- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 36(4); 2012 > Article
-
Original ArticleObesity and Metabolic Syndrome Intracerebroventricular Injection of Metformin Induces Anorexia in Rats
- Chang Koo Lee1, Yoon Jung Choi1, So Young Park1, Jong Yeon Kim1, Kyu Chang Won2, Yong Woon Kim1
-
Diabetes & Metabolism Journal 2012;36(4):293-299.
DOI: https://doi.org/10.4093/dmj.2012.36.4.293
Published online: August 20, 2012
1Department of Physiology, Yeungnam University College of Medicine, Daegu, Korea.
2Department of Internal Medicine, Yeungnam University College of Medicine, Daegu, Korea.
- Corresponding author: Yong Woon Kim. Department of Physiology, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 705-717, Korea. ywkim@med.yu.ac.kr
• Received: February 15, 2012 • Accepted: March 27, 2012
Copyright © 2012 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Metformin, an oral biguanide insulin-sensitizing agent, is well known to decrease appetite. Although there is evidence that metformin could affect the brain directly, the exact mechanism is not yet known.
-
Methods
- To evaluate whether metformin induces anorexia via the hypothalamus, various concentrations of metformin were injected into the lateral ventricle of rats through a chronically implanted catheter and food intake was measured for 24 hours. The hypothalamic neuropeptides associated with regulation of food intake were also analyzed following 1 hour of intracerebroventricular (ICV) injections of metformin.
-
Results
- An ICV injection of metformin decreased food intake in a dose-dependent manner in unrestrained conscious rats. Hypothalamic phosphorylated AMP-activated protein kinase (pAMPK) increased by 3 µg with metformin treatment, but there was no further increase in pAMPK with increases in metformin dosage. The hypothalamic phosphorylated signal transducer and activator of transcription 3 (pSTAT3) increased by 3 µg with metformin treatment, but, there was no further increase in pSTAT3 level following increases of metformin dosage. Hypothalamic proopiomelanocortin was elevated with metformin treatment, while neuropeptide Y was not significantly changed.
-
Conclusion
- Our results suggest that metformin induces anorexia via direct action in the hypothalamus and the increase in pSTAT3, at least in part, is involved in the process. However, hypothalamic pAMPK appears not to contribute to metformin-induced appetite reduction in normal rats. Further studies exploring new pathways connecting metformin and feeding regulation are needed.
- Metformin is the preferred insulin-sensitizing drug in obese diabetes patients due to it suppresses appetite and reduces body weight [1-3]. Metformin acts in the liver and muscle by activating AMP-activated protein kinase (AMPK), and is known to increase the activity of insulin [4-6]. Metformin reportedly activates AMPK through a series of sequential steps through phosphoinositide-3-kinase (PI3K), protein kinase C (PKC)-ζ, and liver kinase B1 [7].
- Appetite suppression by metformin is more prominent in obese model with hyperleptinemia than in lean control [8], and therefore has more clinical significance. However, the mechanism has not yet been completely investigated. Although several reports state that metformin suppresses appetite by increasing the insulin sensitivity of peripheral tissues [2,3], other studies suggest that additional factors affecting the appetite are active in the brain. Kim et al. [8] reported an increase in the phosphorylated signal transducer and activator of transcription 3 (pSTAT3) in the hypothalamus after metformin injections. Billa et al. [9] reported a change in luteinizing hormone and prolactin as a result of metformin action in the pituitary gland of patients with polycystic ovary syndrome. Vigersky et al. [10] reported biochemical or clinical thyroid-stimulating hormone suppression without thyroid hyperactivity in patients who were taking metformin, and discussed the possibility of metformin affecting the brain directly. However, effects on appetite suppression through direct in vivo metformin action in the brain have not yet been reported.
- Currently, metformin is known to increase the only identified target substance, AMPK, in muscles and the liver. However, the mechanism of action in the hypothalamus has yet to be identified [11]. Recently, Chau-Van et al. [12] reported that metformin caused a decrease in pAMPK concentration in primary cultures of hypothalamic cells. Ropelle et al. [13] reported that orally administered metformin increased pAMPK in the hypothalamus. Overall, results for the mechanism of metformin in the hypothalamus are conflicting, thus clarification is necessary.
- The present study was performed in order to investigate the direct effects of metformin on the brain, particularly in the hypothalamus, and the mechanisms of those effects by delivering metformin through a cerebroventricular injection in order for changes in food intake to be observed. The appetite-regulating proteins of the hypothalamus were also analyzed.
INTRODUCTION
- Experimental animals
- Male Sprague-Dawley rats weighing ~250 g were purchased from Samtako (Seoul, Korea). All experimental animals were kept by animal specialists from the Yeungnam University College of Medicine. Animals were given roughly 1 week to acclimate before they were used in the experiment. The rats were exposed to 12-hour light/dark cycles. Light exposure began at 7:00 AM and ended at 7:00 PM. The animals were cared for in accordance with the principles of the Guide to the Care and Use of Experimental Animals of the Yeungnam Medical Center.
- Experimental design
- To investigate whether metformin acts directly on the brain to suppress appetite, various concentrations of metformin (0, 3, 30, or 300 µg) (Sigma Chemical Co., St. Louis, MO, USA) were injected into the left cerebral ventricle and the food intake of the rats was measured and evaluated for 24 hours.
- The dosages of metformin were determined by a modification of previous report [14] that metformin (320 mg/kg/day) caused an increase in in vivo skeletal muscle glucose consumption.
- To determine the mechanism of metformin, varying concentrations were injected into the cerebral ventricle (0, 3, 30, or 300 µg). After 1 hour, the hypothalamus was extracted, the amount of the diet control signals pAMPK, pSTAT3, and PI3K and the expression of proopiomelanocortin (POMC) and neuropeptide Y (NPY) mRNA were measured and compared.
- Placement of a chronic intracerebroventricular (ICV) catheter
- The rats were anethesized with intra-peritoneal injections of 25 mg/kg of tiletamine and zolazepam (Zoletil®; Virbac, Carros, France) and 8 mg/kg of xylazine hydrochloride. After anesthesia, rats were fixed with stereotaxic instruments, an incision was made in the flesh along the midline, and a hole was made 1.3 mm posterior, 1.9 mm lateral, and 3 mm deep from the bregma. A cannula made by 21-gauge syringe needle was inserted into the lateral ventricle and the orifice was occupied with a wire sized to fit inside the cannula. The brain cannula was secured to the surface of the skull using a jewler's screw and acrylic dental cement. Drug injections were given 1 week after surgery to allow for recovery.
- ICV administration of drug
- In order to observe short-term dietary intake, the wire placed earlier was removed in freely moving rats, a pre-made syringe needle that matched the length of the cannula was inserted, and an injection was given over a period of 3 minutes. The syringe was removed 3 minutes after the injection. The injection consisted of 5 µL of saline solution with 0, 3, 30, or 300 µg of metformin. Injections were given at 4:30 PM in order to measure food intake activity at night. At 3, 7, 16, and 24 hours after the injection, food intake was measured.
- In order to observe signal transduction pathways in the hypothalamus after metformin was administered, rats underwent lateral cerebroventricular intubation while anesthetized after which the aforementioned procedure with different amounts of metformin (0, 3, 30, or 300 µg) was administered. Metformin solution was matched with 5 µL applied over 3 minutes and removed 3 minutes afterwards. After 1 additional hour, the hypothalamus was removed and examined.
- Sample collection
- At the end of the experiment, the rats were injected with a double dosage of anesthetic prior to 9:00 AM. The hypothalamus was then harvested, stored in liquid nitrogen, and analyzed using the method employed by Kim et al. [15]. Briefly, the hypothalamus, which is located at the base of the brain along the optic nerve margin and typically 3 mm deep, was collected. The extracted hypothalamus was bisected down the centerline, and was used for analysis of proteins and gene expression.
- Western blot
- Appetite-related neural peptides in the hypothalamus were analyzed using a Western blot. STAT3, pSTAT3, AMPK, pAMPK, and PI3K were targeted for the analysis of signal transduction protein products. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal standard. After 30 µg of hypothalamus protein was separated on a 10% polyacrylamide gel, the nitrocellulose membrane was removed, antibodies for each protein (Cell Signaling, Danvers, MA, USA) were fixed, the membranes were washed, and secondary antibodies prepared. After a combined blot was presented using an ECL kit (Amersham Biosciences, Little Chalfont, UK), quantified analysis was performed using Scion Image Software (NIH, Bethesda, MD, USA). Each blot removed attached antibodies, and another antibody fixing method was performed.
- Real-time polymerase chain reaction (PCR)
- Gene expression of the hypothalamus was analyzed using real-time PCR. To summarize the process of RNA extraction, 1 mL TRI-reagent (Sigma Chemical Co.) was added to the hypothalamic tissue and homogenized using ultrasound. Next, 200 µL of chloroform was added and the mixture was shaken vigorously. The sample was left at room temperature for 5 minutes to allow for the reactions to occur. Afterwards, the sample was centrifuged at 13,200 rpm for 15 minutes at 4℃. An equal volume of isopropanol was added to the separated supernatant and left at room temperature for 10 minutes. The sample was then centrifuged at 13,200 rpm for 10 minutes at 4℃ to pellet precipitated RNA. The precipitated RNA was washed with 1 mL of cold 75% ethanol, centrifuged at 13,200 rpm at 4℃ for 5 minutes, and dried at room temperature. Finally, the remaining RNA was dissolved in 30 µL RNase free water and stored at -70℃. RNA concentration was measured using a Nano-drop (Thermo, Wilmington, MA, USA) between 260 and 280 nm wavelengths. Briefly, to amplify the RNA the isolated 1 µg RNA was reacted for 5 minutes at 70℃, oligo (dT) primer was added to Prime RT Premix (iNtRON, Seongnam, Korea) and was kept at 42℃ for 60 minutes and 70℃ for 10 minutes to produce the cDNA. Afterwards, FastStart Taq DNA polymerase (LightCycler FastStart DNA Master SYBR Green I; Roche, Mannheim, Germany), 3 mM MgCl2, and 0.4 µM of primer was added and amplified in a LightCycler 1.5 Instrument at 95℃ for 10 minutes. After the process was completed, the sample was denatured at 95℃ for 10 seconds, annealed at 54℃ to 60℃ for 5 seconds, and extended at 72℃ for 4 to 9 seconds. This process was repeated 45 times. Once the reactions were completed, to confirm the specificity of the amplification, the temperature was decreased to 65℃, and gradually increased by 0.2℃/sec until the temperature reached 95℃. The PCR products were then analyzed. β-actin was used as an internal standard. The primer sequences used are shown in Table 1.
- Statistical analysis
- Values were expressed as mean±standard error. For the cumulative dietary intake relative to metformin dosage concentration, the concentration dependence was evaluated as the P trend qualification. The comparison of the amount of expression of signaling proteins and neuropeptides in each group was performed using a one-way ANOVA test, and a post-hoc test was performed using Duncan.
METHODS
Experiment 1
Experiment 2
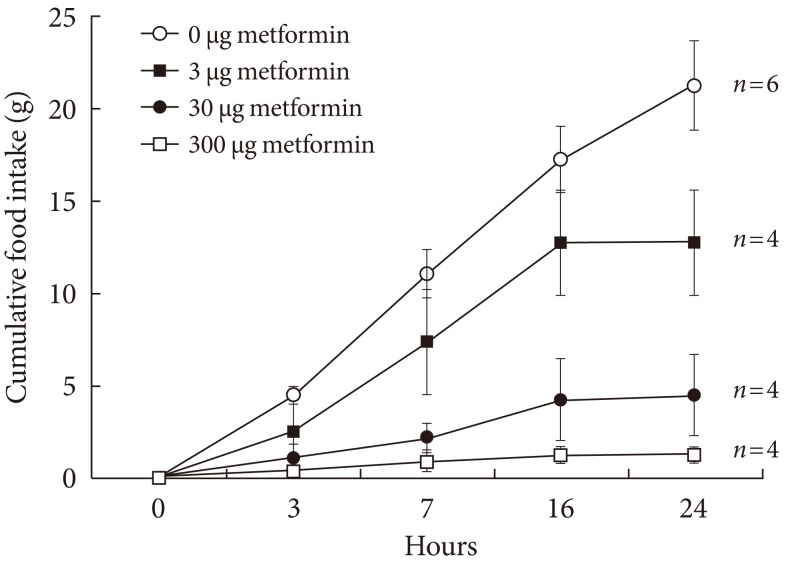
- In order to observe how metformin reduces appetite by acting directly on the brain, a long-term catheter was installed which administered metformin. The 24-hour cumulative food intake was recorded and is shown in Fig. 1. The cumulative food intake appeared to decrease as metformin concentration increased, reflecting concentration dependence.
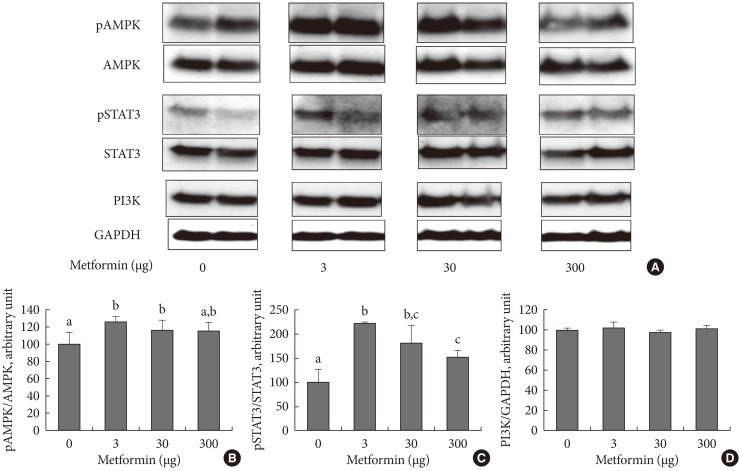
- In order to examine the appetite suppressing mechanism of metformin, the hypothalamus was collected 1 hour after an injection was made into the cerebral ventricle and signaling molecules involved with appetite regulation were analyzed. pAMPK increased in the hypothalamus when 3 µg of metformin was applied, but there was no further change as metformin dosage increased. Phosphorylation of STAT3 increased as a result of metformin, but further changes did not occur as a result of increasing metformin dosage. PI3K did not show any specific changes (Fig. 2).
- The effects of metformin on the appetite controlling hypothalamic peptides POMC and NPY were observed. POMC mRNA, which is known to suppress appetite, increased in the presence of metformin but was not dependent on concentration, and NPY mRNA did not decrease in the presence of metformin (Fig. 3).
RESULTS
- It has been suggested that the appetite suppressing mechanism of metformin can be achieved by being applied directly to the brain [8-10,12]. However, no studies have been published investigating the effects on appetite suppression of metformin administered in vivo directly to the brain.
- In the present study, in order to observe the effects of metformin on the brain, specifically the hypothalamus, metformin was injected directly into the cerebral ventricle. The decrease in food intake was examined for 24 hours after metformin was administered in a dose-dependent manner (Fig. 1). In order to determine whether the loss of appetite due to metformin was reversible, food intake was analyzed 48, 72, and 96 hours after metformin injections (data not shown). Food intake returned to normal after 24 to 48 hours after 3 and 30 µg of metformin was administered, and after 72 to 96 hours after 300 µg of metformin was administered. These results imply that metformin can act directly to suppress appetite in the brain.
- In order to clarify the central appetite-suppressing mechanism of metformin, metformin was injected into the cerebral ventricle, the hypothalamus removed, and appetite-related signaling molecules (AMPK, STAT3, PI3K) levels and neuropeptide (POMC and NPY) expression were analyzed.
- AMPK is a metabolic sensor which detects cellular energy and reacts appropriately. When AMP increases, activation by phosphorylation occurs [16,17]. The increase in pAMPK in skeletal muscle increases energy production during metabolism. However, an increase of pAMPK in the hypothalamus increases appetite. Conversely, the reduction of pAMPK is known to suppress appetite [18,19]. The appetite-increasing effect of phosphorylated AMPK is known to occur through increased NPY, which is blocked by treatment of compound C, a known AMPK inhibitor [12]. AMPK is also controlled by leptin; however, leptin activates AMPK in skeletal muscle and is known to reduce pAMPK in the hypothalamus [11]. In the present study, metformin injections appeared to increase pAMPK. These results were consistent with the results reported by Ropelle et al. [13] that metformin injections caused an increase in pAMPK in cancer cell inoculated rats. However, there was a difference with the in vitro results reported by Chau-Van et al. [12]. The differences were not only due to the difference in the in vitro and in vivo environments, but Chau-Van et al. [12] examined the metformin's effect on a glucose-deprived in vitro condition which elevates pAMPK markedly. In the aforementioned study, when metformin was administered to hypothalamic neurons when glucose levels were normal the pAMPK inhibition effect was not significantly apparent [12]. ICV metformin treatment suppressed appetite, but failed to reduce pAMPK in this study, suggest that the AMPK pathway has little association with reduced appetite or metformin under normal conditions. Further research on the effects of metformin on the hypothalamus in patients with hyperglycemia or hyperlipidemia is required.
- STAT3 is activated by phosphorylation through tyrosine kinase activity associated with leptin receptor autophosphorylation [20]. The increase in phosphorylated STAT3 leads to the increase of POMC and allows for appetite suppression [21]. In the present study, pSTAT3 increased starting at 3 µg of metformin, but when the metformin dosage was increased to 300 µg, pSTAT3 did not increase any further. Although the appetite-suppressing effect of metformin is related with pSTAT3 elevation at least in part, since there was not shown any concentration-dependent increase of pSTAT3 by metformin, this was not considered to be the primary action mechanism. PI3K, another pathway that carry appetite and related signals, activates POMC neurons and is also known to contribute to insulin action [22,23]. In the present study, hypothalamic PI3K did not show any particular change after metformin administration, suggesting detailed studies on mechanisms are required due to the possibility of several forms of action of metformin on the brain. If a new appetite control pathway can be discovered, it could possibly be manipulated for treatment of obesity.
- In the present study, upon metformin injection, through changes in neuropeptides, POMC mRNA increased, but NPY mRNA did not appear to decrease. Changes in POMC mRNA had a tendency to be very similar to changes in pSTAT3. Changes in NPY mRNA had a tendency to be similar to changes in pAMPK observed in the present study and were consistent with reports from other studies [12,24]. Rouru et al. [25] suppressed appetites in Zucker rats using metformin, but reported that there was no reduction in NPY. Taken together, the appetite suppression action of metformin may not be a result of the inhibition of pAMPK and NPY.
- In conclusion, the result of injecting metformin into the lateral ventricle of rats produced concentration-dependent appetite suppression, and the direct suppression of appetite through metformin to the brain could be confirmed. One hour after metformin was injected into the lateral ventricle, hypothalamic pSTAT3 increased, but concentration dependence was not observed. However, metformin did not decrease the amount of hypothalamic pAMPK. Thus, inhibition of appetite by metformin is thought to be partially due to the increase in pSTAT3, although other studies investigating mechanisms besides STAT3, AMPK, and PI3K are considered necessary.
DISCUSSION
-
Acknowledgements
- This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0069055).
ACKNOWLEDGMENTS
- 1. Morin-Papunen LC, Koivunen RM, Tomas C, Ruokonen A, Martikainen HK. Decreased serum leptin concentrations during metformin therapy in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 1998;83:2566-2568. ArticlePubMed
- 2. Paolisso G, Amato L, Eccellente R, Gambardella A, Tagliamonte MR, Varricchio G, Carella C, Giugliano D, D'Onofrio F. Effect of metformin on food intake in obese subjects. Eur J Clin Invest 1998;28:441-446. ArticlePubMedPDF
- 3. Kay JP, Alemzadeh R, Langley G, D'Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism 2001;50:1457-1461. ArticlePubMed
- 4. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167-1174. ArticlePubMedPMC
- 5. Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 2002;51:2420-2425. ArticlePubMedPDF
- 6. Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002;51:2074-2081. ArticlePubMedPDF
- 7. Xie Z, Dong Y, Scholz R, Neumann D, Zou MH. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation 2008;117:952-962. ArticlePubMedPMC
- 8. Kim YW, Kim JY, Park YH, Park SY, Won KC, Choi KH, Huh JY, Moon KH. Metformin restores leptin sensitivity in high-fat-fed obese rats with leptin resistance. Diabetes 2006;55:716-724. ArticlePubMedPDF
- 9. Billa E, Kapolla N, Nicopoulou SC, Koukkou E, Venaki E, Milingos S, Antsaklis A, Adamopoulos DA. Metformin administration was associated with a modification of LH, prolactin and insulin secretion dynamics in women with polycystic ovarian syndrome. Gynecol Endocrinol 2009;25:427-434. ArticlePubMed
- 10. Vigersky RA, Filmore-Nassar A, Glass AR. Thyrotropin suppression by metformin. J Clin Endocrinol Metab 2006;91:225-227. ArticlePubMedPDF
- 11. Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004;428:569-574. ArticlePubMedPDF
- 12. Chau-Van C, Gamba M, Salvi R, Gaillard RC, Pralong FP. Metformin inhibits adenosine 5'-monophosphate-activated kinase activation and prevents increases in neuropeptide Y expression in cultured hypothalamic neurons. Endocrinology 2007;148:507-511. ArticlePubMed
- 13. Ropelle ER, Pauli JR, Zecchin KG, Ueno M, de Souza CT, Morari J, Faria MC, Velloso LA, Saad MJ, Carvalheira JB. A central role for neuronal adenosine 5'-monophosphate-activated protein kinase in cancer-induced anorexia. Endocrinology 2007;148:5220-5229. ArticlePubMedPDF
- 14. Borst SE, Snellen HG, Ross H, Scarpace PJ, Kim YW. Metformin restores responses to insulin but not to growth hormone in Sprague-Dawley rats. Biochem Biophys Res Commun 2002;291:722-726. ArticlePubMed
- 15. Kim YW, Choi DW, Park YH, Huh JY, Won KC, Choi KH, Park SY, Kim JY, Lee SK. Leptin-like effects of MTII are augmented in MSG-obese rats. Regul Pept 2005;127:63-70. ArticlePubMed
- 16. Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 2004;279:12005-12008. ArticlePubMed
- 17. Lee K, Li B, Xi X, Suh Y, Martin RJ. Role of neuronal energy status in the regulation of adenosine 5'-monophosphate-activated protein kinase, orexigenic neuropeptides expression, and feeding behavior. Endocrinology 2005;146:3-10. ArticlePubMedPDF
- 18. Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem 2004;279:19970-19976. ArticlePubMed
- 19. Kim MS, Park JY, Namkoong C, Jang PG, Ryu JW, Song HS, Yun JY, Namgoong IS, Ha J, Park IS, Lee IK, Viollet B, Youn JH, Lee HK, Lee KU. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med 2004;10:727-733. ArticlePubMedPDF
- 20. Shek EW, Scarpace PJ. Resistance to the anorexic and thermogenic effects of centrally administrated leptin in obese aged rats. Regul Pept 2000;92:65-71. ArticlePubMed
- 21. Scarpace PJ, Matheny M, Tumer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience 2001;104:1111-1117. ArticlePubMed
- 22. Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 2008;118:1796-1805. ArticlePubMedPMC
- 23. Hill JW, Xu Y, Preitner F, Fukuda M, Cho YR, Luo J, Balthasar N, Coppari R, Cantley LC, Kahn BB, Zhao JJ, Elmquist JK. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology 2009;150:4874-4882. ArticlePubMedPMCPDF
- 24. Scarpace PJ, Matheny M, Tumer N, Cheng KY, Zhang Y. Leptin resistance exacerbates diet-induced obesity and is associated with diminished maximal leptin signalling capacity in rats. Diabetologia 2005;48:1075-1083. ArticlePubMedPDF
- 25. Rouru J, Pesonen U, Koulu M, Huupponen R, Santti E, Virtanen K, Rouvari T, Jhanwar-Uniyal M. Anorectic effect of metformin in obese Zucker rats: lack of evidence for the involvement of neuropeptide Y. Eur J Pharmacol 1995;273:99-106. ArticlePubMed
REFERENCES
Fig. 1Cumulative food intake following injection of various dosages of metformin into the lateral ventricle through a chronically implanted catheter in conscious unrestrained rats. Values are presented as mean±standard error. The cumulative food intake following metformin injection decreased in a dose-dependent manner analyzed by the P trend test with ANOVA (P trend <0.05).
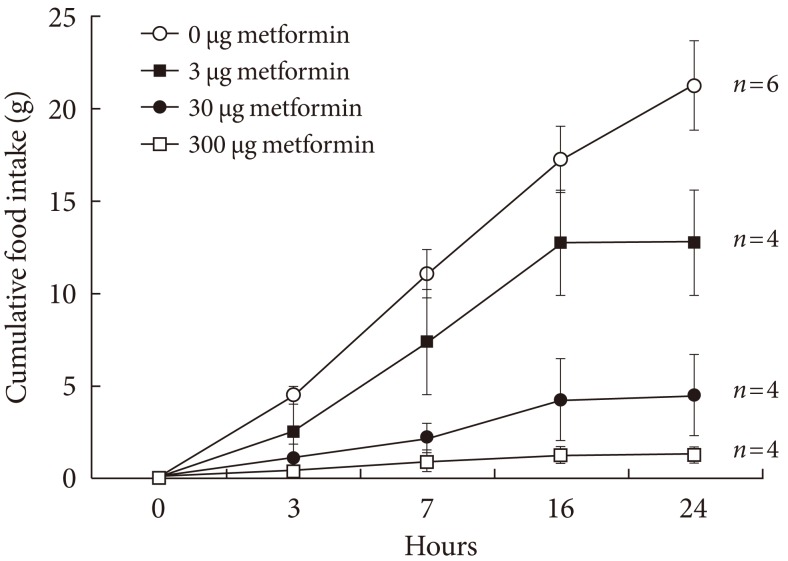

Fig. 2The hypothalamic levels of phosphorylated AMP-activated protein (pAMPK) and phosphorylated signal transducer, activator of transcription 3 (pSTAT3), and phosphoinositide-3-kinase (PI3K) following intracerebroventricular injection of metformin. Representative blots are shown in (A) and the densitometric data are denoted in (B), (C), and (D). Values are presented as mean±standard error of 6, 5, 4, and 6 in 0, 3, 30, or 300 µg of metformin-treated rats, respectively. Values that do not share a common superscript are significantly different at P<0.05.
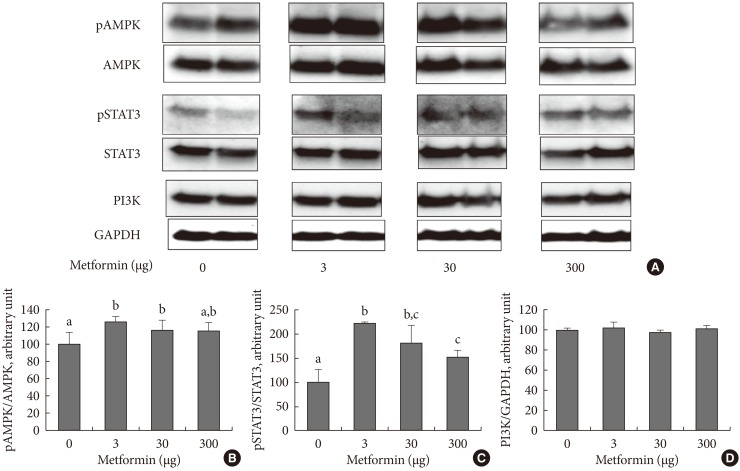

Fig. 3Expression of proopiomelanocortin (POMC) (A) and neuropeptide Y (NPY) (B) in the hypothalamus following intracerebroventricular injection of metformin. Expression was measured by real-time polymerase chain reaction. Bars are mean±standard error. The number of cases are 6, 5, 4, and 6 in 0, 3, 30, or 300 µg of metformin-treated rats, respectively. Values that do not share a common superscript are significantly different at P<0.05.
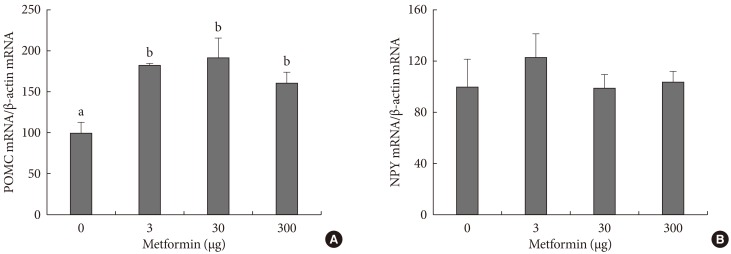

Figure & Data
References
Citations
Citations to this article as recorded by 

- Metabolic and Metabolomic Effects of Metformin in Murine Model of Pulmonary Adenoma Formation
Andrew C. Elton, Vannesa Cedarstrom, Arman Quraishi, Beverly Wuertz, Kevin Murray, Todd W. Markowski, Donna Seabloom, Frank G. Ondrey
Nutrition and Cancer.2023; 75(3): 1014. CrossRef - Steroidogenic Effect of Luteinizing Hormone Receptor Agonists and Metformin in Male Rats with Androgenic Deficiency Caused by Diet-Induced Obesity
A. A. Bakhtyukov, K. V. Derkach, I. A. Lebedev, V. N. Sorokoumov, A. O. Shpakov
Journal of Evolutionary Biochemistry and Physiology.2023; 59(5): 1810. CrossRef - Steroidogenic Effect of Luteinizing Hormone Receptor Agonists and Metformin in Male Rats with Androgenic Deficiency Caused by Diet-Induced Obesity
A. A. Bakhtyukov, K. V. Derkach, I. A. Lebedev, V. N. Sorokoumov, A. O. Shpakov
Российский физиологический журнал им И М Сеченова.2023; 109(10): 1414. CrossRef - Metformin in nucleus accumbens core reduces cue‐induced cocaine seeking in male and female rats
Amy Chan, Alexis Willard, Sarah Mulloy, Noor Ibrahim, Allegra Sciaccotta, Mark Schonfeld, Sade M. Spencer
Addiction Biology.2022;[Epub] CrossRef - Knockdown of Endogenous Nucb2/Nesfatin-1 in the PVN Leads to
Obese-Like Phenotype and Abolishes the Metformin- and Stress-Induced Thermogenic
Response in Rats
Daniel Stephan, Natalie Taege, Riccardo Dore, Julica Folberth, Olaf Jöhren, Markus Schwaninger, Hendrik Lehnert, Carla Schulz
Hormone and Metabolic Research.2022; 54(11): 768. CrossRef - Modulation of hypothalamic AMPK phosphorylation by olanzapine controls energy balance and body weight
Vitor Ferreira, Cintia Folgueira, Maria Guillén, Pablo Zubiaur, Marcos Navares, Assel Sarsenbayeva, Pilar López-Larrubia, Jan W. Eriksson, Maria J. Pereira, Francisco Abad-Santos, Guadalupe Sabio, Patricia Rada, Ángela M. Valverde
Metabolism.2022; 137: 155335. CrossRef - Metformin acts on the gut-brain axis to ameliorate antipsychotic-induced metabolic dysfunction
Xiaorong Wang, Huimin Huang, Yiyi Zhu, Shaoli Li, Peifen Zhang, Jiajun Jiang, Caixi Xi, Lingling Wu, Xingle Gao, Yaoyang Fu, Danhua Zhang, Yiqing Chen, Shaohua Hu, Jianbo Lai
BioScience Trends.2021; 15(5): 321. CrossRef - Therapeutic effect of treatment with metformin and/or 4-hydroxychalcone in male Wistar rats with nonalcoholic fatty liver disease
Selene de Jesús Acosta-Cota, Elsa Maribel Aguilar-Medina, Rosalío Ramos-Payán, José Guadalupe Rendón Maldonado, José Geovanni Romero-Quintana, Julio Montes-Avila, Juan I. Sarmiento-Sánchez, Carolina Gabriela Plazas-Guerrero, Marcela J. Vergara-Jiménez, Ar
European Journal of Pharmacology.2019; 863: 172699. CrossRef - The evidence of metabolic-improving effect of metformin in Ay/a mice with genetically-induced melanocortin obesity and the contribution of hypothalamic mechanisms to this effect
Kira Derkach, Irina Zakharova, Inna Zorina, Andrey Bakhtyukov, Irina Romanova, Liubov Bayunova, Alexander Shpakov, Guillermo López Lluch
PLOS ONE.2019; 14(3): e0213779. CrossRef - Effect of Metformin on Antipsychotic-Induced Metabolic Dysfunction: The Potential Role of Gut-Brain Axis
Chao Luo, Xu Wang, Hanxue Huang, Xiaoyuan Mao, Honghao Zhou, Zhaoqian Liu
Frontiers in Pharmacology.2019;[Epub] CrossRef - Metformin alters signaling induced crosstalk and homeostasis in the carcinogenesis paradigm “Epistemology of the origin of cancer”
Björn L.D.M. Brücher, Ijaz S. Jamall, Obul R. Bandapalli
4open.2019; 2: 12. CrossRef - Melatonin potentiates the effects of metformin on glucose metabolism and food intake in high‐fat‐fed rats
Rosana F. Dantas‐Ferreira, Helene Raingard, Stephanie Dumont, Carole Schuster‐Klein, Beatrice Guardiola‐Lemaitre, Paul Pevet, Etienne Challet
Endocrinology, Diabetes & Metabolism.2018;[Epub] CrossRef -
Molecular Mechanisms of the Effects of Metformin on the Functional Activity of Brain Neurons
A. O. Shpakov, K. V. Derkach
Neuroscience and Behavioral Physiology.2018; 48(8): 969. CrossRef - Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: in vitro and in vivo studies
Ali Taghizadehghalehjoughi, Ahmet Hacimuftuoglu, Meltem Cetin, Afife Busra Ugur, Bianca Galateanu, Yaroslav Mezhuev, Ufuk Okkay, Numan Taspinar, Mehmet Taspinar, Abdullah Uyanik, Betul Gundogdu, Maryam Mohammadzadeh, Kemal Alp Nalci, Polychronis Stivaktak
Nanomedicine.2018; 13(13): 1595. CrossRef - Effect of Betahistine and Metformin on Antipsychotic-Induced Weight Gain: An Analysis of Two Clinical Trials
Dongyu Kang, Zhihui Jing, Ranran Li, Gangrui Hei, Tiannan Shao, Li Li, Mengxi Sun, Ye Yang, Ying Wang, Xiaoyi Wang, Yujun Long, Xiansheng Huang, Renrong Wu
Frontiers in Psychiatry.2018;[Epub] CrossRef - Metformin: not only per os
Lev M. Berstein
Expert Review of Endocrinology & Metabolism.2018; 13(2): 63. CrossRef - МЕТАБОЛИЧЕСКИЕ ПОКАЗАТЕЛИ И ФУНКЦИОНАЛЬНОЕ СОСТОЯНИЕ СИГНАЛЬНЫХ СИСТЕМ ГИПОТАЛАМУСА И ВЛИЯНИЕ НА НИХ МЕТФОРМИНА У МЫШЕЙ С МУТАЦИЕЙ AY/A, ГЕНЕТИЧЕСКИ ПРЕДРАСПОЛОЖЕННЫХ К ОЖИРЕНИЮ, "Доклады Академии наук"
К.В. Деркач, И.О. Захарова, И.В. Романова, И. И. Зорина, А.Л. Михрина, А.О. Шпаков
Доклады Академии Наук.2017; (4): 488. CrossRef - Beneficial effects of metformin on energy metabolism and visceral fat volume through a possible mechanism of fatty acid oxidation in human subjects and rats
Ichiro Tokubuchi, Yuji Tajiri, Shimpei Iwata, Kento Hara, Nobuhiko Wada, Toshihiko Hashinaga, Hitomi Nakayama, Hiroharu Mifune, Kentaro Yamada, M. Faadiel Essop
PLOS ONE.2017; 12(2): e0171293. CrossRef - Metabolic parameters and functional state of hypothalamic signaling systems in AY/a mice with genetic predisposition to obesity and the effect of metformin
K. V. Derkach, I. O. Zakharova, I. V. Romanova, I. I. Zorina, A. L. Mikhrina, A. O. Shpakov
Doklady Biochemistry and Biophysics.2017; 477(1): 377. CrossRef - Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice
Joanne S. Allard, Evelyn J. Perez, Koji Fukui, Priscilla Carpenter, Donald K. Ingram, Rafael de Cabo
Behavioural Brain Research.2016; 301: 1. CrossRef - Intracerebroventricular Metformin Decreases Body Weight But Has Pro-oxidant Effects and Decreases Survival
Luis Valmor Portela, Jussania Gnoatto, Andressa Wigner Brochier, Clarissa Branco Haas, Adriano Martimbianco de Assis, Afonso Kopczynski de Carvalho, Gisele Hansel, Eduardo Rigon Zimmer, Jean Pierre Oses, Alexandre Pastoris Muller
Neurochemical Research.2015; 40(3): 514. CrossRef - Metformin alleviates hepatosteatosis by restoring SIRT1-mediated autophagy induction via an AMP-activated protein kinase-independent pathway
Young Mi Song, Yong-ho Lee, Ji-Won Kim, Dong-Sik Ham, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2015; 11(1): 46. CrossRef - Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: Interference of AMPK/PGC-1α pathway
Ghorbangol Ashabi, Fariba Khodagholi, Leila Khalaj, Mahdi Goudarzvand, Masoumeh Nasiri
Metabolic Brain Disease.2014; 29(1): 47. CrossRef - Acute oral metformin enhances satiation and activates brainstem nesfatinergic neurons
Thaïs Rouquet, Pierre Clément, Stéphanie Gaigé, Catherine Tardivel, Julien Roux, Michel Dallaporta, Bruno Bariohay, Jean-Denis Troadec, Bruno Lebrun
Obesity.2014; : n/a. CrossRef - Metformin—mode of action and clinical implications for diabetes and cancer
Ida Pernicova, Márta Korbonits
Nature Reviews Endocrinology.2014; 10(3): 143. CrossRef - Effects of metformin on weight loss
Steven K. Malin, Sangeeta R. Kashyap
Current Opinion in Endocrinology, Diabetes & Obesity.2014; 21(5): 323. CrossRef - The effect of ghrelin on MK-801 induced memory impairment in rats
Fatemeh Goshadrou, Mojtaba Kermani, Abdolaziz Ronaghi, Samad Sajjadi
Peptides.2013; 44: 60. CrossRef

 KDA
KDA
 PubReader
PubReader Cite
Cite