Effect of Eplerenone, a Selective Aldosterone Blocker, on the Development of Diabetic Nephropathy in Type 2 Diabetic Rats
Article information
Abstract
Background
Aldosterone antagonists are reported to have beneficial effects on diabetic nephropathy by effective blocking of the renin-angiotensin-aldosterone system. We investigated the renoprotective effect of the selective aldosterone receptor blocker eplerenone, the angiotensin converting enzyme inhibitor lisinopril, and combined eplerenone and lisinopril treatment in type 2 diabetic rats.
Methods
Animals were divided into six groups as follows: Otsuka Long-Evans Tokushima Fatty (OLETF) rat control, OLETF rats treated with a low dose of eplerenone (50 mg/kg/day), OLETF rats treated with a high dose of eplerenone (200 mg/kg/day), OLETF rats treated with lisinopril (10 mg/kg/day), OLETF rats treated with a combination of both drugs (eplerenone 200 mg/kg/day and lisinopril 10 mg/kg/day), and obese non-diabetic Long-Evans Tokushima Otsuka rats for 26 weeks.
Results
Urinary albumin excretion was significantly lower in the lisinopril group, but not in the eplerenone group. Urinary albumin excretion was decreased in the combination group than in the lisinopril group. Glomerulosclerosis and renal expression of type I and type IV collagen, plasminogen activator inhibitor-1, transforming growth factor-β1, connective tissue growth factor, and fibronectin mRNA were markedly decreased in the lisinopril, eplerenone, and combination groups.
Conclusion
Eplerenone and lisinopril combination showed additional benefits on type 2 diabetic nephropathy compared to monotherapy of each drug.
INTRODUCTION
Diabetic nephropathy is one of the common complications of diabetes, and often leads to end-stage renal insufficiency. The renin-angiotensin-aldosterone system (RAAS) has been known as a key mechanism of progressive renal injury in diabetic nephropathy. Drugs blocking RAAS including angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II (Ang II) type 1 receptor blockers (ARB) have been exhibited to reduce proteinuria, tubulointerstitial fibrosis and glomeruolosclerosis [1,2]. However, neither ACEI nor ARB abrogate the progression of the diabetic nephropathy. This suggests that ACEI or ARB do not fully suppress aldosterone in some patients, so called 'aldosterone escape phenomenon' [3,4].
Studies in experimental rat models have shown that aldosterone participates to the progression of kidney disease through hemodynamic and direct cellular actions and antagonists of aldosterone retard the progression or cause regression of existing glomerulosclerosis independently of effects on blood pressure [5-10]. Chrysostomou et al. [11] showed that spironolactone add-on therapy in diabetes patients who were taking ACEI had improvement in proteinuria. This suggests that more efficient blocking of RAAS by combination of ACEI and aldosterone receptor blocker may have additive renoprotective effects in diabetic nephropathy. However, the non-selective aldosterone receptor blocker, spironolactone also works on progesterone and androgen receptors to cause menstrual disorders in females, and gynecomastia in men. On the other hand, the selective aldosterone receptor blocker, eplerenone selectively acts on the mineralocorticoid receptor to minimize these side effects [12].
In the present study, we compared the effects of ACEI (lisinopril), eplerenone, and combination of lisinopril and eplerenone on urinary albumin excretion, glomerulosclerosis and the expression of various pro-fibrotic cytokines in type 2 diabetic rat models.
METHODS
Animals
Fourteen-week-old Otsuka Long Evans Tokushima Fatty (OLETF) rats (type 2 diabetes animal model) and Long Evans Tokushima Fatty (LETO) rats (non-diabetic obese animal model) were supplied by the Tokushima Research Institute (Otsuka Pharmaceutical, Tokushima, Japan). Rats had free access to rat chow and tap water, and were caged individually within a temperature and light-controlled environment. They were placed into the OLETF control group (OLETF); the OE 50 group: 50 mg/kg eplerenone per day (Pfizer Inc., New York, NY, USA); and the OE 200 group: 200 mg/kg eplerenone per day; OA group: 10 mg/kg lisinopril per day (Hyundai Pharm., Seoul, Korea); OAE group: combined treatment of 10 mg/kg/day lisinopril and 200 mg/kg/day eplerenone; and LETO group. The dosage of eplerenone and lisinopril was based on the results of a previous study [13,14]. Drug administration was continued until the rats were 40 weeks old, and body weight and blood pressure were measured at the end of the study period. Blood pressure was measured by tail-cuff plethysmography (LE 5001-Pressure Meter; Letica SA, Barcelona, Spain), and the mean value of 5 measurements were used. Rats were intraperitoneally injected with 50 mg/kg pentobarbital. After anesthesia, both kidneys were extracted, and one kidney was fixed in paraffin while the remaining kidney was frozen in liquid nitrogen and preserved for the future analysis. All trials were conducted in accordance with the animal management guidelines of the Korea University College of Medicine.
Biochemical analysis
Urinary albumin concentration was measure by competitive ELISA kit (Shibayagi, Shibukawa, Japan), and urinary creatinine was determined by the modified Jaffe method. Urinary albumin excretion was quantified by a urinary albumin-to-creatinine ratio and reported in micrograms of albumin per milligram of creatinine (µg/mg Cr).
Glucose tolerance test
Forty-week-old rats were fasted for 10 hours and administered 2.0 g/kg glucose intraperitoneally at 8:00 AM. Then, blood samples were taken from the tail vein and blood glucose was measured after 0, 30, 60, 90, and 120 minutes (Roche-Diagnostic, Pleasanton, CA, USA).
Histological analysis
One kidney was stained with periodic acid-Schiff (PAS) immediately after extraction, fixed in paraffin, and cut into 4 µm segments. An anti-quantitative score index [15] was used in order to evaluate the glomerulosclerosis score with PAS-stained tissue. Scores between 0 and 4 were given to glomeruli based on the assessment of degrees of sclerosis (no lesion: 0, sclerosis <25%: 1+, sclerosis 25% to 50%: 2+, sclerosis 50% to 75%: 3+, sclerosis >75%: 4+). One renal pathologist assessed over 50 glomeruli from each rat in a blinded manner. The degree of sclerosis of each glomerulus was averaged and compared.
RNA extraction and analysis of gene expression
Total RNA was extracted from renal cortical tissue with TRIZOL reagent (Invitrogen, Carlsbad, CA, USA), and further purified using an RNeasy mini kit (Qiagen, Valencia, CA, USA). The nucleotide sequence of each primer was as follows:
Transforming growth factor-β (TGF-β): sense 5'-ATACAGGGCTTTCGATCCAGG-3' and anti-sense 5'-GTCCAGGCTCCAAATATAGG-3'; Type IV collagen: sense 5'-TAGGTGTCAGCAATTAGGCAGG-3' and anti-sense 5'-CGGACCACTAT GCTTGAAGTGA-3'; Type I collagen: sense 5'-TCACCTACAGCACGCTTG-3' and anti-sense 5'-GGTCTGTTTCCAGGG TTG-3'; Plasminogen activator inhibitor-1 (PAI-1): sense 5'-ATGAGATCAGTACTGCGGACGCCATCTTTG-3' and anti-sense 5'-GCACGGAGATGGTGCTACCATCAGACTTGT-3'; Connective tissue growth factor (CTGF): sense 5'-CTGAAAGAATAGCTGGCTTCA-3' and anti-sense 5'-CTGGTACTAGCTGAGGTCAT-3'; Fibronectin: sense 5'-CTGGGATGCTCCTGCTGTCAC-3' anti-sense 5'-CTGTTTGATCTGGACCTG CAG-3'.
Using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA), mRNA was reverse transcribed into cDNA, and the specificity of each polymerase chain reaction product was measured using a melting curve analysis. Then, a single band was confirmed through agarose gel electrophoresis. Real-time reverse transcription-polymerase chain reaction was performed by running for 10 minutes at 50℃, for 5 minutes at 95℃ and denaturation for 10 seconds at 95℃, and then annealing and extension at 60℃ for 30 seconds for 45 cycles. The results showed the β-actin expression level as a relative value. Gene expression was quantified using a Bio-Rad iCycler system (Bio-Rad).
Statistical analysis
Results are presented as the value±standard deviation, and the natural log was taken for values which did not follow the normal distribution. One-way ANOVA and Duncan post-hoc analyses were used for comparison of more than two groups. Testing for statistical significance was performed using SPSS for windows version 17.0 (SPSS Inc., Chicago, IL, USA). Statistical significance levels with P values under 0.05 were considered significant.
RESULTS
Comparison of the clinical data at the end of the study
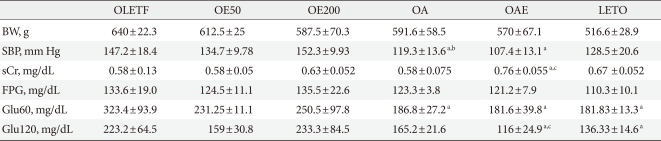
There was no significant difference in the weight between the drug-treated groups and the control group. The systolic blood pressure in the OA and OAE group was significantly lower than that of OLETF group. In contrast, there was no difference between the OE200 group and the OLETF group. High doses of eplerenone did not show a significant decrease in blood pressure. Plasma creatinine levels are significantly increased in the OAE group. Fasting plasma glucose did not differ among the groups; however, 60 minutes plasma glucose after glucose loading was significantly lower in the lisinopril treated groups (OA and OAE) than in the OLETF group. Plasma glucose after 120 minutes of glucose loading was significantly lower in the OAE group compared to the OLETF group. Body weight, blood glucose, and urinary albumin excretion values were significantly lower in the LETO group compared to the all OLETF groups (Table 1).
Twenty-four hour urinary albumin excretion
Twenty-four hour urinary albumin excretion adjusted for urinary creatinine was significantly lower in the OA and OAE groups than the OLETF group, and it was lowest in the OAE group, suggesting the additive effect of lisinopril and eplerenone. Although eplerenone monotherapy groups (OE50, OE200) showed a decreasing trend of albuminuria compared to the OLETF group, it was not statistically significant. On the other hand, the OA group presented lower albuminuria than the OE200 group (Fig. 1).
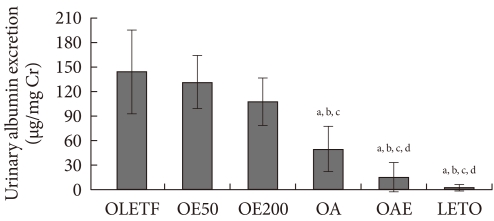
Effect of eplerenone and lisinopril on urinary albumin excretion. Twenty-four hour urinary albumin excretion was corrected by urine creatinine concentration. Values are presented as mean±standard deviation. OLETF, Otsuka Long-Evans Tokushima Fatty (OLETF) rat control; OE50, OLETF rats treated with eplerenone at 50 mg/kg/day; OE200, OLETF rats treated with eplerenone at 200 mg/kg/day; OA, OLETF rats treated with lisinopril at 10 mg/kg/day; OAE, OLETF rats treated with lisinopril at 10 mg/kg/day and eplerenone at 200 mg/kg/day; LETO, non-diabetic control. aP<0.05 vs. OLETF, bP<0.05 vs. OE50, cP<0.05 vs. OE200, dP<0.05 vs. OA.
Histological findings
Fig. 2A shows representative histologic findings in the experimental groups. Diabetic OLETF rats showed more severe glomerulosclerosis than LETO rats. Eplerenone treatment significantly reduced the degree of glomerulosclerosis and mesangial cell proliferation, compared to the untreated OLETF rats. Similar findings were observed in the OA and OAE groups. The glomerulosclerosis index of the OE200, OA, and OAE groups were significantly lower compared to the OLETF control group (Fig. 2B). However, there was no statistically significant difference in the degree of glomerulosclerosis among the OE200, OA, and OAE groups. Although eplerenone and lisinopril both improved glomerulosclerosis, additional benefits could not be determined in the OAE group.
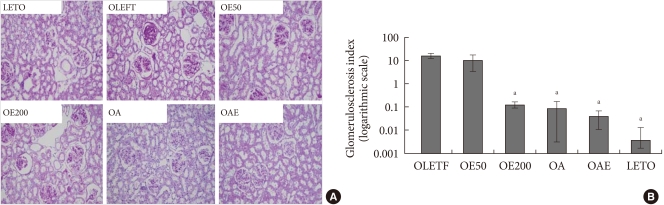
Renal histologic findings and glomerulosclerosis index according to the treatment groups. (A) Renal histologic findings (periodic acid-Schiff [PAS] staining). (B) Glomerulosclerosis index according to the study groups on a logarithmic scale. PAS staining of glomeruli showed marked glomerulosclerosis in the OLETF group compared to treatment groups (×200). Glomerulosclerosis indices are presented as mean±standard deviation. Statistical analysis was done after logarithmic transformation. OLETF, Otsuka Long-Evans Tokushima Fatty (OLETF) rat control; OE50, OLETF rats treated with eplerenone at 50 mg/kg/day; OE200, OLETF rats treated with eplerenone at 200 mg/kg/day; OA, OLETF rats treated with lisinopril at 10 mg/kg/day; OAE, OLETF rats treated with lisinopril at 10 mg/kg/day and eplerenone at 200 mg/kg/day; LETO, non-diabetic control. aP<0.05, vs. OLETF.
Expression of pro-fibrotic and pro-inflammatory cytokines
The expressions of type I and type IV collagen mRNA were lower in the OE50, OE200, OA, and OAE groups than in the OLETF group. Combination of lisinopril and eplerenone (OAE) group showed the decreased expression of type I and type IV collagen than lisinopril monotherapy (OA) group. The expressions of PAI-1, TGF-β, and CTGF mRNA in the OE50, OE100, OA, and OAE groups were lower than in the OLETF groups. However, their expressions were not different between the OA group and the OAE groups. Fibronectin mRNA expression was also lower in the OE50, OE200, and OA groups than in the OLETF groups (Fig. 3).
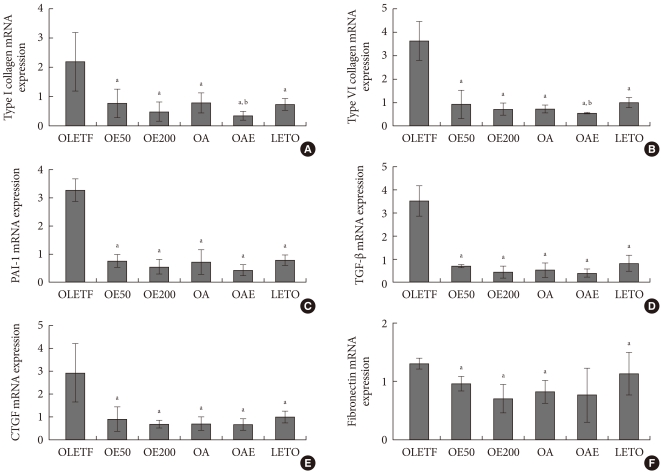
mRNA expression of pro-fibrotic and pro-inflammatory cytokines in renal cortical tissues in experimental animals. mRNA expression of (A) type I collagen, (B) type IV collagen, (C) plasminogen activator inhibitor-1 (PAI-1), (D) transforming growth factor-β (TGF-β), (E) connective tissue growth factor (CTGF), and (F) fibronectin are presented relatively compared to β-actin mRNA expression. Values are presented as mean±standard deviation. Statistical analysis was done after logarithmic transformation. OLETF, Otsuka Long-Evans Tokushima Fatty (OLETF) rat control; OE50, OLETF rats treated with eplerenone at 50 mg/kg/day; OE200, OLETF rats treated with eplerenone at 200 mg/kg/day; OA, OLETF rats treated with lisinopril at 10 mg/kg/day; OAE, OLETF rats treated with lisinopril at 10 mg/kg/day and eplerenone at 200 mg/kg/day; LETO, non-diabetic control. aP<0.05, vs. OLETF, bP<0.05, vs. OA.
DISCUSSION
In this study, we investigated the effects of eplerenone and lisinopril monotherapy and their combination on diabetic nephropathy in type 2 diabetic rat models. Even in low doses, eplerenone reduced the expression of type I collagen, type IV collagen, PAI-1, TGF-β, and fibronectin. High doses of eplerenone improved glomerulosclerosis, and had a tendency to decrease albuminuria. Moreover, eplerenone and lisinopril combination therapy lowered the expression of type I collagen and type IV collagen mRNA and albuminuria more than lisinopril monotherapy. The present study suggests that aldosterone receptor blocker and ACEI combination therapy in type 2 diabetic rat models prevents the progression of diabetic nephropathy, which is consistent with several studies [8,15-19]. Recently, Lee et al. [19] showed that spironolactone and losartan combination therapy reduced the expression of TGF-β, type IV collagen mRNA, and glomerulosclerosis in type 2 diabetic rat models. In addition, Kang et al. [20] also reported that eplerenone and enalapril combination therapy had additional effects on reducing urinary albumin excretion and glomerulosclerosis.
Aldosterone is known to stimulate the production of Ang II and independently cause toxicity and fibrosis of blood vessels through several mechanisms [4-7]. Aldosterone increases the production of fibronectin in mesangial cells through the Smad2-dependent TGF-β1 pathway [21], and promotes the expression of collagen genes through ERK1/2 in rat kidney fibroblasts [22]. In addition, aldosterone increases the expression of PAI-1 via serum- and glucocorticoid-inducible protein kinase 1 (SGK1), which is involved in signal transduction causing fibrosis of mesangial cells [23,24]. In the present study, low doses of eplerenone decreased the expression of these pro-fibrotic cytokines, and reduced glomerulosclerosis in the high doses. However, the reduction of albuminuria could not be confirmed. It could be due to small sample size of rat model, or due to another unknown hemodynamic and metabolic factors affecting albuminuria.
In this study, lisinopril monotherapy had a more significant effect on blood pressure and albuminuria reduction compared to eplerenone. In addition, the blood glucose level 60 minutes after glucose loading in the lisinopril group was lower than in the eplerenone group, and after 120 minutes, the combination group showed a lower blood glucose value than the lisinopril monotherapy group. In the previous study, suppression of Ang II by ACEI and ARB promoted the differentiation of adipocytes and stimulated peroxisome proliferator-activated receptor-γ (PPAR-γ), improving insulin resistance [25,26]. In addition, when Miana et al. [27] used high doses of eplerenone, blood glucose levels were improved, which was reported to be associated with PPAR-γ. In the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) trials, although ramipril was ineffective in reducing the incidence of diabetes in patients with impaired glucose tolerance, the proportion of subjects who regressed to the euglycemia was significantly higher in the ramipril group [28]. This finding partially demonstrated the hypoglycemic effects of ACEI; however, such effects of aldosterone inhibitors have not yet been identified in clinical studies.
Eplerenone and lisinopril combination therapy was more effective in reducing urinary creatinine-adjusted 24-hour albumin excretion than lisinopril monotherapy. When the similarities in the blood pressure between the lisinopril monotherapy group and the combination group were considered, an additional effect of the combination therapy was independent from the hemodynamic effects of reduced blood pressure. There were no differences of the expression of pro-inflammatory and pro-fibrotic cytokines among combination group, high dose eplerenone group and lisinopril monotherapy group. Combination of ACEI and eplerenone may possess additional renal protective mechanism besides reducing the expression of these cytokines.
However, a greater increase in plasma creatinine was observed in the combination therapy group compared to the lisinopril monotherapy group. In the previous study to show the additive effect of enalapril and eplerenone on reducing albuminuria, although the creatinine clearance rate was initially decreased, it was recovered to the baseline level after 8 weeks of treatment [29]. Reduction of the glomerular filtration rate through the relaxation of the efferent arteriole during the initial period of ACEI therapy is well known [30], and the relaxation effect on renal arterioles from aldosterone antagonists is also known [31]. Thus, when eplerenone and lisinopril combination therapy is constantly used, renal perfusion is reduced, and plasma creatinine might increase. Unlike this study, Mavrakanas et al. [32] showed that ramipril and eplerenone combination therapy in streptozotocin-induced diabetic rats increased creatinine clearance, and neither drug presented the improvement in mesangial hypertrophy. However, as their experimental period was 8 weeks after streptozotocin injection, it might be too short to demonstrate the beneficial effects of the treatment on histological changes.
In summary, eplerenone monotherapy exhibited the reduced expression of pro-inflammatory and pro-fibrotic cytokines, although reduction of albuminuria was not evident. However, combination of lisinopril and eplerenone presented additive effect in reducting albuminuria compared to the monotherapy of each drug. Based on this investigation, in order to minimize side effects and maximize the reno-protective effects of the combination therapy, prospective studies on the side effects and proper dosage of ACEI and aldosterone antagonist combination therapy are necessary. Furthermore, further studies to demonstrate the beneficial effects of this combination therapy on the late stage of diabetic nephropathy are also needed.
ACKNOWLEDGMENTS
This study was supported by A grant (N.H.K., 2009) from the Korean Diabetes Association.
Notes
No potential conflict of interest relevant to this article was reported.