Glycemic Effects of Once-a-Day Rapid-Acting Insulin Analogue Addition on a Basal Insulin Analogue in Korean Subjects with Poorly Controlled Type 2 Diabetes Mellitus
Article information
Abstract
Background
The present study investigates the efficacy in glycemic control by adding once-a-day glulisine to glargine as a basal plus regimen and factors influencing glycemic control with the basal plus regimen in Korean subjects with type 2 diabetes.
Methods
In the present retrospective study, subjects previously treated with the basal plus regimens for at least 6 months were reviewed. Changes in glycemic profiles and clinical parameters were evaluated.
Results
A total of 87 subjects were ultimately enrolled in this study. At baseline, mean glycated hemoglobin (A1c) and glycated albumin were 8.5% (8.0% to 9.6%) and 25.2±7.6%, respectively. After treatment with the basal plus regimen, patients had significant reductions of A1c at 6 months (0.8±0.1%, P<0.001) and their postprandial glucose levels were decreased by 48.7±10.3 mg/dL (P<0.001). Multiple logistic regression showed old age (odds ratio [OR], 1.25; 95% confidence interval [CI], 1.02 to 1.55), high initial A1c (OR, 22.21; 95% CI, 2.44 to 201.78), and lower amounts of glargine (OR, 0.85; 95% CI, 0.76 to 0.99), and glimepiride (OR, 0.23; 95% CI, 0.06 to 0.93) at baseline were independently associated with good responders whose A1c reduction was more than 0.5%.
Conclusion
The authors suggest a basal plus regimen may be effective in reducing glucose levels of subjects with old age, high initial A1c, and patients on low doses of glimepiride and glargine. Despite the use of high doses of hypoglycemic agents, elderly patients with poorly-controlled diabetes are preferred for early initiation of the basal plus regimen.
INTRODUCTION
Tight glycemic control, as close to the non-diabetic range as possible, without side effects from glucose-lowering agents, such as hypoglycemia, may prevent or delay micro- and macro-vascular complications in patients with type 2 diabetes mellitus (T2DM) [1-3]. If patients fail to achieve or sustain the target glycemic goal, individualized addition of oral anti-diabetic drugs (OADs) or initiation of basal insulin such as glargine or detemir with the aim of achieving a fasting blood glucose (AC) level of less than or equal to 100 mg/dL should be considered according to a consensus statement [4]. Accordingly, for the progressive decline in β-cell function in T2DM [5], basal insulin-based combination of OADs or short-acting insulin is commonly initiated as a regimen for achieving or sustaining a glycemic target [6]. Particularly in Korean subjects, the secretory dysfunction of β-cells is a major contributing factor to the development and aggravation of hyperglycemia in T2DM [7-9]. However, little information is currently available regarding the effects of adding once-a-day glulisine (rapid-acting insulin analogue) to glargine as a basal plus regimen in Korean patients with T2DM. In addition, no evidence has been documented regarding the characteristics of Korean diabetic patients who responded satisfactorily to the basal plus regimen after failure of combination therapy with basal insulin and OADs.
Therefore, the present study analyzed the effects of the basal plus regimen, and the clinical and metabolic characteristics of T2DM patients who were inadequately controlled by the basal insulin-based therapy combined with OADs. The present study investigated the efficacy in glycemic control of the basal plus regimen and factors influencing glycemic control after 6 months of using the basal plus regimen in Korean subjects with T2DM.
METHODS
Patients and research design
In this retrospective study, T2DM patients who satisfied all of the following inclusion criteria were analyzed: 1) subjects registered in the Severance Hospital Diabetes Insulin Education Registry between January 2009 and September 2010; and 2) patients who recently added a once-daily dose of glulisine to basal glargine, a basal insulin analogue injection (basal plus regimen) during the study period. Subjects were excluded for any of the following reasons: subjects with 1) malignancy, 2) severe liver disease corresponding to Child-Pugh class C, 3) chronic kidney disease at stages 4 and 5 (estimated glomerular filtration rate of less than 30 mL/min per 1.73 m2), 4) insulin regimen using more than twice daily administration of glulisine during the study period, 5) discontinuance of the injection of glulisine or glargine, 6) pregnancy, and 7) admitted to the hospital during the study due to another disease. Registered patients received comprehensive education regarding self-management skills, self-monitoring of blood glucose levels, treatment of hypoglycemia, insulin administration, and lifestyle modification at the Severance Diabetes Center. The study protocol was approved by the ethics committee of the Yonsei University College of Medicine.
Changes in glycated hemoglobin (A1c) and glycated albumin (GA) at 3 and 6 months after initiation of the basal plus regimen were evaluated. Dose titrations and changes in concomitant OAD use were also analyzed. Davidson et al. [10] reported that a 24-week-basal plus regimen decreased A1c levels by 0.45% in patients with T2DM who had failed with basal insulin treatment. Based on this data, subjects with A1c reduction of more than 0.5% were considered as good responders. Ultimately enrolled subjects were classified into two groups according to change in basal A1c after 6 months of treatment (Group I, A1c reduction <0.5%; Group II, A1c reduction ≥0.5%).
Laboratory measurements
A1c was measured by high performance liquid chromatography using Variant II Turbo testing system (Bio-Rad Laboratories, Hercules, CA, USA). Serum GA was determined by an enzymatic method using an albumin-specific proteinase, ketoamine oxidase, and albumin assay reagent (LUCICA GA-L; Asahi Kasei Pharma Co., Tokyo, Japan), as well as the Hitachi 7699 P module autoanalyzer (Hitachi Instruments Service, Tokyo, Japan). Plasma glucose was measured using the glucose oxidase method. Plasma triglycerides (TG), total cholesterol, high density lipoprotein cholesterol (HDL-C), blood urea nitrogen (BUN), creatinine (Cr), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels were assayed using a routine Hitachi 7600 autoanalyzer (Hitachi Instruments Service). Low density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation. Pancreatic β-cell secretory function was assessed according to 1) C-peptide increment (ΔC-peptide=postprandial C-peptide-fasting C-peptide) and 2) incline of C-peptide increment (ΔC-peptide/fasting C-peptide) [11].
Statistical analyses
All continuous variables were expressed as mean±standard deviation or median with the interquartile ranges, when variables were not normally distributed. Whether each variable was normally distributed using the Kolmogorov-Smirnov test was examined. The Student's t-test, Mann-Whitney U test, Pearson's chi-square test, and multiple logistic regression analysis were performed to assess the association between responsiveness to the basal plus regimen and various clinical and laboratory parameters, as appropriate. Changes in A1c, GA, AC, and postprandial glucose (PC) at 3 and 6 months after initiation of the basal plus regimen were compared using a paired t-test with the Bonferroni procedure for multiple comparisons. Since A1c, as well as doses of glulisine, glimepiride and metformin showed non-normality based on the Kolmogorov-Smirnov test, the Wilcoxon signed rank test was used to test those variables. The data was analyzed using two sided P values, and a P value less than 0.05 (0.025 in paired t-test with Bonferroni correction) was considered to be statistically significant. All statistical analyses were conducted using the SPSS software for Windows version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Among 124 Korean type 2 diabetic subjects who satisfied the inclusion criteria, 37 subjects were excluded because 14 subjects used rapid-acting insulin more than twice a day, 13 patients discontinued glulisine injection, and 10 patients were admitted to the hospital. Ultimately, 87 patients (50 men, 37 women) were enrolled in the present study.
Clinical and laboratory characteristics of patients at baseline
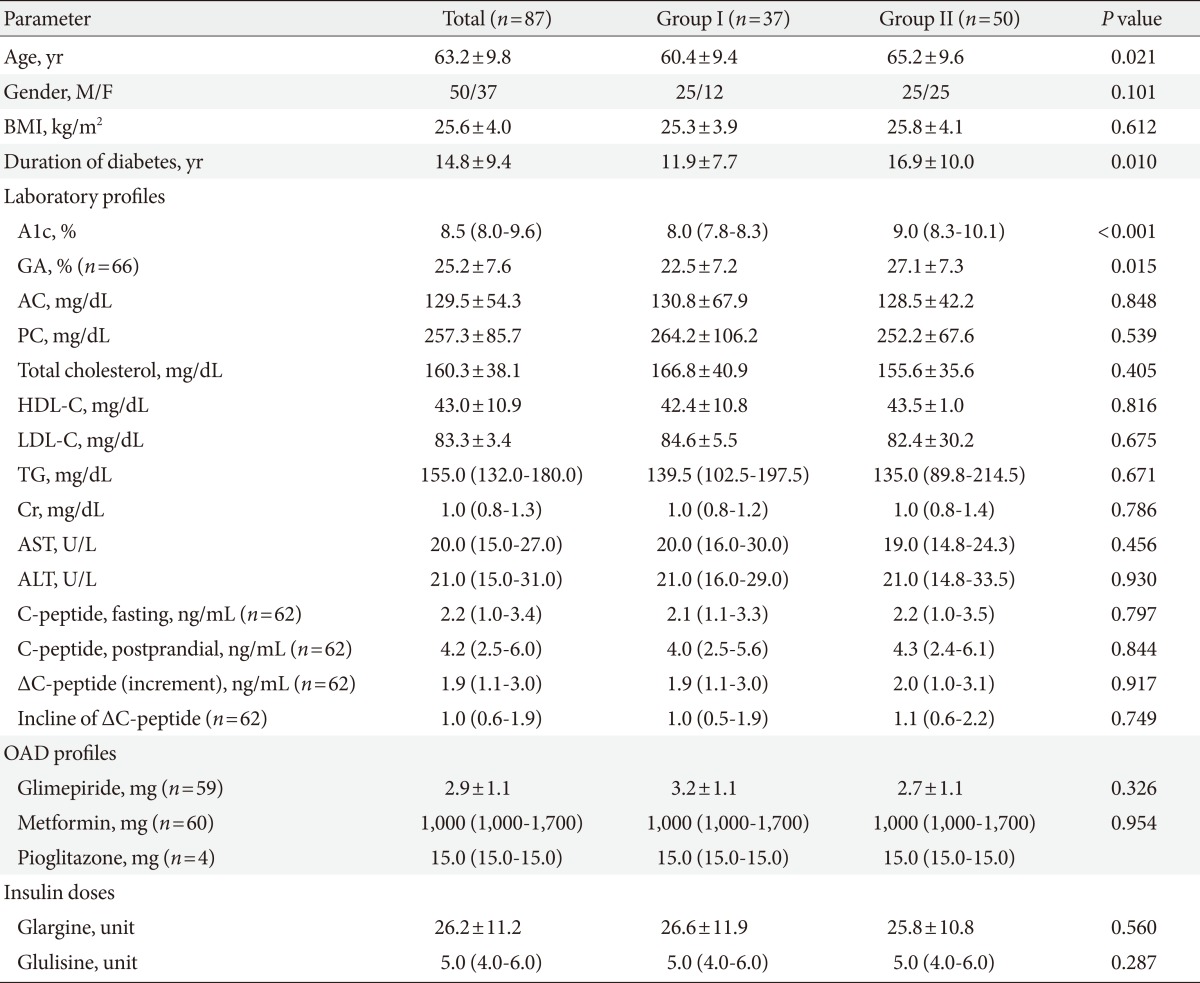
The patients' demographic and clinical characteristics are summarized in Table 1. Mean age and duration of diabetes were 63.2±9.8 and 14.8±9.4 years, respectively. Body mass index (BMI) was 25.6±4.0 kg/m2. The initial levels of A1c, GA, AC, and 2 hour PC after a main meal were as follows: 8.5% (8.0% to 9.6%), 25.2±7.6%, 129.5±54.3 mg/dL, and 257.3±85.7 mg/dL, respectively. Forty-seven subjects used sulfonylurea with a dose of 2.9±1.1 mg glimepiride. The average doses of glargine and glulisine were 26.2±11.2 and 5.0 units (4.0 to 6.0 units), respectively.
Patients were classified into two groups according to A1c change after 6 months of treatment (Group I <0.5%, n=37; Group II ≥0.5%, n=50). The distributions of gender and BMI among subjects were relatively even. There were no significant differences in AC, PC, Cr, AST, ALT levels, lipid profiles, and doses of medications including glimepiride, metformin, glargine, and glulisine. When comparing to Group I, the patients in Group II were older (P=0.021) and had longer duration of diabetes (P=0.01). Group II had more subjects with higher levels of A1c and GA (P<0.001 and P=0.015, respectively) than Group I. With respect to insulin secretory function of beta cells, C-peptide increment and incline of ΔC-peptide levels were not significantly different between the two groups.
Glycemic control with basal plus regimen at 3 and 6 months
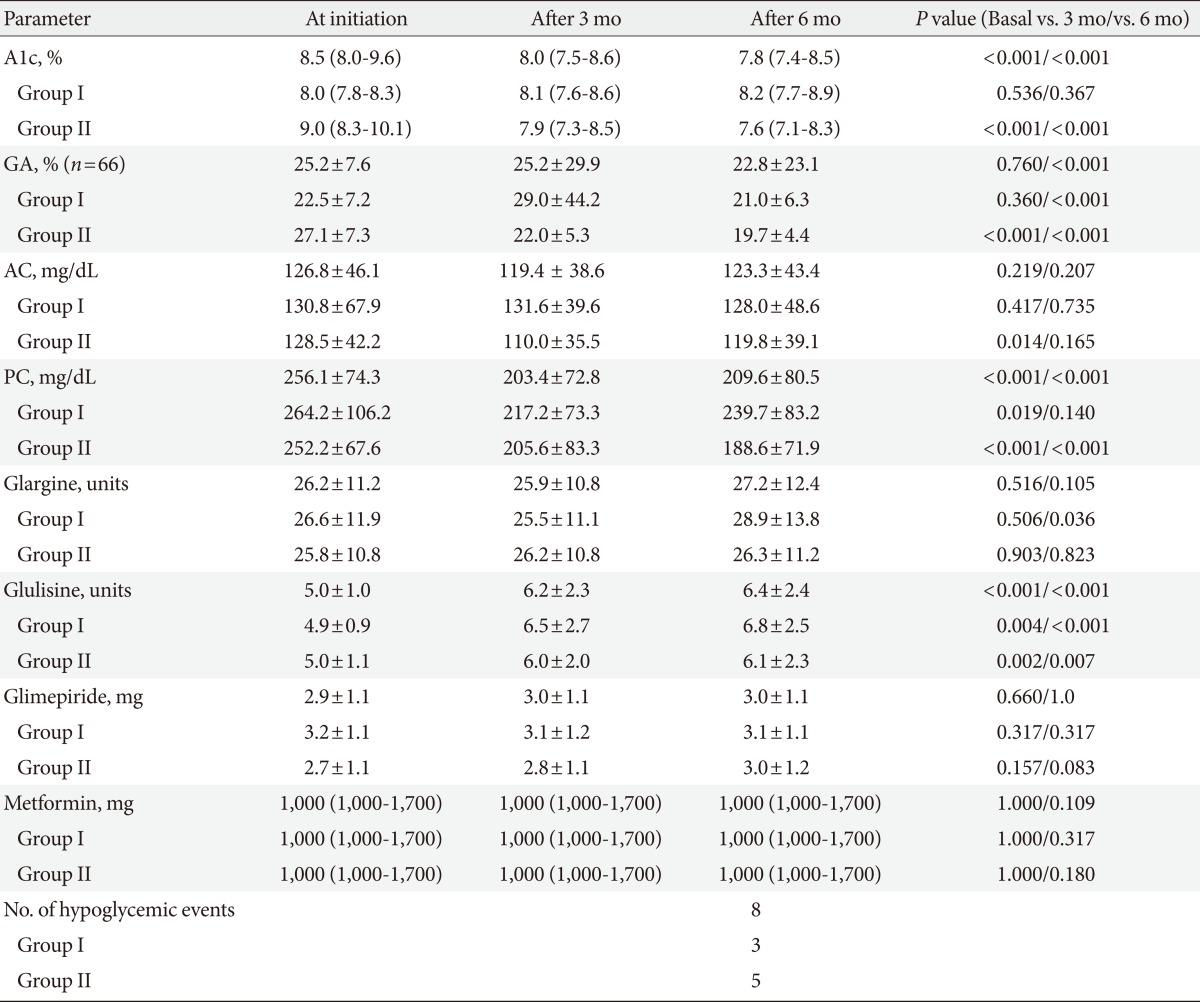
After treatment with the basal plus regimen, glucose parameters including A1c, GA, AC, and PC were improved in all subjects (Table 2). A1c was significantly decreased by 0.8±0.1% (P<0.001) at 3 months, but there was no significant change in GA. After 6 months, A1c and GA were improved by 0.8±0.1% (P<0.001) and 5.2±0.7% (P<0.001) in all patients, respectively (Fig. 1). PC levels were significantly decreased 52.8±9.4 mg/dL (P<0.001) at 3 months, and 48.7±10.3 mg/dL (P<0.001) at 6 months after initiating the basal plus regimen, respectively. However, AC levels were only slightly decreased by 7.5±5.9 mg/dL (P=0.208) and 6.6±5.2 mg/dL (P=0.205) at 3 and 6 months, respectively, which were not statistically significant.

Glucose-lowering effect of basal bolus regimen during 6 months. (A) Changes in glycated hemoglobin (A1c) during 6 months of treatment between Group I and Group II. (B) Changes in glycated albumin (GA) during 6 months of treatment between Group I and Group II.
Group II showed significant improvement in glycemic control in comparison to Group I. After the first 3 months of treatment, A1c was decreased by 1.2±0.1% and GA showed a reduction of 4.9±5.7% in Group II (both P<0.001). However, there were no significant differences in A1c and GA among subjects in Group I. At 6 months, A1c increased by 0.1±0.1% in Group I (P=0.234), while decreasing by 1.5±0.1% (P<0.001) in Group II. GA decreased by 1.9±0.4% (P<0.001) in Group I and by 7.3±1.0% (P<0.001) in Group II after 6 months of treatment. Compared to the glucose levels at baseline, there were no significant differences in AC and PC in Group I. However, in Group II, PC level had significantly decreased during the study period (52.9±13.0 mg/dL, 64.3±12.0 mg/dL at 3 and 6 months, respectively, both, P<0.001). Hypoglycemic events with minor episodes were reported in 8 patients (9.2%) and no severe hypoglycemic events occurred during the study period.
Independent parameters to achieve better glycemic control
In order to investigate the independent variables that were able to predict the likelihood of A1c reduction with the basal plus regimen in uncontrolled diabetic subjects currently on basal insulin-based combination of OADs, multiple logistic regression analyses were performed. The authors included clinically important conventional variables (BMI, initial dose of glargine, glulisine, glimepiride, and metformin) and established the parameters of age, duration of diabetes, and A1c which were significantly different between the two groups based on the results of Table 1 as independent factors. As a result, old age (odds ratio [OR], 1.25; 95% confidence interval [CI], 1.02 to 1.55), high initial A1c (OR, 22.21; 95% CI, 2.44 to 201.78), and low doses of initial glargine (OR, 0.85; 95% CI, 0.76 to 0.99), and glimepiride (OR, 0.23; 95% CI, 0.06 to 0.93) were found to be independently associated with an A1c reduction of more than 0.5% after initiating the basal plus regimen (Fig. 2).

Multiple logistic regression models for the variables independently associated with better response to basal plus regimen in patients. The reference group for calculation of the OR was Group I. OR, odds ratio; CI, confidence interval; A1c, glycated hemoglobin; DM, diabetes mellitus; BMI, body mass index.
DISCUSSION
Little controversy remains regarding early intensive glycemic control with basal insulin-based combination of OADs or short-acting insulin to prevent or delay diabetic complications [1,3,12]. However, when, how, and in whom to initiate insulin therapy are still subjects of debate. Accordingly, initiation of insulin therapy has been dependent on the factors of A1c, fasting glucose, postprandial glucose level, co-morbidity, patient age and remaining pancreas insulin secretory function [13].
Concerning how and in whom to initiate insulin, no documented data is currently available regarding the effects of the addition of rapid-acting insulin analogue to basal insulin (basal plus or bolus regimen) in Korean patients with T2DM. Although numerous studies have reported that timely initiation of single-dose basal insulin treatment is a convenient, effective, and recommended strategy [4], basal insulin-based regimen combined with OADs tends to fail in achieving glycemic goals as time passes, because of the natural course of decline in endogenous β-cell function [5,14]. Adding injections of rapid-acting insulin before a meal is physiologically reasonable to be effective in limiting a high glycemic surge after food intake. With respect to the frequency of rapid-acting insulin injections, multiple injections before meals are logical and expected to control glycemic levels within a non-diabetic range. However, due to discomfort and fear of hypoglycemia, patients were reluctant to apply multiple administrations of insulin [15,16]. Furthermore, Zambanini et al. [17] reported that increased injection frequency may induce anxiety and injection avoidance. In this regard, a single injection of rapid-acting insulin is one alternative for patients with poorly controlled T2DM. Davidson et al. [10] also reported the effects of onetime or multiple injections of preprandial rapid-acting insulin on patients with secondary failure of basal insulin. They found that A1c reductions in patients given single daily injections (0.44%) were not inferior to twice (0.36%) or thrice (0.43%) daily injections.
Based on the metabolic characteristics of T2DM in the Korean population and the natural decline of innate β-cell function [7,8], we hypothesized that earlier implementation of the basal plus regimen would be more likely to attain and sustain optimal glycemic targets. Attention was focused on the anticipated reduction of glucose levels in subjects following this regimen and investigating predictive characteristics of patients who responded satisfactorily to the basal plus regimen after failure of the basal insulin therapy.
The present clinical study with poorly controlled subjects who previously were on a basal insulin therapy demonstrated two main findings: 1) implementation of a single preprandial injection of glulisine at the main meal in subjects with basal insulin failure was effective in lowering A1c, with a reduction of 0.8±0.1% after 6 months of administration; and 2) elderly patients with poorly controlled T2DM who had used a smaller amount of glargine or glimepiride showed significant improvements in A1c with the basal plus regimen.
Significant reductions of A1c at 3 and 6 months (both 0.8±0.1%) were observed in all subjects. After initiation of the basal plus regimen, PC levels were significantly decreased in patients of Group II, but not Group I. According to the correlation analysis, the reduction of A1c after 6 months of therapy was significantly associated with a decrease in PC levels after 6 months (r=0.254, P=0.021), but not with changes in AC (r= 0.054, P=0.682). The result indicates the addition of glulisine may contribute to a reduction of A1c by lowering the glycemic surge after a meal.
During the 6 months of the basal plus regimen, 12 patients (13.8%) achieved an A1c lower than 7% in our study population. Similar to Group II, subjects who attained an optimal glycemic target of A1c <7% were significantly older than subjects with A1c ≥7% (subjects with A1c <7% vs. subjects with A1c ≥7%; 68.2±9.8 years vs. 62.2±10.1 years, P=0.029). However, they had lower levels of A1c at baseline comparing to subjects with A1c ≥7% (8.1±0.4% vs. 9.0±1.2%, P=0.012).
The characteristics of subjects with significant reduction of A1c were a high initial value of A1c (>9.0%), old age (>65 years), and low levels of glargine (<20 units) and glimepiride (<4 mg). The results suggest the implementation of rapid-acting insulin at a deteriorated stage, due to gradual deterioration of β-cell secretory function by increased glimepiride, contributes to low efficacy in reduction of A1c.
The present study has several limitations, which should be complemented by further investigation. First, the number of study subjects was relatively small, and information regarding patients' compliance with medication, life style modification and factors related to adverse effects of insulin including weight gain, was uncollectable because this was a retrospective study. Second, the analysis of clinical data was conducted over a relatively short study period. As a result, our goal of glycemic control was lower (A1c reduction >0.5%) than other studies (A1c reduction >1%). In addition, because the study population consisted of poorly controlled Korean patients who had a longer duration of diabetes (>10 years) and predominantly featured an insulin secretory defect over insulin resistance, the results may not be applicable to other ethnic groups with T2DM, especially when the major etiologic factor is insulin resistance. Therefore, the present findings should be interpreted with caution. However, this is the first clinical study to investigate the glucose-lowering efficacy and its implications of the basal plus regimen for Korean patients with T2DM.
In conclusion, the data from the present study showed initiation of a basal plus regimen may be effective in lowering glucose levels of subjects with old age, high initial A1c, and a history of using smaller amounts of glargine or glimepiride. Further studies on the clinical efficiency and safety of the basal plus regimens for type 2 diabetic patients will make this study's observations useful in the clinical setting.
Notes
No potential conflict of interest relevant to this article was reported.