Contributing Factors to Diabetic Brain Injury and Cognitive Decline
Article information
Abstract
The link of diabetes with co-occurring disorders in the brain involves complex and multifactorial pathways. Genetically engineered rodents that express familial Alzheimer's disease-associated mutant forms of amyloid precursor protein and presenilin 1 (PSEN1) genes provided invaluable insights into the mechanisms and consequences of amyloid deposition in the brain. Adding diabetes factors (obesity, insulin impairment) to these animal models to predict success in translation to clinic have proven useful at some extent only. Here, we focus on contributing factors to diabetic brain injury with the aim of identifying appropriate animal models that can be used to mechanistically dissect the pathophysiology of diabetes-associated cognitive dysfunction and how diabetes medications may influence the development and progression of cognitive decline in humans with diabetes.
INTRODUCTION
Type 2 diabetes mellitus accelerates age-related cognitive decline [12] and increases the risk for dementia [34567]. Pathophysiological processes causing diabetic brain injury and cognitive decline begin years before actual manifestation of symptoms occurs [89]. Compared to cognitive impairment in individuals without diabetes, cognitive decline in humans with diabetes can be influenced by the type of diabetes (type 1 diabetes mellitus or type 2 diabetes mellitus), duration of diabetes, antidiabetic medications and presence of other diabetic-related complications [1011]. Here, we focus on contributing factors to diabetic brain injury with the aim of identifying appropriate animal models that can be used to mechanistically dissect the pathophysiology of diabetes-associated cognitive dysfunction and how diabetes medications may influence the development and progression of cognitive decline in humans with diabetes.
CONTRIBUTING FACTORS TO DIABETIC BRAIN INJURY IN HUMANS
Common diabetes-associated pathological processes and complications that can have deleterious impact on brain function are summarized in Fig. 1.
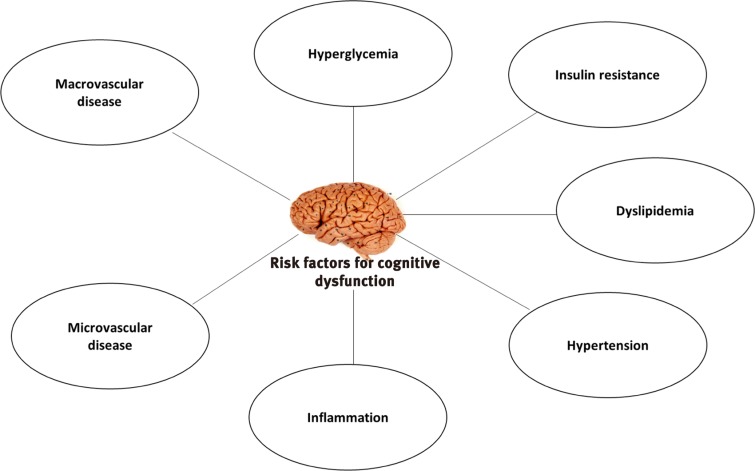
Risk factors for cognitive dysfunctions in diabetes. Figure showing main risk factors involved in cognitive dysfunction in diabetes. These risk factors may be associated with different types of cognitive dysfunction in diabetes.
Hyperglycemia
Efficient delivery of glucose to brain cells is critical for brain function [12]. Glucose uptake by the brain cells appears to be independent of insulin; however neurons are known to express receptors for insulin and insulin related peptide (insulin-like growth factor 1 [IGF-1]) [12]. Both insulin and IGF-1 play important roles in neuronal development and affect cognition [12]. Insulin resistance and consequent abnormal blood glucose levels as observed in type 2 diabetes mellitus can have detrimental effects on brain and cognitive function [1314]. Fluctuation in glucose levels or peaks, which is common in diabetes, increases the risk for cognitive decline. Targeting glucose peaks has been proposed as therapeutic strategy for prevention of diabetes-associated cognitive dysfunction [14]. In laboratory animals, glucose lowering compounds such as metformin, thiazolidinediones and compounds targeting the glucagon like peptide-1 receptor have beneficial effects on cognition [10]. It was reported that agonists of peroxisome proliferator-activated receptor gamma (PPARγ), a ligand-activated transcriptional factor, improved brain function in a rat model of streptozotocin-induced diabetes [15]. Decreased activity of PPARγ and its cofactor peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) causes mitochondrial dysfunction and oxidative stress in the settings of type 2 diabetes mellitus and in brain disorders. Glucose lowering thiazolidinediones, such as rosiglitazone and pioglitazone, act as agonist for PPARγ and improve cognitive function. Glucagon-like peptide 1 (GLP-1) receptor agonists are known elicit neuroprotective effects in animal models of stroke, Alzheimer's disease (AD) and Parkinson's disease [16]. GLP-1 receptor agonists reduced neuro-inflammation and increased neuro-survival [16]. Metformin reduces tau hyper-phosphorylation by inducing protein phosphatase 2A (PP2A) activity, a major tau phosphatase [17].
Dyslipidemia
Elevated levels of plasma triglycerides and cholesterol may play a role in diabetes-associated risk for poor cognition function [1819]. Pharmacological interventions to ameliorate dyslipidemia may influence the progression of cognitive decline in individuals with diabetes and cerebrovascular disease [1819]. Cholesterol modulates the activity of enzymes involved (β and γ-secretases) in amyloid precursor protein (APP) processing and thus it can affect production of amyloid β (Aβ) protein. Because β and γ-secretases enzymes are membrane bound enzymes, the high cholesterol content in membrane lipid rafts could facilitate clustering of these enzymes with their substrate, thereby promoting cleavage of precursors of Aβ protein into amyloidogenic forms [20].
Hypertension
Cerebrovascular complications in individuals with diabetes are frequently linked to the presence of hypertension [2122]. The use of anti-hypertensive medications may diminish the risk of dementia in diabetic patients by 4% to 24% [23]; however, the underlying molecular mechanisms remain unknown [24].
Inflammation and blood-brain barrier injury
Cerebrovascular accumulation of toxic lipids, advanced glycation end products (AGEs) and aggregated proteins trigger inflammatory responses and secretion of inflammatory mediators in the circulation [25262728]. Inflammatory responses are associated with blood-brain barrier (BBB) breakdown [29]. BBB injury further exposes brain parenchyma to neurotoxic blood proteins, thrombin, fibrin, plasmin, hemoglobin, and iron from lysed red blood cells. A leaky BBB causes abnormal neuronal activity [29] that plays a role in diabetes-associated neurological deficits [30].
Chronically elevated levels of C-reactive proteins, interleukin 6 (IL-6), fibrinogen, and tumor necrosis factor α are strongly correlated with cognitive decrement in diabetic patients [31323334]. Reducing systemic inflammation promotes brain health [31323334].
Vascular contributions to cognitive impairment and dementia
Macrovascular disease such as myocardial infarction or stroke negatively affects cognitive performance [835]. Microvascular abnormalities are also frequent in patients with type 2 diabetes mellitus [835]. Patients with diabetes and individuals with normal metabolic function showing retinal microvascular abnormalities are at high risk of cognitive decline [36]. Due to similarities between retina cells and cerebrovascular cells, retinopathy can be used as a marker and therapeutic target for microangiopathy and cerebral small vessel disease [37]. It is speculated that cerebral small vessel disease might be due to long term endothelium dysfunction, capillary loss and subsequent ischemia leading to white matter disease and neurological deficits [252829]. White matter disease of vascular origin is commonly associated with vascular contributions to cognitive impairment and dementia (VCID) [252829].
Vascular endothelial cell dysfunction is linked to vascular accumulation of toxic lipids [26], AGEs [27] and aggregated proteins [25]. Interaction of AGEs with endothelial cells increases generation of reactive oxygen species (ROS) [27] and impairs the production of vasodilatory substances resulting in a reduced cerebral blood flow [38]. Elevated ROS damage cellular structures and activate matrix metalloproteinases enzyme, which further induce cytoskeletal reorganization and vascular remodeling [29]. Cytoskeletal reorganization increases the vascular permeability by disrupting tight junction proteins in endothelium, which increases energy depletion and alters neural viability [2938].
Systemic amylin dyshomeostasis
Amylin, or islet APP, is a pancreatic hormone that is synthesized and co-secreted with insulin by pancreatic β-cells and participates in the central regulation of satiety [39]. Individuals with pre-diabetic insulin resistance have hypersecretion of amylin (and insulin) leading to amylin oligomerization and pancreatic amylin amyloid. Amylin dyshomeostasis plays an important role in the development and progression of type 2 diabetes mellitus [394041]. Deposits of amylin amyloid were detected in extra-pancreatic tissues, including the heart [42], kidneys [43] and the brain [4445464748]. Because amylin amyloid is toxic [394041], the presence of amylin deposition in brains of individuals with AD suggests that the development of drugs that could limit amylin deposition in the brain may provide benefit to patients with AD or mild cognitive impairment.
Calcium dysregulation in diabetes and dementia
Altered Ca2+ signaling contributes in brain dysfunction through multiple mechanisms [49]. Erickson et al. [50], showed that diabetes causes Ca2+/calmodulin-dependent protein kinase II (CaMKII) modification at Ser279 through o-linked N-acetylglucosamine (O-GlcNAc). This modification of CaMKII has been detected both in hearts and brains of humans with type 2 diabetes mellitus [50].
DIABETIC INJURY IN LABORATORY ANIMALS
Mouse and rat models for diabetes and AD are generated by gene manipulation or/and pharmacological intervention (Table 1). In mice genetically modified to develop parenchymal deposition of Aβ and intraneuronal accumulation of hyperphosphorylated tau, brain pathology and behavior changes are accelerated by diabetic states induced by streptozotocin injection or by diet interventions [51525354555657]. Mice generated by crossing obese db/db mice with AD mice develop microhemorrhages and accelerated behavior changes compared to AD mice [55].
Brain insulin resistance
Brain insulin resistance increases the activity of β-secretase and γ-secretase [5455] promoting cerebral Aβ deposition and tau hyperphosphorylation [51525354555657]. Intracerebroventricular administration of insulin increased learning ability in normal mice [5859]; however, a similar treatment showed no significant effect on brain function in diabetic mice [59]. Cerebral accumulation of Aβ and phosphorylation of tau in mice were reduced by pharmacological interventions that ameliorate insulin resistance [60].
White matter disease
In a rat model for type 2 diabetes mellitus transgenic for human islet amyloid polypeptide (the HIP rat) [25], the development of diabetes is associated with amylin-mediated vascular endothelial dysfunction leading to axonal demyelination, white matter rarefaction and neurological deficits [25]. Phenylalanine and tyrosine, two main precursors of neurotransmitters, were greatly decreased in brains of diabetic HIP rats compared to non-diabetic littermates [61]. These results [2561] indicate amylin dyshomeostasis as a direct molecular link between pancreatic pathology in diabetes and VCID.
Peroxidative neuronal injury and neuroinflammation
In addition to amylin plaques and mixed amylin-Aβ deposits, brains of diabetic patients with AD show amylin immunoreactive deposits inside the neurons [62]. Neuronal amylin formed adducts with 4-hydroxynonenal (4HNE), a marker of peroxidative membrane injury, and increased synthesis of the proinflammatory cytokine IL-1β [62]. These pathological changes were mirrored in rats expressing human amylin in pancreatic islets (HIP rats) and mice intravenously injected with aggregated human amylin, but not in hyperglycemic rats secreting wild-type non-amyloidogenic rat amylin [62]. In cultured primary hippocampal rat neurons, aggregated amylin increased IL-1β synthesis via membrane destabilization and subsequent generation of 4HNE [62]. These effects were blocked by membrane stabilizers and lipid peroxidation inhibitors [62]. Thus, elevated circulating levels of aggregated amylin negatively affect the neurons causing peroxidative membrane injury and aberrant inflammatory responses independent of other confounding factors of diabetes. Schematic view of effect of amylin dyshomeostasis on brain is shown in Fig. 2.
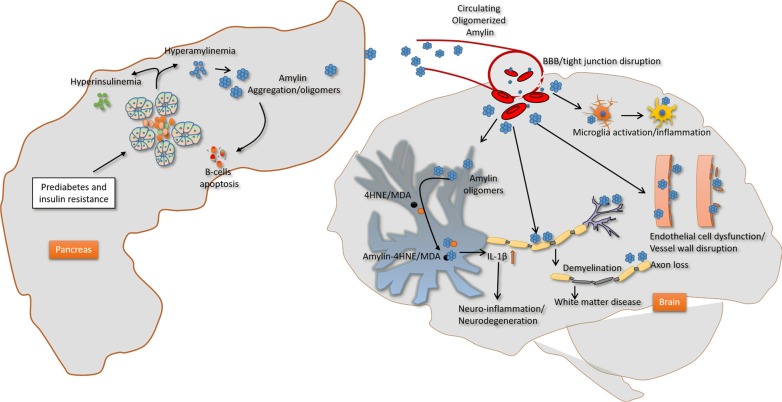
Proposed mechanism underlying the impact of amylin dyshomeostasis on the brain. Prediabetic hyperamylinemia in humans promotes amylin oligomerization within the pancreatic secretory pathway and consequent secretion of oligomerized amylin in the blood (amylin dyshomeostasis), which causes brain microhemorrhages leading to neuroinflammation and hypoxic-ischemic brain injury. BBB, blood-brain barrier; 4HNE, 4 hydroxynonenal; MDA, malondialdehyde; IL-1β, interleukin 1β.
CONCLUSIONS
In conclusion, preclinical data and epidemiological studies show a consistent association of diabetes with cognitive decline. Since some diabetes-associated factors (hyperglycemia, insulin resistance) are also extending in non-diabetic populations, the association of diabetes with cognitive impairment should also be studies in non-diabetic populations.
The link of diabetes with co-occurring disorders in the brain involves complex and multifactorial pathways. Laboratory animals can help to better understand the relationship between diabetes and cognitive impairments, which can further be used to develop new treatment strategies to slow the progression of pathological processes and cognitive decline. Since diabetes-associated amylin dyshomeostasis appears central to white matter injury in AD [25], we propose that an appropriate combination of human amylin-expressing non-AD rats and AD rats have the potential to uncover: (1) cellular and molecular mechanisms that underlie the effects of amylin dyshomeostasis on small blood vessels and white matter; (2) phenotypical characteristics of the interaction between amylin dyshomeostasis and Aβ pathology; and (3) the interplay between mixed VCID-AD and known amylin-mediated vascular injury [63]. Such knowledge is critical for designing novel interventions based on our hypothesis of the central role of amylin dyshomeostasis in the development of mixed VCID-AD.
ACKNOWLEDGMENTS
This research was supported by: National Institutes of Health AG057290, AG053999 and Alzheimer's Association VMF-15-363458.
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.


