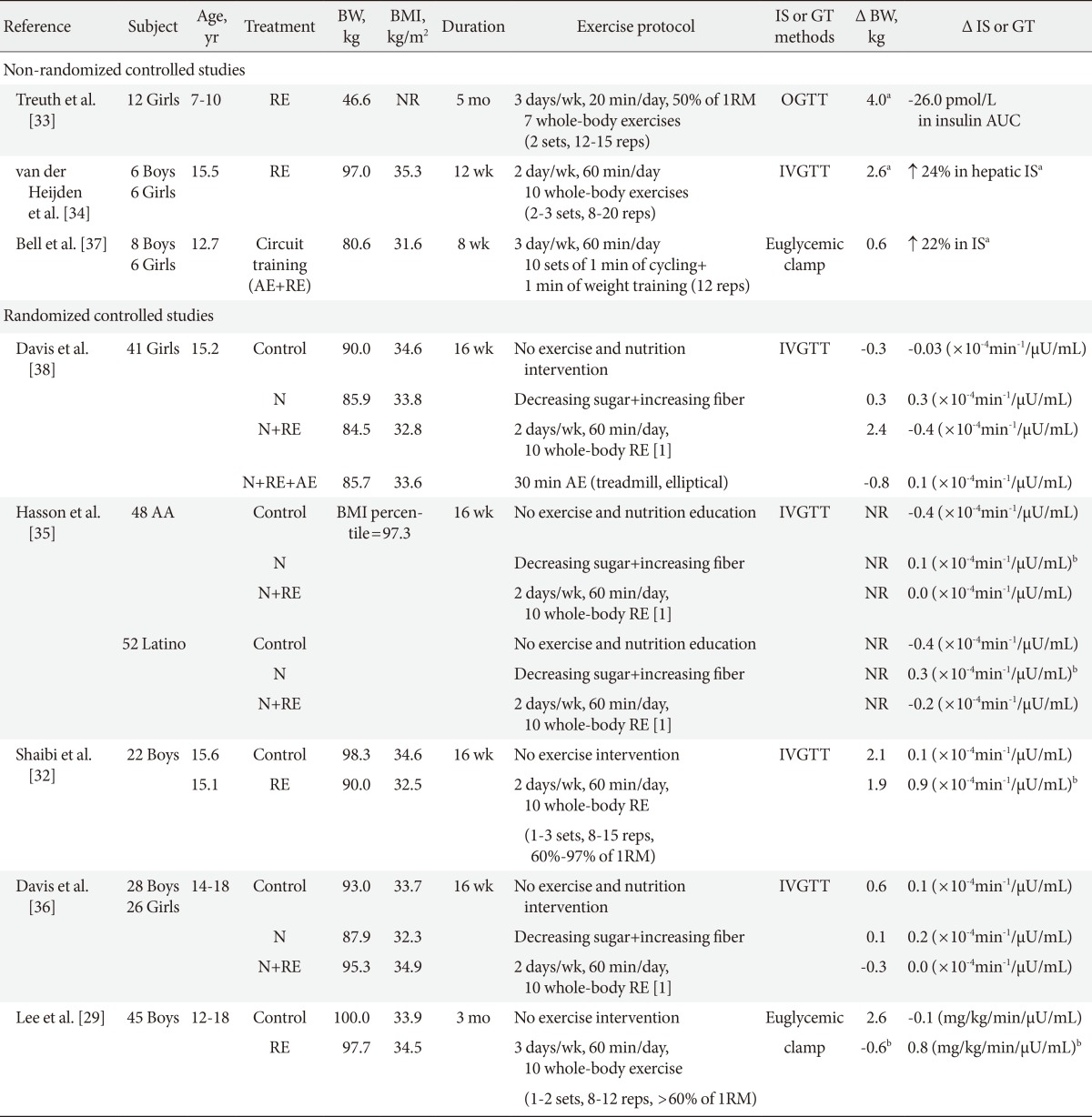
Effect of resistance exercise alone or in combination with aerobic exercise on insulin sensitivity and glucose tolerance in obese youth
Unlike favorable metabolic benefits observed in obese adolescent boys [29,32], others report no beneficial effects of resistance exercise on oral glucose tolerance and peripheral insulin sensitivity in obese girls [33] and a mixed sample of obese boys and girls [34-36]. Goran's group [35,36] reported no improvements in insulin sensitivity in response to a 16-week resistance exercise (2 days/wk, 60 min/session) combined with healthy diet (decreasing sugar and increasing fiber intake) in obese African-American and Hispanic adolescent boys and girls. Similarly, Treuth et al. [33] demonstrated significant increases in total and abdominal subcutaneous fat after a 5-month resistance training, which resulted in no improvements in glucose and insulin AUC in obese prepubertal girls (7 to 10 years). Although the disparate findings are unclear in the current literature, inclusion of children of both sexes in the analyses [34-36], significant weight gain [33] and increased energy compensation in response to exercise training [35,36] may in part explain no improvements in insulin sensitivity.
To date, we are aware of two studies [37,38] wherein the effect of combined resistance and aerobic exercise on insulin sensitivity was examined in obese youth. In a nonrandomized controlled trial, Bell et al. [37] examined the effects of circuit training (3 times/wk, 60 min/session) on insulin sensitivity using the hyperinsulinemic euglycemic clamp technique in a mixed sample of obese boys and girls (n=14). In this study, each exercise session included 1 minute of cycle ergometry (65% to 85% of maximum heart rate) followed by a 1 minute of resistance training (12 repetitions/min, 55% to 65% of maximum voluntary contraction) and two sets of 10 resistance exercise stations. Significant increases in insulin sensitivity (22%) and reductions in submaximal heart rate responses (indicative of improvement in cardiorespiratory fitness) were observed independent of changes in body composition (e.g., total fat and lean body mass). Conversely, no significant effects of combination of aerobic and resistance training on insulin sensitivity and glucose tolerance were reported in obese Hispanic girls [38].
CONCLUSIONS
Insulin resistant overweight/obese children and adolescents have a high prevalence of the metabolic syndrome [8] and an atherogenic lipoprotein profile of small dense low density lipoprotein, small high density lipoprotein, and large very low density lipoprotein [39]. Our group previously demonstrated that for a given body mass index or total fat, insulin resistance is higher in those with high visceral fat [40] and/or high waist circumference [41]. Although lifestyle intervention is the primary intervention strategy to treat obese youth, the role of physical activity alone to reduce the risk of type 2 diabetes remains unclear.
Currently, few randomized controlled studies are available that examined the independent effect of aerobic exercise [29,30,42], resistance exercise [29,32,35,36] or combination of both exercises [38] on glucose tolerance or insulin sensitivity in overweight and obese youth and their findings are inconclusive to date. Although there is evidence of dose-response relationships between aerobic exercise and oral glucose tolerance in children [30], the effect of aerobic exercise alone on in vivo insulin sensitivity has not been firmly established. Within the currently limited evidence, two randomized controlled trials suggest the beneficial effects of resistance exercise on insulin sensitivity and skeletal muscle mass in obese adolescent boys, independent of calorie restriction [29,32].
Given the variations in the study designs, the population studied (single gender vs. both gender combined; prepubertal vs. pubertal) and small sample size, intervention studies have failed to conclusively demonstrate the independent effects of exercise on insulin sensitivity and glucose tolerance in children and adolescents. As insulin resistance occurs with puberty [5], pubertal stage (e.g., Tanner stage) should be examined and considered during data analyses/interpretations. As many of the studies did not concurrently assess abdominal fat, a strong independent factor of insulin resistance in youth [40,41], it is unclear whether the changes in insulin sensitivity and glucose tolerance are mediated by the changes in abdominal fat. Due to the lack of intervention studies comparing the effects of different exercise modalities, the optimal mode of exercise to improve insulin sensitivity is currently unknown in children and adolescents.
Notes
No potential conflict of interest relevant to this article was reported.