Improvement of Glycosylated Hemoglobin in Patients with Type 2 Diabetes Mellitus under Insulin Treatment by Reimbursement for Self-Monitoring of Blood Glucose
Article information
Abstract
Background
In Korea, the costs associated with self-monitoring of blood glucose (SMBG) for patients with type 2 diabetes mellitus (T2DM) under insulin treatment have been reimbursed since November 2015. We investigated whether this new reimbursement program for SMBG has improved the glycemic control in the beneficiaries of this policy.
Methods
Among all adult T2DM patients with ≥3 months of reimbursement (n=854), subjects without any changes in anti-hyperglycemic agents during the study period were selected. The improvement of glycosylated hemoglobin (HbA1c) was defined as an absolute reduction in HbA1c ≥0.6% or an HbA1c level at follow-up <7%.
Results
HbA1c levels significantly decreased from 8.5%±1.3% to 8.2%±1.2% during the follow-up (P<0.001) in all the study subjects (n=409). Among them, 35.5% (n=145) showed a significant improvement in HbA1c. Subjects covered under the Medical Aid system showed a higher prevalence of improvement in HbA1c than those with medical insurance (52.2% vs. 33.3%, respectively, P=0.012). In the improvement group, the baseline HbA1c (P<0.001), fasting C-peptide (P=0.016), and daily dose of insulin/body weight (P=0.024) showed significant negative correlations with the degree of HbA1c change. Multivariate analysis showed that subjects in the Medical Aid system were about 2.5-fold more likely to improve in HbA1c compared to those with medical insurance (odds ratio, 2.459; 95% confidence interval, 1.138 to 5.314; P=0.022).
Conclusion
The reimbursement for SMBG resulted in a significant improvement in HbA1c in T2DM subjects using insulin, which was more prominent in subjects with poor glucose control at baseline or covered under the Medical Aid system.
INTRODUCTION
Self-monitoring of blood glucose (SMBG) is an essential tool to ensure optimal blood glucose control. Major clinical trials have shown that SMBG can improve glycemic control among patients with both type 2 diabetes mellitus (T2DM) [12] and type 1 diabetes mellitus (T1DM) [34]. In particular, SMBG is important for all insulin-treated patients to minimize the risks of both hyper- and hypoglycemic episodes and to reach their glycemic goals [5]. The American Diabetes Association first published guidelines for SMBG in 1987 [6] and the current recommendations suggest regular SMBG based on the situation of each patient [7]. Considering the importance of SMBG for diabetes care, the American Diabetes Association recommends that the government should make the process accessible and affordable for all patients who require it. Moreover, it suggests that insurers and third-party payers reimburse the medication and supplies related to the daily care of diabetes [8].
In Korea, restricted reimbursement for SMBG was started in July 2011 by the National Health Insurance Corporation of Korea (NHI). As the NHI has covered the entire population since 1989, it covered almost all reimbursements for candidates in Korea. However, at that time, the reimbursement for SMBG was limited to glucose test strips prescribed for patients with T1DM. In November 2015, a new nationwide reimbursement program of SMBG for T2DM patients was introduced. The range of subjects was expanded to all diabetic patients under insulin treatment, and the covered supplies were expanded to include blood glucose test strips, lancets, insulin syringes, and pen needles. This extended reimbursement program of SMBG was applied to both beneficiaries of the Medical Insurance service and those under the Medical Aid Program of the NHI; the Medical Aid Program is provided for low-income individuals as a part of social welfare programs. The proportions of beneficiaries of the Medical Insurance service and Medical Aid system in the NHI are 97% and 3%, respectively [9].
The current study aimed to investigate whether the introduction of this new reimbursement program for SMBG has improved the glycemic control in T2DM patients under insulin treatment.
METHODS
Patients
T2DM patients who had visited the Seoul Metropolitan Government Seoul National University Boramae Medical Center and started SMBG reimbursement between November 16, 2015, and January 29, 2016, were eligible for study inclusion. The index date was defined as the day of the first reimbursement for SMBG. The inclusion criteria were (1) T2DM patients over 20 years old receiving treatment with insulin, (2) no changes in the type of insulin or oral anti-diabetic drugs from 3 months before the index date to the end of the study period, (3) available glycosylated hemoglobin (HbA1c) data from before and after ≥3 months from the index date, and (4) a HbA1c level at the index date ≥7.0%. We excluded subjects with a duration of insulin treatment of less than 12 months before the index date or with a history of malignancy or systemic steroid treatment. Patients with any changes in the type of insulin or oral anti-diabetic drugs from 3 months prior to the index date and during the study period were excluded, although those with only changes in dose were allowed. For each patient, the baseline and follow-up data until 6 months after the first reimbursement for SMBG were collected retrospectively from electronic medical records. This study was conducted in accordance with the provisions of the Declaration of Helsinki for the participation of human subjects in research and was approved by the Institutional Review Board of Seoul Metropolitan Government Seoul National University Boramae Medical Center, and informed consent was waived for this retrospective study (No. 20160902/26-2016-118/092).
Clinical and biochemical measurements
The plasma glucose and lipid concentrations were measured enzymatically using a Hitachi Automatic Analyzer B2400 (Hitachi, Tokyo, Japan), and HbA1c was measured using a 200FR system (Toshiba, Tokyo, Japan). Improvement in HbA1c was defined as an absolute reduction in HbA1c ≥0.6% from the baseline or an HbA1c level <7.0% at follow-up. Patients who met this definition were classified as the improvement group; otherwise, they were classified as the no improvement group. A mean improvement in HbA1c of 0.6% (6.6 mmol/mol) is in keeping with other studies of T2DM patients under insulin treatment [1011].
Statistical analysis
Continuous variables are described as mean±standard deviation (SD) or median values with interquartile range (IQR). Dichotomous variables are described as counts and percentages (%). To determine the differences in baseline clinical characteristics according to the improvement in HbA1c, Pearson's chi-square test for categorical variables and the independent t-test or the Wilcoxon-Mann-Whitney test for continuous variables were used. The HbA1c and fasting serum glucose levels and the mean insulin dose at baseline and at the 3- and 6-month follow-up were examined, and the last-observation-carried-forward method was used to handle data missing because of data censoring. The glycemic profile before and after reimbursement for SMBG was compared using paired t-tests. Variables that were significantly associated with improvement in HbA1c in the univariate analysis (P<0.05) were subsequently entered into logistic regression models to determine the adjusted odds ratios for independent predictors of the improvement in HbA1c. Relationships between the reduction in the HbA1c level and other parameters were evaluated using Spearman's correlation coefficient. All statistical analyses were performed using SPSS version 20.0 (IBM Co., Armonk, NY, USA). Missing values were handled using the last-observation-carried-forward method, and statistical significance was defined as two-sided P values <0.05.
RESULTS
Baseline clinical characteristics of the study subjects
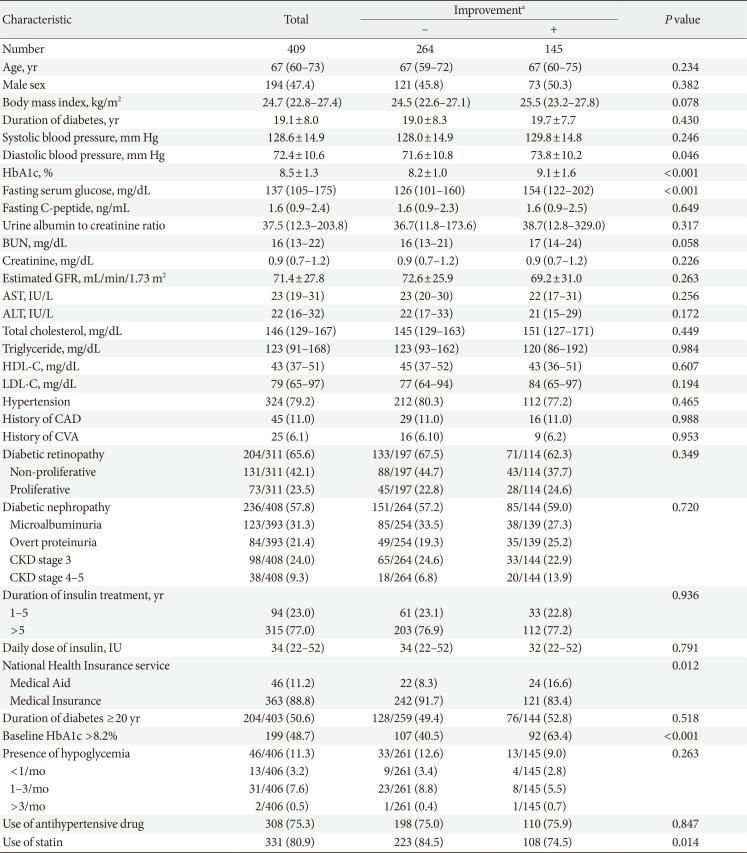
Among the total number of T2DM patients with reimbursement for SMBG (n=854), 409 patients with T2DM were included in the analysis (Supplementary Fig. 1). The median follow-up duration was 6.1 months (IQR, 5.6 to 6.5 months). The median age of the study participants was 67 years (IQR, 60 to 73 years), the mean duration of diabetes was 19.1±8.0 years, and the median HbA1c was 8.2% (IQR, 7.5% to 9.2%). Among all study participants, 77.0% had taken insulin for >5 years. Those under the Medical Insurance and Medical Aid systems accounted for 88.8% (363/409) and 11.2% (46/409) of the subjects, respectively (Table 1). Patients under the Medical Aid system were younger and had higher body mass index, higher HbA1c and fasting serum glucose levels, higher frequency of diabetic retinopathy, and higher daily doses of insulin than those with Medical Insurance (Supplementary Table 1). The most prevalent concomitant oral anti-diabetic medication was metformin, which was taken by 74.8% of the study subjects. Sulfonylurea and dipeptidyl peptidase-4 inhibitors were prescribed for 43.5% and 23.5% of the subjects, respectively (Supplementary Table 2).
Changes in glycemic control after reimbursement for SMBG
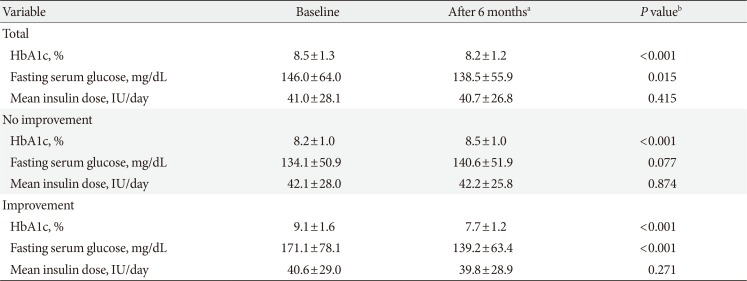
After 6 months of reimbursement for SMBG, HbA1c levels significantly decreased from a baseline value of 8.5%±1.3% to 8.2%±1.2% (P<0.001), and fasting serum glucose levels also decreased from a baseline of 146.0±64.0 to 138.5±55.9 mg/dL (P=0.015) (Table 2). Sensitivity analyses according to the insulin regimen confirmed that HbA1c levels significantly decreased regardless of the type of regimen (Supplementary Table 3).

Comparison of glycemic profile between before and after reimbursement for self-monitoring of blood glucose
Of the 409 patients, 145 (35.5%) showed significant improvements in HbA1c: 136 achieved an absolute reduction in HbA1c ≥0.6%, 47 had HbA1c levels at follow-up <7%, and 38 were satisfied with both conditions. In subjects with improvement, the mean HbA1c and fasting serum glucose levels at baseline were 9.1% and 171.1 mg/dL, and the mean changes in those levels during the follow-up were –1.4% and –31.9 mg/dL, respectively (P<0.001 for both) (Table 2). In contrast, HbA1c in the no improvement group significantly increased, from 8.2%±1.0% to 8.5%±1.0% (P<0.001). The mean daily dose of insulin was unchanged both in subjects with and without improvement. Regarding dosage changes in oral anti-diabetic drugs, most patients maintained the same dosage during the study period (93.2% of the improvement group and 96.6% of the no improvement group) (Supplementary Table 4). Moreover, there were no significant differences in the proportion of patients with increased or decreased dosages of oral anti-diabetic drugs between the two groups.
Compared to the subjects without improvement, those with improvement had significantly higher baseline HbA1c (9.1±1.6 mg/dL vs. 8.2±1.0 mg/dL, P<0.001). They also showed a higher diastolic blood pressure at baseline (Table 1); however, the statistical significance was lost when the subgroup analysis was performed in patients who were not taking anti-hypertensive drugs (P=0.417). Furthermore, 52.2% (24/46) of the subjects under the Medical Aid system showed improvement in their HbA1c levels, which was a higher proportion than in those with Medical Insurance (33.3%, 121/363; P=0.012).
We compared the trends of the change in HbA1c levels during the study period between both groups and found that subjects in the improvement group showed a significant gradual reduction in HbA1c levels (P<0.001 from the paired t-test between baseline vs. 3 months and 3 months vs. 6 months) (Fig. 1). Considering that significantly higher baseline HbA1c levels in the improvement group might affect the change in HbA1c levels during the follow-up period, we also compared the change in HbA1c levels from –3 months. HbA1c levels at –3 months were not different between the two groups (Supplementary Table 5). HbA1c levels at 6 months were significantly lower than HbA1c levels at –3 months in the improvement group; in contrast, the no improvement group showed significantly increased levels of HbA1c during the same period (Supplementary Table 5). Furthermore, the amount of changes in HbA1c levels during that period was significantly lower in the improvement group (–0.67%±1.21% vs. 0.12%±1.03%, P<0.001).
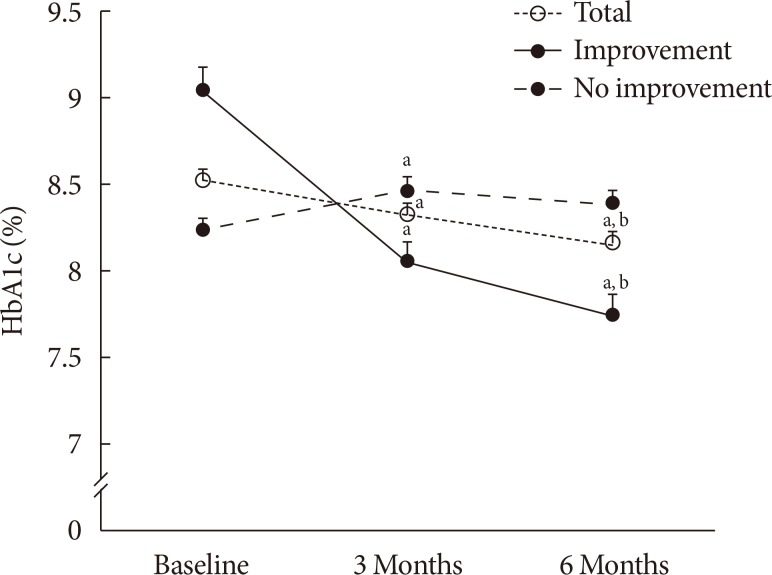
The change in HbA1c during the follow-up periods. Comparisons of the glycosylated hemoglobin (HbA1c) levels at baseline and 3 and 6 months after the first reimbursement for self-monitoring of blood glucose as determined by the paired t-test. All data are expressed as mean±SEM. aP<0.05 vs. baseline, bP<0.05 vs. 3 months.
Correlations between changes in HbA1c levels and clinical variables
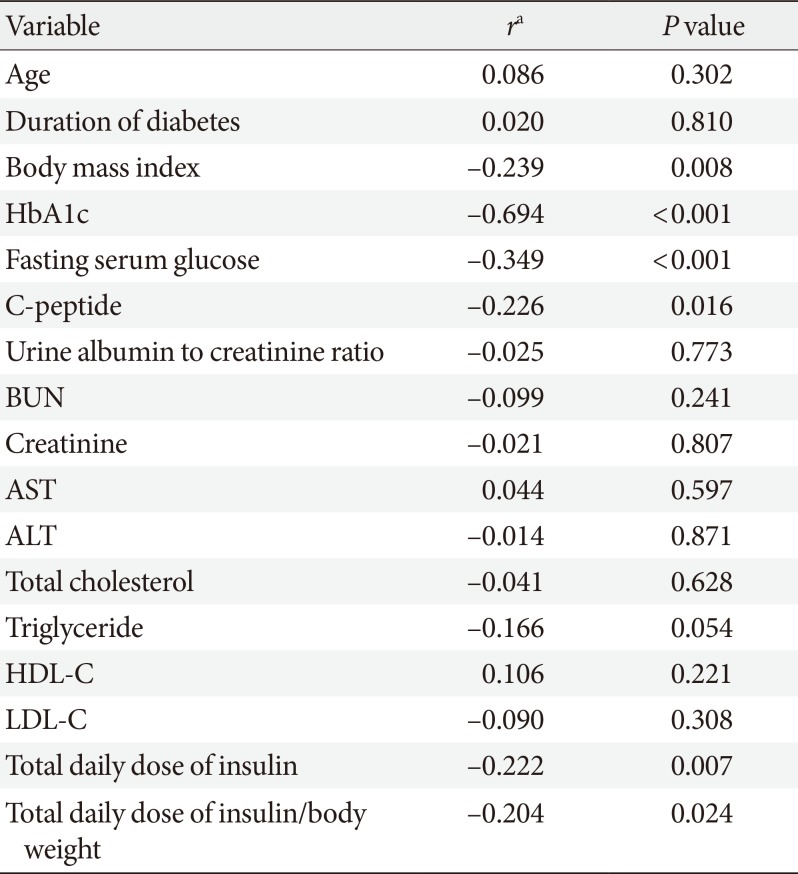
Subsequently, we investigated whether any clinical parameters determined changes in HbA1c levels in the improvement group. Baseline HbA1c, baseline fasting serum glucose level, body mass index, fasting C-peptide, daily dose of insulin, and body weight-adjusted daily dose of insulin showed significant negative correlations with the degree of HbA1c change (Table 3). That is, increase in these variables was associated with decrease in HbA1c levels. Especially, baseline HbA1c and fasting serum glucose levels showed moderate-to-strong inverse correlations with changes in HbA1c (r=–0.694, P<0.001; r=–0.349, P<0.001, respectively).
Determinants for improvement of the HbA1c level
To evaluate the predictors of improvement in HbA1c, multivariate analysis was performed using the baseline clinical factors that significantly differed between those with and without improvement, namely the type of NHI service benefit, baseline HbA1c, fasting serum glucose, and use of statins. The baseline HbA1c level was categorized as ≤8.2% and >8.2% according to the median level. All the above variables remained statistically significant determinants for the improvement in HbA1c even after adjustment for age, sex, and potential confounders (Model 3, Table 4). In addition, to confirm the effects of the Medical Aid system, variables with significant differences between the groups of Medical Aid and Medical Insurance, including body mass index, presence of diabetic retinopathy, and daily insulin dose, were additionally adjusted (Model 4). T2DM patients supported by the Medical Aid system were about 2.5-fold more likely to show an improvement in HbA1c levels compared to patients with Medical Insurance (odds ratio, 2.459; 95% confidence interval, 1.138 to 5.314; P=0.022).
DISCUSSION
Korea's newly introduced reimbursement program of SMBG significantly improved glycemic control in T2DM patients treated with insulin. In particular, patients with high baseline HbA1c levels had a greater reduction in HbA1c, and those reimbursed under the Medical Aid system were more likely to achieve improvement in glycemic control. Furthermore, HbA1c reduction was maintained for 6 months after the initiation of the reimbursement program.
SMBG is crucial for evaluating drug response, adjusting insulin doses, and preventing hypoglycemia. It helps control diabetes in a number of ways, including providing information regarding the appropriate food choices and quantities, assisting in insulin dosing, and identifying dynamic blood glucose profiles that can lead to improvements in glycemic control [1213], lower risks of long-term diabetic complications [414], and reduce health care costs [8]. A negative association between the frequency of SMBG and the blood glucose levels was also reported among insulin-treated T2DM patients [15] as well as T1DM [316], although current evidence regarding the usefulness of SMBG in those with T2DM who do not take insulin is insufficient [16171819].
Despite the clinical importance of SMBG in insulin-treated diabetic patients, reimbursement policies vary between countries [20]. In China, glucometers and test strips for SMBG are currently not covered by government-provided medical insurance [21]. In contrast, many European and North American countries largely cover the cost for SMBG, even for those without insulin treatment. The United States and Canada offer 75% to 80% reimbursement for SMBG equipment and supplies, although the amount of supplies that are covered differs between insulin-dependent and non-insulin-dependent beneficiaries [2223]. Similarly, in Australia, patients with diabetes are eligible for reimbursement; however, those with T2DM who do not use insulin are reimbursed only for the initial 6 months due to limited evidence of the benefits of SMBG in this population [24]. France and Italy provide reimbursement for all T2DM patients under insulin treatment without any restrictions, with the exception of the number of tests for T2DM patients without insulin treatment [25].
A limited number of studies have investigated the cost-effectiveness of the reimbursement of SMBG. Our data provide evidence that each country's reimbursement policies should be refined considering the population distribution of the socioeconomic status and burden of diabetes, as well as the merits of improving glycemic control. Interestingly, the patients covered by the Medical Aid system were more likely to achieve improvement in glycemic control in our study, suggesting that the cost of SMBG is an important barrier for poorer patients and that the amount of reimbursement or the refinement of the beneficiaries of this health policy should be adjusted after analysis of the long-term cost-effectiveness.
In this study, glucose control in T2DM patients under insulin treatment was improved by reimbursement for SMBG, although there was no difference in the dose of insulin or oral anti-diabetic drugs after the reimbursement, even in the improvement group. This suggests that the observed improvement might be caused by modified behavior, including food intake, exercise, and taking medication regularly, rather than increasing doses of medication. In particular, subjects with high HbA1c levels showed greater improvement in HbA1c after reimbursement. This significant correlation remained when we analyzed the data according to the relative proportions of the improvement of HbA1c.These results suggest that the new reimbursement program may provide motivation to improve diabetic care, especially in uncontrolled diabetic patients with previously poor compliance.
Some possible limitations of our study need to be discussed. First, we did not analyze the actual frequency of SMBG in our study subjects before and after the reimbursement, which resulted in the inability to confirm that reimbursement for SMBG increased the frequency of SMBG. Second, as all T2DM patients under insulin treatment are eligible for the reimbursement, our study could not have control subjects, that is, subjects without reimbursement. Instead, we compared the glucose level before and after the reimbursement for SMBG in each subject and analyzed the factors affecting the amount of change in glucose levels. Assessment of the improvement glucose levels in each patient might also be important, as the benefit from the reimbursement for SMBG in each individual can be assessed and there is a lower risk of confounders. Furthermore, analysis of the change in glucose levels after the reimbursement for SMBG without considering the actual frequency of SMBG in each patient might be more suitable to appraise the benefits of reimbursement for SMBG policy in the real-world setting. Third, there was a difference in baseline HbA1c levels between the groups of Medical Aid and Medical Insurance, and it can be a confounder. However, we could overcome this factor by adjusting the HbA1c level in the multivariate analysis. In addition, considering a higher baseline HbA1c in the improvement group compared to the no improvement group, we compared the change in HbA1c levels not only from the baseline but also from the –3 months; there was no difference between the two groups, confirming that the current definition of improvement of HbA1c could successfully differentiate individuals with HbA1c improvement among the study subjects. Finally, this study analyzed glucose levels for only 6 months after the initiation of reimbursement for SMBG. The long-term benefits should be assessed in future studies, as the health policy associated with the reimbursement for SMBG might need to be adjusted accordingly.
In conclusion, the new reimbursement program for SMBG in Korea improved the glycemic control in T2DM patients under insulin treatment; this improvement was especially prominent in subjects with poor glycemic control at the baseline or covered under the Medical Aid system.
ACKNOWLEDGMENTS
This study was supported by the Creative Industrial Technology Development Program (No. 10053249) and the Korea Meteorological Administration Research and Development Program (No. KMIPA 2015-5120).
Notes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Baseline characteristics according to the type of National Health Insurance service
Supplementary Table 2
Baseline characteristics of types of prescription drugs according to improvement of glycosylated hemoglobin
Supplementary Table 3
Comparison of glycemic profile between at baseline and 6 months after reimbursement for self-monitoring of blood glucose according to the type of insulin regimen
Supplementary Table 4
Dosage changes in insulin and oral anti-diabetic drugs during the study period according to improvement in glycosylated hemoglobin
Supplementary Table 5
The trends of the change in HbA1c during the study period in subjects with and without improvement in HbA1c
Supplementary Fig. 1
Flow chart of patients included in the study. T2DM, type 2 diabetes mellitus; SMBG, self-monitoring of blood glucose; OAD, oral antidiabetic drug; HbA1c, glycosylated hemoglobin.