
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 36(1); 2012 > Article
-
Original ArticleRole of HbA1c in the Screening of Diabetes Mellitus in a Korean Rural Community
- Jae Hyun Kim1, Gun Woo Kim1, Mi Young Lee1, Jang Yel Shin1, Young Goo Shin1, Sang Baek Koh2, Choon Hee Chung1,3
-
Diabetes & Metabolism Journal 2012;36(1):37-42.
DOI: https://doi.org/10.4093/dmj.2012.36.1.37
Published online: February 17, 2012
1Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
2Department of Preventive Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
3Institute of Lifestyle Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea.
- Corresponding author: Choon Hee Chung. Department of Internal Medicine, Yonsei University Wonju College of Medicine, 162 Ilsan-dong, Wonju 220-701, Korea. cchung@yonsei.ac.kr
Copyright © 2012 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Recently, the measurement of glycated hemoglobin (HbA1c) was recommended as an alternative to fasting plasma glucose or oral glucose tolerance tests for diagnosing diabetes mellitus (DM). In this study, we analyzed HbA1c levels for diabetes mellitus screening in a Korean rural population.
-
Methods
- We analyzed data from 10,111 subjects from a Korean Rural Genomic Cohort study and generated a receiver operating characteristic curve to determine an appropriate HbA1c cutoff value for diabetes.
-
Results
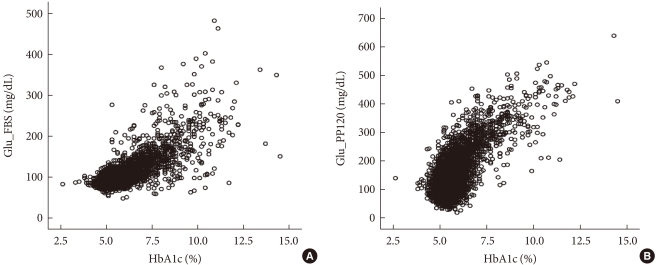
- The mean age of the subjects was 56.3±8.1 years. Fasting plasma glucose and 2-hour plasma glucose after 75 g oral glucose tolerance tests were 97.5±25.6 and 138.3±67.1 mg/dL, respectively. The mean HbA1c level of the subjects was 5.7±0.9%. There were 8,809 non-DM patients (87.1%) and 1,302 DM patients (12.9%). A positive relationship between HbA1c and plasma glucose levels and between HbA1c and 2-hour plasma glucose levels after oral glucose tolerance tests was found in a scatter plot of the data. Using Youden's index, the proper cutoff level of HbA1c for diabetes mellitus screening was 5.95% (sensitivity, 77%; specificity, 89.4%).
-
Conclusion
- Our results suggest that the optimal HbA1c level for DM screening is 5.95%.
- The prevalence of diabetes is increasing worldwide. In many cases, chronic complications in the advanced stage are found during diagnosis [1]. In addition, cases of microvascular and macrovascular complications have been found in pre-diabetes such as impaired fasting glucose or impaired glucose tolerance [2]. Early detection and active treatment can help in preventing the progression of complications and are therefore considered to be very important for controlling diabetes mellitus.
- Until now, oral glucose tolerance tests have been used to measure fasting or postprandial blood glucose in the diagnosis of diabetes. However, short-term blood glucose observations require fasting prior to blood sampling which can be uncomfortable for patients. In addition, since blood sugar levels can be affected by exercise, diet, and illness, the patient's condition, and also the temperature at which blood samples are stored at, can make the interpretation of results difficult. Therefore, many studies have investigated the diagnosis of diabetes using glycated hemoglobin (HbA1c) because more accurate tests and simple diagnostic criteria are required for screening of diabetes. The glycated hemoglobin test measures how much glucose is bound to hemoglobin in red blood cells. The test reflects the level of glucose control over the previous 2 to 3 months and is used as an indicator for the risk of complications in patients diagnosed with diabetes [3]. In addition, this method is not affected by diet and can be performed at any time; thus, the level of glycated hemoglobin in patients who have diabetes can be used to determine blood glucose levels more accurately than existing tests for the diagnosis of diabetes. Despite its accuracy, however, the glycated hemoglobin test has not been considered reliable due to a lack of standardization. Therefore, it has not been included in the diagnostic criteria for diabetes [4]. However, in many recent studies, when glycated hemoglobin is compared with fasting blood glucose, it does not fall behind the other diabetes screening methods and is considered a useful part of the diagnostic criteria [5]. In addition, as the measuring process for glycated hemoglobin has become more accurate and standardized, the American Diabetes Association (ADA) in 2010 adopted glycated hemoglobin level over 6.5% as a new diagnostic criteria for diabetes [6]. However, there is still controversy over whether this glycated hemoglobin level is applicable to Korean adults suitably. Therefore, in this study, the glycated hemoglobin levels of patients who received 75 g oral glucose tolerance tests were comparatively analyzed in order to determine the appropriate glycated hemoglobin levels for screening or diagnosis of diabetes in Korean adults.
INTRODUCTION
- Between January 2005 and January 2007, this study was performed on 10,111 patients who belonged to the Korean Genomic Research Cohort. Data on age, gender, blood pressure, and body mass index (BMI), calculated by dividing body weight (kg) by height squared (m2), were collected for the all patients. Cholesterol, triglycerides, high density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C), liver enzymes, fasting blood glucose, 75 g oral glucose tolerance tests, 2-hour blood glucose samples, and glycated hemoglobin were investigated. Glycated hemoglobin was measured using a high-performance liquid chromatography (HPLC) Variant II Turbo (Bio-Rad, Hercules, CA, USA). Using a specific standardized measurement set established through the National Glycohemoglobin Standardization Program (NGSP), calibrations were made and the coefficient of variation was between 1.3% (normal) and 0.8% (abnormal).
- Using Youden's index, the distribution curve and the relative character curve for the levels of glycated hemoglobin of patients diagnosed with diabetes were generated and used to obtain glycated hemoglobin values showing the appropriate sensitivity and specificity were investigated. Diabetes was defined as an 8-hour fasting blood glucose over 126 mg/dL and a 75 g oral glucose tolerance test over 200 mg/dL after 2 hours based on criteria set by the Korean Diabetes Association [7].
- Statistical analysis was performed using SPSS program version 12.0 for Windows (SPSS Inc., Chicago, IL, USA). Statistical values were expressed as mean±standard deviation. Threshold values for glycated hemoglobin obtained from the analysis of relative characteristic curves were used to predict diabetes.
METHODS
- Clinical characteristics of study participants
- The mean age of the patients in this study was 56.3±8.1 years, and the gender distribution was 4,091 (40.5%) males and 6,020 (59.5%) females. The mean fasting level of blood glucose was 97.5±25.6 mg/dL, and the mean blood glucose level measured by 75 g oral glucose tolerance test after 2 hours was 138.3±67.1 mg/dL. The mean glycated hemoglobin value for all the patients was 5.7±0.9% (Table 1). The mean glycated hemoglobin for males and females were 5.7±0.9% and 5.7±0.8%, respectively, a statistically significant result (P=0.017).
- Evaluation of cutoff value (threshold)
- According to the results from fasting plasma glucose and 75 g oral glucose tolerance tests, 8,809 (87.1%) of the patients belonged to the non-diabetes group. Among them, 1,850 (18.3%) had impaired fasting glucose and 1,951 (19.3%) had impaired glucose tolerance. 1,302 (12.9%) of the patients belonged to the diabetes group. The distribution of fasting plasma glucose and glycated hemoglobin, and the distribution of glycated hemoglobin measured from blood glucose after 2-hour 75 g oral glucose tolerance tests all showed a positive correlation (Fig. 1). When glycated hemoglobin levels used to predict diabetes were analyzed using a relative characteristic curve, the sensitivity and specificity were 77.0% and 89.4%, respectively, and appropriate levels for selection criteria were observed (Fig. 2).
- This study was a cross-sectional analysis. We did not investigate diabetic microvascular complications, and providing suggestions for diagnostic criteria was difficult. However, with results that showed a specificity of 97.5% and a corresponding glycated hemoglobin value of 6.4% (Table 2), both of which are appropriate values, we believe that this study can be used as an indicator for predicting diabetes.
RESULTS
- The glycated hemoglobin value reflects glycemic control over the previous 2 to 3 months, and there is a positive correlation between fasting plasma glucose and blood glucose after 2 hours in 75 g oral glucose tolerance test. In addition, glycated hemoglobin measurements are more reproducible than fasting blood glucose, and the coefficient of variation within the population is 1.7% compared to 5.7% for fasting blood glucose [8,9]. Furthermore, in the study results from the Diabetes Control and Complication Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS), the authors noted that measuring glycated hemoglobin was a useful indicator in predicting the occurrence of complications with diabetes [10,11]. Thus, glycated hemoglobin measurements are measurements are receiving spotlights in screening and diagnosing diabetes recently. Studies that compare the existing tests to glycated hemoglobin measurement are being developed and being assessed for their usefulness. In a study of 4,935 participants in the U.S. National Health and Nutrition Examination Survey (NHANES) for 6 years (1999 to 2004), fasting blood glucose was used for the diagnosis of diabetes. On the basis of this study, the suggested ranges for glycated hemoglobin are as follows: over 5.8% indicates impaired glucose metabolism, 6.1% to 6.9% is considered to be pre-diabetes, and over 7% is considered to be diabetes [12]. In addition, several studies have reported a relationship between glycated hemoglobin and diabetic retinopathy [13,14], and in 2010, through NGSP standardization [15], the ADA proposed that glycated hemoglobin levels greater than 6.5% should be diagnosed as diabetes [6]. In the 2002 Japan National Diabetes survey based on impaired glucose metabolism, glycated hemoglobin greater than 6.1% is classified as probable DM, 5.6% to 6.0% as possible DM, and less than 5.6% as normal [16].
- There have been many recent studies in Korea regarding the use of glycated hemoglobin levels for the diagnosis of diabetes. Kim et al. [17] performed a study on 205 patients with normal fasting blood glucose who had never been diagnosed with glucose metabolism impairments. Among those patients, the mean glycated hemoglobin value between the diabetes group and the non-diabetes group was 6.1±0.7% and 5.7±0.5%, respectively, a statistically significant difference. In addition, this study reported that diabetes could be predicted through glycated hemoglobin measurements. In a study based on fasting blood glucose performed by Ku et al. [18] on 19,178 patients who did not have diabetes, the threshold glycated hemoglobin value for predicting diabetes was 5.9%. In addition, using a 2-hour oral glucose tolerance test with the threshold value set at 6.1% for glycated hemoglobin, sensitivities and specificities were 84.6%/85.9% and 86.5%/85.8%, respectively. In a study by Bae et al. [19] performed on 1,482 patients who had not previously been diagnosed with diabetes, the glycated hemoglobin threshold value for predicting diabetes was set at 5.95%, and the sensitivity and specificity were reported to be 60.8% and 85.6%, respectively. In a retrospective study by Jung et al. [20] performed on 1,474 diabetes patients, a glycated hemoglobin threshold value of 6.75% and a specificity of 85% were suggested for the diagnosis of diabetes. In our study results, the glycated hemoglobin threshold was 5.95%. However, the specificity must be higher in order for glycated hemoglobin to be used as a diagnostic criteria [21]; therefore, the baseline for predicting diabetes was set at 6.4%, which corresponds to the specificity of 97.5%. This baseline prediction value is used in many studies, and when used for the diagnosis of diabetes, the specificity value is considered to be high [22-26].
- However, there are several problems when glycated hemoglobin is used for the prediction of diabetes in Korean adults, primarily because the measurement of glycated hemoglobin has not yet been standardized in Korea. As a result, the differences between compared measurements cause many difficulties in the analysis of the results. Standards must be preceded by selection criteria or by diagnostic criteria.
- In addition, Korean diets are different from Western diets, and this can result in a difference in blood glucose after meals which can also influence glycated hemoglobin. According to the 2008 National Health Statistics [27], the daily consumption of carbohydrates for Koreans is 293.3 g. According to the 2006 U.S. National Health and Nutrition Examination Survey [28], Americans consume an average of 265 g of carbohydrates per day. Thus, Koreans may have higher blood glucose levels after meals compared to Americans. In addition, Korean diabetics have fewer beta cells compared to American diabetics, and there are studies regarding the amount of insulin secreted after meals [29]. Other factors that may cause differences between Korean and American diabetics include culture and socio-economic differences, susceptibility to diabetes, enzymatic activity, and intracellular accumulations of glycated hemoglobin resulting from varying degrees of non-enzymatic glycosylation [30,31].
- The threshold value of 5.95% for glycated hemoglobin set by the authors of this study was not significantly different from previous studies. However, African Americans consistently showed higher glycated hemoglobin than other races in studies performed by Saudek et al. [2] and Selvin et al. [32], and this was also true in the normal fasting blood glucose state. Such racial difference is caused by the use of glycated hemoglobin as a diagnostic criterion; therefore, the use of this method for the diagnosis of diabetes may be problematic. Also, because glycated hemoglobin is measured directly from red blood cells, factors affecting the survival of red blood cells such as hemolytic anemia, aplastic anemia, hemorrhage, and splenectomy may disrupt the normal relationship between blood glucose and glycated hemoglobin. Hemolytic anemia and acute hemorrhaging may cause glycated hemoglobin to drop, and patients with aplastic anemia or who have received splenectomy have older red blood cells; therefore, regardless of blood glucose concentrations, glycated hemoglobin concentrations may increase [2]. Additionally, high doses of salicylate or vitamin C, and deficiencies of vitamin E or iron may also affect glycated hemoglobin [2].
- Because of these problems, appropriate glycated hemoglobin values have not been established for the diagnostic criteria of diabetes in Koreans; however, this topic is still considered controversial. Despite the problems associated with using glycated hemoglobin, there are many benefits of using it for the diagnosis and prediction of diabetes and its complications compared to the existing tests. Therefore, we believe glycated hemoglobin will play a large role in the diagnosis of diabetes in the future. However, more research regarding the effects of racial differences and other factors on glycated hemoglobin is needed in order for it to become more widely used in the diagnosis of diabetes.
- This study has the following limitations. First, the cohort data was collected from individuals living in rural communities, so regional lifestyle differences may have influenced the results. Second, the greater number of females than males in this study may have also influenced the results. Because of the limitations of the glycated hemoglobin test, large-scale prospective studies using supplemental varied data will be required in the future. Furthermore, additional studies on threshold for increased occurrence of diabetes-associated microvascular complications will be needed to obtain a more precise diabetes diagnostic criterion.
- In this study, the appropriate levels of glycated hemoglobin were evaluated for the screening and diagnosis of diabetes. Using a relativity curve, the threshold value for glycated hemoglobin was 5.95%, and the sensitivity and specificity for the selection criteria were 77% and 89.4%, respectively. Also, the corresponding specificity of 97.5% for a glycated hemoglobin value of 6.4% was considered appropriate for diagnostic predictions. On the basis of this study, glycated hemoglobin should be considered as a method that can replace or complement existing screening methods for the diagnosis of diabetes.
DISCUSSION
- 1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414-1431. ArticlePubMedPDF
- 2. Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 2008;93:2447-2453. ArticlePubMedPDF
- 3. Sung KC, Rhee EJ. Glycated haemoglobin as a predictor for metabolic syndrome in non-diabetic Korean adults. Diabet Med 2007;24:848-854. ArticlePubMedPMC
- 4. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183-1197. ArticlePubMedPDF
- 5. Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med 2007;24:333-343. ArticlePubMed
- 6. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl 1):S62-S69. ArticlePubMedPMCPDF
- 7. Korean Diabetes Association. Diagnosis and classification of diabetes. Clin Diabetes 2005;6:132-140.
- 8. Petersen PH, Jorgensen LG, Brandslund I, De Fine Olivarius N, Stahl M. Consequences of bias and imprecision in measurements of glucose and HbA1c for the diagnosis and prognosis of diabetes mellitus. Scand J Clin Lab Invest Suppl 2005;240:51-60. ArticlePubMed
- 9. Sacks DB, Bruns DE, Goldstein DE, Maclaren NK, McDonald JM, Parrott M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin Chem 2002;48:436-472. ArticlePubMedPDF
- 10. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977-986. ArticlePubMed
- 11. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-853. ArticlePubMed
- 12. Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999 2004 NHANES population. Diabetes Care 2007;30:2233-2235. ArticlePubMedPDF
- 13. van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Polak BC. Risk factors for incident retinopathy in a diabetic and nondiabetic population: the Hoorn study. Arch Ophthalmol 2003;121:245-251. ArticlePubMed
- 14. Tapp RJ, Tikellis G, Wong TY, Harper CA, Zimmet PZ, Shaw JE. Australian Diabetes Obesity and Lifestyle Study Group. Longitudinal association of glucose metabolism with retinopathy: results from the Australian Diabetes Obesity and Lifestyle (AusDiab) study. Diabetes Care 2008;31:1349-1354. PubMedPMC
- 15. Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE. NGSP Steering Committee. The national glycohemoglobin standardization program: a five-year progress report. Clin Chem 2001;47:1985-1992. PubMed
- 16. Nakagami T, Tominaga M, Nishimura R, Yoshiike N, Daimon M, Oizumi T, Tajima N. Is the measurement of glycated hemoglobin A1c alone an efficient screening test for undiagnosed diabetes? Japan National Diabetes Survey. Diabetes Res Clin Pract 2007;76:251-256. PubMed
- 17. Kim SY, Park JH, Kang SM, Jin HY, Baek HS, Park TS. Value of HbA1c for diabetic screening in subject with normal fasting glucose. Korean Diabetes J 2008;32(Suppl 2):S218.
- 18. Ku YH, Yoo SH, Jung HS, Lim S, Moon MK, Choi SH, Jang HC, Park KS, Kim SY, Lee HK, Cho YM. Diagnostic value of HbA1c different clinical setting with different prevelence of diabetes mellitus. Korean Diabetes J 2008;32(Suppl 8):S311.
- 19. Bae JC, Rhee EJ, Choi ES, Kim JH, Kim WJ, Yoo SH, Park SE, Park CY, Lee WY, Oh KW, Park SW, Kim SW. The cutoff value of HbA1c in predicting diabetes in Korean adults in a university hospital in Seoul. Korean Diabetes J 2009;33:503-510.Article
- 20. Jung JH, Kim ST, Cho YZ, Lee HN, Kim JY, Kim JH, Lim DM, Lee KW, Kim BJ, Park KY. Acceptability of HbA1c values as a diagnostic tool for diabetes mellitus in Korea. Korean J Med 2010;79:673-680.
- 21. Engelgau MM, Narayan KM, Herman WH. Screening for type 2 diabetes. Diabetes Care 2000;23:1563-1580. ArticlePubMedPDF
- 22. Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, Flegal KM, Eberhardt MS, Goldstein DE. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care 2000;23:187-191. ArticlePubMedPDF
- 23. Eid WE, Pottala JV. Value of hemoglobin A1c in diagnosing diabetes mellitus within a chronic disease management system illustrated by the receiver operating characteristic curve. Endocr Pract 2010;16:14-20. ArticlePubMed
- 24. Larsen ML. The utility of glycated hemoglobin in identification of impaired glucose tolerance. Diabetes Res 1989;12:67-70. PubMed
- 25. Verrillo A, de Teresa A, Golia R, Nunziata V. The relationship between glycosylated haemoglobin levels and various degrees of glucose intolerance. Diabetologia 1983;24:391-393. ArticlePubMedPDF
- 26. Weykamp CW, Penders TJ, Miedema K, Muskiet FA, van der Slik W. Standardization of glycohemoglobin results and reference values in whole blood studied in 103 laboratories using 20 methods. Clin Chem 1995;41:82-86. ArticlePubMedPDF
- 27. Korean National Health and Nutrition Examination Survey: the 4th report (2008). Ministry of Health and Welfare; Korea Centers for Disease Control and Prevention updated 2009 Dec 12. Available from: http://www.bokjiro.go.kr/data/statusView.do?board_sid=297&data_sid=209708&searchSort=REG_DESC&pageIndex=1&searchWrd=%EA%B5%AD%EB%AF%BC%EA%B1%B4%EA%B0%95%ED%86%B5%EA%B3%84&searchCont=&pageUnit=10.
- 28. National Health and Nutrition Examination Survey (NHANES) 2005-2006. Centers for Disease Control and Prevention updated 2011 Apr 29. Available from: http://www.cdc.gov/nchs/nhanes/nhanes 2005-2006/nhanes05_06.htm.Article
- 29. Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 2003;88:2300-2308. PubMed
- 30. McCarter RJ, Hempe JM, Chalew SA. Mean blood glucose and biological variation have greater influence on HbA1c levels than glucose instability: an analysis of data from the Diabetes Control and Complications Trial. Diabetes Care 2006;29:352-355. PubMed
- 31. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, Lachin JM, Montez MG, Brenneman T, Barrett-Connor E. Diabetes Prevention Program Research Group. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453-2457. ArticlePubMedPDF
- 32. Selvin E, Zhu H, Brancati FL. Elevated A1C in adults without a history of diabetes in the U.S. Diabetes Care 2009;32:828-833. ArticlePubMedPMCPDF
REFERENCES

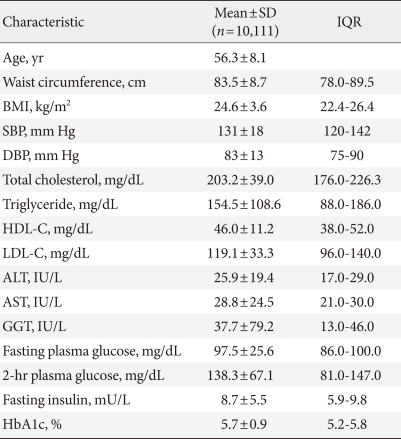
SD, standard deviation; IQR, inter quartile range; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase.
Figure & Data
References
Citations

- Hyperinsulinemia: an early biomarker of metabolic dysfunction
Rama A. Vaidya, Sharvari Desai, Panchali Moitra, Sheryl Salis, Shubhada Agashe, Rekha Battalwar, Anushree Mehta, Jagmeet Madan, Soumik Kalita, Shobha A. Udipi, Ashok B. Vaidya
Frontiers in Clinical Diabetes and Healthcare.2023;[Epub] CrossRef - A Novel Earwax Method to Measure Acute and Chronic Glucose Levels
Andrés Herane-Vives, Susana Espinoza, Rodrigo Sandoval, Lorena Ortega, Luis Alameda, Allan H. Young, Danilo Arnone, Alexander Hayes, Jan Benöhr
Diagnostics.2020; 10(12): 1069. CrossRef - Risk Factors for Underdiagnosis of Diabetes Based on the Korean National Health and Nutrition Examination Survey 2013-2015
Deulle Min, Eunhee Cho
Asia Pacific Journal of Public Health.2019; 31(5): 404. CrossRef - Recent advances of medical journals in Korea and and further development strategies: Is it possible for them to publish Nobel Prize-winning research?
Sun Huh
Journal of the Korean Medical Association.2018; 61(9): 524. CrossRef - The clinical value of HbA1c in combination with FPG in the early screening of the elderly with type 2 diabetes
Lihua Liu, Wenqing Chen, Minghua Dong, Lixia Jiang, Wei Qiu, Jian Li, Xiaoting Luo, Zhengchun Huang, Qin Wu, Qinfeng Wu, Shuiqin Chen, Lu Ou-Yang, Shumei Li, J.Q. Cheng, H.L. Moffitt, I. Kim, Z.T. Chi, J. Zhang
BIO Web of Conferences.2017; 8: 01030. CrossRef - The Cutoff Value of HbA1c in Predicting Diabetes and Impaired Fasting Glucose
Seyoung Kwon, Youngak Na
The Korean Journal of Clinical Laboratory Science.2017; 49(2): 114. CrossRef - Performance of HbA1c for the prediction of diabetes in a rural community in Korea
B. M. Song, H. C. Kim, J. Y. Lee, J.‐M. Lee, D. J. Kim, Y.‐H. Lee, I. Suh
Diabetic Medicine.2015; 32(12): 1602. CrossRef - The Relationship between BMI and Glycated Albumin to Glycated Hemoglobin (GA/A1c) Ratio According to Glucose Tolerance Status
Ji Hye Huh, Kwang Joon Kim, Byung-Wan Lee, Dong Wook Kim, Eun Seok Kang, Bong Soo Cha, Hyun Chul Lee, Marta Letizia Hribal
PLoS ONE.2014; 9(2): e89478. CrossRef - Additional perspectives on chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka – lessons learned from the WHO CKDu population prevalence study
Jennifer Hoponick Redmon, Myles F Elledge, Donna S Womack, Rajitha Wickremashinghe, Kamani P Wanigasuriya, Roshini J Peiris-John, Joseph Lunyera, Kristin Smith, James H Raymer, Keith E Levine
BMC Nephrology.2014;[Epub] CrossRef - Diagnostic Efficiency of Hemoglobin A1c for Newly Diagnosed Diabetes and Prediabetes in Community-Based Chinese Adults Aged 40 Years or Older
Kai Liang, Yu Sun, Wen-juan Li, Xiu-ping Zhang, Cheng-qiao Li, Wei-fang Yang, Ze-qiang Ma, Ai-xia Ma, Hui-zhen Zheng, Jun Song, Peng Lin, Xin-guo Hou, Li Chen
Diabetes Technology & Therapeutics.2014; 16(12): 853. CrossRef - Diagnostic accuracy of HbA1c in diabetes between Eastern and Western
Shuang Yan, Siying Liu, Yashuang Zhao, Wencui Zhang, Xiaohui Sun, Jianing Li, Fuli Jiang, Jiaming Ju, Ning Lang, Yingqi Zhang, Weiyu Zhou, Qiang Li
European Journal of Clinical Investigation.2013; 43(7): 716. CrossRef

 KDA
KDA
 PubReader
PubReader Cite
Cite





