
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 44(3); 2020 > Article
-
Original ArticleMetabolic Risk/Epidemiology Association between Cigarette Smoking and New-Onset Diabetes Mellitus in 78,212 Koreans Using Self-Reported Questionnaire and Urine Cotinine
-
Ji Hye Kim1
 , Dae Chul Seo1
, Dae Chul Seo1 , Byung Jin Kim1
, Byung Jin Kim1 , Jeong Gyu Kang2, Seung Jae Lee1, Sung Ho Lee1, Bum Soo Kim1, Jin Ho Kang1
, Jeong Gyu Kang2, Seung Jae Lee1, Sung Ho Lee1, Bum Soo Kim1, Jin Ho Kang1 -
Diabetes & Metabolism Journal 2020;44(3):426-435.
DOI: https://doi.org/10.4093/dmj.2019.0068
Published online: November 1, 2019
1Division of Cardiology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding author: Byung Jin Kim. Division of Cardiology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 03181, Korea. bjjake.kim@samsung.com
- *Ji Hye Kim and Dae Cheol Seo contributed equally to this study as first authors.
Copyright © 2020 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- No study has assessed association between cigarette smoking and new-onset diabetes mellitus (NODM) incidence using two different smoking classification systems: self-reported questionnaire and urine cotinine. The objective of this longitudinal study was to evaluate NODM risk using the above two systems in Korean adults.
-
Methods
- Among individuals enrolled in Kangbuk Samsung Health Study and Cohort Study who visited between 2011 and 2012 at baseline and 2014 at follow-up, 78,212 participants without baseline diabetes mellitus were followed up for a median of 27 months. Assessment of NODM incidence was made at the end of follow-up period. Cotinine-verified current smoking was having urinary cotinine ≥50 ng/mL.
-
Results
- Percentages of self-reported and cotinine-verified current smokers were 25.9% and 23.5%, respectively. Overall incidence of NODM was 1.5%. According to multivariate regression analyses, baseline self-reported current smoking (relative risk [RR], 1.33; 95% confidence interval [CI], 1.07 to 1.65) and cotinine-verified current smoking (RR, 1.27; 95% CI, 1.08 to 1.49) increased NODM risk compared to baseline self-reported never smoking and cotinine-verified current non-smoking. Higher daily amount and longer duration of smoking were also associated with increased NODM risk (P for trends <0.05). In particular, self-reported current smokers who smoked ≥20 cigarettes/day (RR, 1.62; 95% CI, 1.25 to 2.15) and ≥10 years (RR, 1.34; 95% CI, 1.08 to 1.67) had the highest RRs for NODM. These results remained significant in males, although there was no gender interaction.
-
Conclusion
- This longitudinal study showed that baseline self-reported and cotinine-verified current smoking were associated with increased risks of NODM, especially in males.
- Diabetes mellitus (DM), a chronic metabolic disorder affecting approximately 285 million adults worldwide in 2010, is estimated to affect more than 439 million people by 2030 [1]. Cigarette smoking is a problematic social and health issue affecting more than 1.1 billion people worldwide [2]. Cigarette smoking is known to promote DM development by chronic systemic and pancreatic β-cell inflammation, aggravation of abdominal fat accumulation, increased sympathetic activity, and direct toxic damage of pancreatic β-cells [345]. Smoking also increases risks of microvascular and macrovascular complications by inflammation and endothelial dysfunction in already diagnosed diabetic patients [367].
- As proven in many longitudinal studies, smoking is a definite major risk factor of many cardiovascular diseases, including hypertension, myocardial infarction, and stroke [89101112]. Several studies have shown valid association between smoking and DM [13141516]. However, direct causal association between the two in Asian population has not been reported, although smoking rates are higher in Asians compared to those in Caucasians.
- In previous studies, classification of smoking status was usually based on self-reported questionnaire as it is easy and simple. However, this may result in underestimation of actual smokers and misclassification of smoking groups due to inaccurate self-report of current smoking status [1718]. Cotinine, a main metabolite of nicotine, is a biomarker with high sensitivity and specificity used for cigarette smoking assessment [192021]. A cross-sectional study has shown positive association between environmental smoking and DM in self-reported never smokers using serum cotinine, demonstrating the effectiveness of cotinine in verifying smoking exposure [22]. Therefore, the accuracy of smoking status classification can be enhanced by co-use of traditional self-reported questionnaires and objective biomarkers such as urinary cotinine.
- The objective of the present large longitudinal study was to further elucidate the association between cigarette smoking and DM by assessing incidence of new-onset diabetes mellitus (NODM) in study cohort without baseline DM using both self-reported questionnaire and urine cotinine.
INTRODUCTION
- Study population
- Initially, 131,010 Korean adults in Kangbuk Samsung Health Study (KSHS) and Kangbuk Samsung Cohort Study (KSCS) who visited between 2011 and 2012 were included in the study. Among them, 49,431 participants were excluded due to follow-up loss in 2014, 1,082 were excluded due to missing urinary cotinine and DM data, and 2,530 were excluded due to having baseline DM. Finally, 78,212 participants (46,148 males) with mean age of 38.3±5.5 years were included in the study. Their median follow-up was 27 months (range, 12 to 44 months) (Supplementary Fig. 1).
- KSHS is a retrospective cohort study of Korean adults who received annual or biennial examination at Total Healthcare Centers of Kangbuk Samsung Hospital from 2002 to 2011. KSCS is an ongoing prospective cohort study including the above study population since 2012. This study was approved by Institutional Review Board of Kangbuk Samsung Hospital (Approval no.: 2016-05-051) and informed consent was waived by the board.
- Anthropometry and laboratory tests
- Information of underlying medical history including metabolic syndrome, hypertension and DM, baseline and follow-up smoking status (never, former, current smoking), amount (<10, 10 to 19, ≥20 cigarettes/day) and duration (<10, ≥10 years) of cigarette smoking, daily alcohol consumption (g/day), and percentage of individuals who consumed alcohol more than three times per week and exercised more than five times per week were evaluated with self-reported questionnaires. Waist circumference was measured at mid-level between lowest rib and iliac crest for central obesity assessment. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Systolic and diastolic blood pressures were measured by a trained nurse using standardized sphygmomanometer.
- Laboratory examinations including serum glucose, glycosylated hemoglobin (HbA1c), total cholesterol, triglycerides (TG), low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), high-sensitivity C-reactive protein (hs-CRP), blood urea nitrogen (BUN), and uric acid were conducted using an automated chemistry analyzer (Modular DPP; Roche Diagnostics, Tokyo, Japan) after at least 10 hours of fasting. Serum creatinine level was assessed with isotope dilution mass spectroscopy traceable method using Modular D2400 (Roche Diagnostics). Insulin resistance was assessed as homeostasis model assessment of insulin resistance (HOMA-IR) with the following equation: [fasting blood glucose (mmol/L)×fasting insulin (µIU/mL)]/22.5. Urine cotinine was measured after at least 10 hours of smoking cessation using DRI Cotinine Assay (Microgenics, Fremont, CA, USA) and Modular P800 (Roche Diagnostics).
- Definition of ‘self-reported’ and ‘cotinine-verified’ smoking status
- Self-reported smoking status was classified according to self-reported response to the following question: What is the total number of cigarettes you smoked until now? Self-reported never smoking was defined as never smoking or smoking less than a total of five packs in one's life but currently non-smoking. Self-reported former smoking was defined as smoking more than a total of five packs in one's life but currently non-smoking. Self-reported current smoking was defined as currently smoking.
- Cotinine-verified smoking status was classified according to urine cotinine level. Cotinine-verified current smoking was defined as having urine cotinine ≥50 ng/mL. Cut-off level of 50 ng/mL was used to classify cotinine-verified current non-smoking and cotinine-verified current smoking based on recommendations of Society for Research on Nicotine and Tobacco (SRNT) [23]. According to our previous study, cut-off level of 50 ng/mL yielded high sensitivity (84.8%) and specificity (98.2%) for classifying never and current smoking status [24].
- Definition of diabetes mellitus and new-onset diabetes mellitus
- DM was defined as having at least one of the following: fasting serum glucose ≥126 mg/dL (7.0 mmol/L), HbA1c ≥6.5% (48 mmol/mol), and taking antidiabetic medication(s). NODM was defined as having no DM at baseline but developing DM at follow-up.
- Statistical analysis
- Data are expressed as mean±standard deviation or as median with interquartile ranges for continuous variables. Categorical variables are expressed as percentages (%). Serum TG, hs-CRP, HOMA-IR, and amount of daily alcohol consumption were log-transformed to correct for skewed distributions. Data in tables are expressed in untransformed data for easier interpretation.
- Prevalence rate in each category of self-reported and cotinine-verified smoking status was calculated using descriptive statistics. Comparative baseline characteristic analysis of self-reported smoking groups was conducted using analysis of variance (ANOVA) or chi-square test. Comparative analyses between cotinine-verified current non-smokers and current smokers and between those with and without NODM were conducted using Student t-test or chi-square test. Multivariate cox-hazard regression analyses were performed to assess the association of NODM incidence with each category of self-reported and cotinine-verified smoking status. Multivariate model was adjusted for variables with a univariate relationship (P<0.05), including age, sex, waist circumference, BMI, vigorous exercise (≥5 times per week), daily alcohol consumption, systolic blood pressure, BUN, creatinine, glucose, HDL-C, LDL-C, TG, and hs-CRP. Statistical analyses were performed with IBM SPPS version 24 (IBM Corp., Armonk, NY, USA). Statistical significance was considered when two-sided P value was less than 0.05.
METHODS
- Characteristics of participants according to baseline self-reported and cotinine-verified smoking status
- According to baseline self-reported smoking questionnaire, prevalence rates of never smokers, former smokers, and current smokers were 55.5%, 18.6%, and 25.9%, respectively. The prevalence rate of baseline cotinine-verified current smokers was 23.5%.
- Characteristics of baseline self-reported and cotinine-verified smoking groups are shown in Table 1, Supplementary Table 1. All variables showed statistically significant differences. In particular, both self-reported and cotinine-verified current smoking groups had less favorable lifestyle patterns including higher rates of alcohol consumption and lower rates of vigorous exercise compared to the never smoking group.
- Baseline characteristics of participants according to NODM status
- The NODM group subjects were older with higher proportions of males, those with underlying metabolic syndrome and hypertension than the non-NODM group. Subjects in the NODM group had less favorable cardiometabolic and renal profiles than those without NODM. They also had higher proportions of self-reported former smokers, self-reported current smokers, and cotinine-verified current smokers than those without NODM (Table 2).
- Incidence of NODM according to baseline self-reported and cotinine-verified smoking status
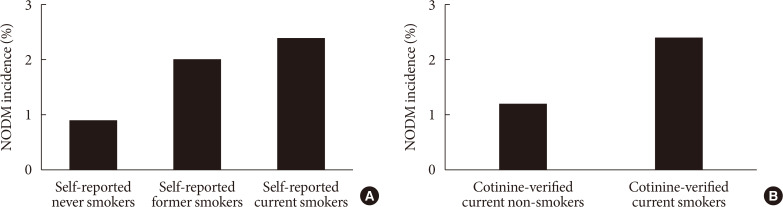
- Overall incidence of NODM was 1.5%. NODM incidence in baseline self-reported former smokers and self-reported current smokers was significantly higher than that in self-reported never smokers (2.0% or 2.4% vs. 0.9%, P<0.001), with self-reported current smokers having the highest incidence (Fig. 1A). NODM incidence in cotinine-verified current smokers was twice as high as that in cotinine-verified current non-smokers (2.4% vs. 1.2%, P<0.001) (Fig. 1B).
- Association between self-reported and cotinine-verified smoking status and NODM
- In age- and sex-adjusted Cox-hazard regression analysis, cotinine-verified current smoking significantly increased the risk for NODM (relative risk [RR], 1.38; 95% confidence interval [CI], 1.22 to 1.57) (Table 3). In multivariate model, the RR was slightly attenuated. However, it remained statistically significant (RR, 1.27; 95% CI, 1.08 to 1.49). One increase in log-transformed cotinine level also increased the risk for NODM (RR, 1.04; 95% CI, 1.01 to 1.06). In the multivariate model, self-reported current smoking was also significantly associated with increased risk for NODM compared to self-reported never smoking (RR, 1.33; 95% CI, 1.07 to 1.65) whereas self-reported former smoking was not (RR, 1.18; 95% CI, 0.93 to 1.48). These results remained significant in males, although there was no gender interaction (P=0.108) (Supplementary Table 2).
- Associations of NODM with smoking amount and duration
- Higher amount and longer duration of smoking dose-dependently increased RR for NODM (P<0.05). In particular, self-reported current smokers who smoked ≥20 cigarettes/day (RR, 1.64; 95% CI, 1.25 to 2.15) and ≥10 years (RR, 1.34; 95% CI, 1.08 to 1.67) had the highest RR for NODM compared to self-reported never smoking (Table 3).
RESULTS
- According to this longitudinal study, there were higher proportions of baseline self-reported former and current smokers and cotinine-verified current smokers in those who developed NODM than those who did not. Risks for NODM were significantly higher in both self-reported and cotinine-verified current smoking groups compared to the self-reported never smoking and cotinine-verified current non-smoking group. Also, higher amount and longer duration of smoking were dose-dependently associated with increased risk of NODM.
- Similar to results of our study, many previous cross-sectional and longitudinal studies have also stated that cigarette smoking is a risk factor for DM, with risk being as high as 30% to 40% in current smokers compared to never smokers [3467]. Major difference between these above studies and ours was that the majority of previous studies classified smoking status using only self-reported questionnaire or information from medical records, which was the simplest and most conventional tool for smoking status classification. However, this method might hinder accurate classification of smoking status as not all study participants might have answered questionnaires truthfully due to social or medical disapproval. This misclassification error rate may be between 0.9% to 9.8% depending on different studies which is large enough to produce bias and wrong interpretation of study results. Our study minimized this classification error by employing both questionnaire (‘self-reported’) and urinary cotinine (‘cotinine-verified’) smoking classification systems. Our study showed that the predictability of cotinine-based categorization was slightly higher than questionnaire-based one according to Harrell's C index (C index=0.882 [95% CI, 0.867 to 0.896] vs. C index=0.879 [95% CI, 0.865 to 0.894]). The practical advantages of urine cotinine are that it is very easy to use, accurate (high sensitivity 84.8%, specificity 98.2%) [24], quick (1 to 2 minutes for results), non-invasive, and cheap (1 to 2 dollars). It is currently widely used in many public health centers and hospital smoke cessation clinics for smoking status evaluation. It is also an approved biomarker for smoking status assessment in ‘Korean National Environmental Health Study.’ Therefore, employment of dual smoking classification system is the key strength of this study.
- Urine cotinine level may also increase in secondhand smokers (SHSs) and e-cigarette users. However, in this study the percentage of self-reported never-smokers with urine cotinine >50 ng/mL were 1.7%, which includes possible misclassified actual current smokers as well as SHS exposed non-smokers. Therefore, the actual percentage of SHS is assumed to be lower. There is currently no cut-off urine cotinine value to distinguish SHS from current smokers. However, many studies comment that less than 20 ng/mL is usually detected in SHS [2526]. Also, the percentage of nicotine containing e-cigarette use in among Korean population in 2011 to 2012 were less than 2%. Therefore, the possibility that secondary smoking or e-cigarette acted as bias in our study seems low.
- Consistent with results of this study, our previous cross-sectional study has evaluated the relationship of DM prevalence with self-reported and cotinine-verified smoking and found a positive association between the two [13]. However, our previous study had a cross-sectional design which could not validate the causal relationship between the two. Since the present study has a longitudinal design to evaluate the risk of NODM in a study population without baseline DM, it can be speculated that current smoking may be a possible causal factor of NODM development.
- Another significant finding of this study was that current smokers who smoked more than 20 cigarettes per day and those who smoked longer than 10 years had 64% and 34% higher risks, respectively, of NODM development compared to never smokers. In particular, current smokers who smoked more than 20 cigarettes had approximately 50% increased risk of NODM compared to those who smoked less than 10 cigarettes per day. Dose-dependent DM risk elevation is a consistent finding in many previous studies [272829]. Processes of pancreatic β-cell dysfunction and insulin resistance are aggravated and accelerated in individuals who are exposed to increased amount and longer duration of nicotine. Therefore, assessment of amount and longevity of smoking or nicotine exposure should be conducted thoroughly when determining the risk of NODM development.
- An interesting finding of this study was that self-reported former smoking did not increase the risk of NODM development. There have been conflicting opinions on whether former smoking is associated with increased DM risk [283031]. In early stages of smoking cessation, weight gain, especially that in the central abdominal region, can lead to impaired glucose tolerance, insulin resistance, and DM. However, in a long-term aspect, smoking cessation is associated with overall reduction in DM risk [30]. Results of the present study suggest that effects of smoking on insulin resistance, glucose control, and consequent DM development may be reversible. However, because lifestyle patterns and metabolic syndrome factors including blood pressure, TG, HDL-C and glucose levels in former smokers were slightly better than current smokers, this could have had a counterbalancing effect on association between smoking and NODM incidence.
- Results of our study did not show significant interaction between genders, although it was more evident in males (P for interaction=0.108). The reason for this may be due to relatively smaller proportions of females who were self-reported and cotinine-verified current smokers in this study (self-reported: 2.6%; cotinine-verified: 5.8%). For more accurate analysis, future study should be conducted with similar gender proportions of current smokers. However, this is hard to realize as there is a big percentage difference between Korean male and female current smokers (male vs. female current smokers in Korea in 2016: 40.7% vs. 6.4%) [32].
- This study has some limitations. First, diagnosis of NODM in this study did not include 2-hour plasma glucose level, one of criteria in DM diagnosis guideline by American Diabetes Association. This may have underestimated NODM incidence. In addition, median follow-up period of 27 months might be insufficient for accurate evaluation of NODM as progression from normal to impaired glucose tolerance and subsequent DM might occur throughout a period of 5 to 15 years [33]. The average age of study population was approximately 38 years. However, DM prevalence in Korea is approximately twice in those over 65 years compared to that in those over 30 years (29.8% vs. 14.4%) [34]. This indicates that NODM incidence might have been underestimated. Also, information on duration and amount of former smoking in self-reported former smokers is not available. The above limitations might be the reason for weak statistical power in analyzing the association between self-reported former smoking and DM. Therefore, future studies with longer follow-up period and additional information on former smokers are needed. Second, genetic components, which were not evaluated in this study, may have affected NODM development. Current studies have identified more than 50 loci associated with pancreatic β-cell dysfunction and DM development, including peroxisome proliferator-activated receptor gamma (PPARγ) gene [3536]. As NODM in mid-thirties is a considerably early phenomenon compared to average DM development process, genetic factors might have confounding influence in this study. However, gene analysis would be difficult to conduct in large-scaled population-based study due to ethical and cost issues. Third, combined effects of abdominal obesity evaluated by waist circumference, lack of physical exercise, higher percentage of frequent alcohol consumption, and co-morbid cardiometabolic diseases might have affected results of our study as they are known risk factors for DM. However, most of the above factors were adjusted in our multivariate analyses and results were consistent with statistical significance.
- The major strength of our study was that it was the first and the largest observational study of Asian population to evaluate the association between cigarette smoking and DM risk by employing two different smoking classification methods to minimize classification error.
- In conclusion, this longitudinal study shows that baseline self-reported and cotinine-verified current smoking are associated with increased risks of NODM in Korean adults compared to baseline never smoking while former smoking does not show statistically significant association. NODM risk is also increased dose-dependently in current smokers with higher daily amount and longer duration of smoking. Therefore, cigarette smoking cessation should be actively recommended and educated to patients in clinical settings as it may be a reversible and modifiable risk factor of DM development.
DISCUSSION
-
Acknowledgements
- None
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS:
Conception or design: J.H.K., D.C.S., B.J.K., J.K., S.J.L., S.H.L.
Acquisition, analysis, or interpretation of data: J.H.K., B.J.K., J.K., J.H.K.
Drafting the work or revising: J.H.K., D.C.S., B.J.K., B.S.K., J.H.K.
Final approval of the manuscript: J.H.K., D.C.S., B.J.K., J.K., S.J.L., S.H.L., B.S.K., J.H.K.
NOTES
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Supplementary Table 2
- 1. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4-14. ArticlePubMed
- 2. World Health Organization. Prevalence of tobacco smoking cited 2019 Sep 29. Available from: https://www.who.int/gho/tobacco/use/en/.
- 3. Chang SA. Smoking and type 2 diabetes mellitus. Diabetes Metab J 2012;36:399-403. ArticlePubMedPMC
- 4. Akter S, Goto A, Mizoue T. Smoking and the risk of type 2 diabetes in Japan: a systematic review and meta-analysis. J Epidemiol 2017;27:553-561. ArticlePubMedPMC
- 5. Seet RC, Loke WM, Khoo CM, Chew SE, Chong WL, Quek AM, Lim EC, Halliwell B. Acute effects of cigarette smoking on insulin resistance and arterial stiffness in young adults. Atherosclerosis 2012;224:195-200. ArticlePubMed
- 6. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking: 50 years of progress. A report of the surgeon general. Atlanta: Centers for Disease Control and Prevention; 2014.
- 7. Eliasson B. Cigarette smoking and diabetes. Prog Cardiovasc Dis 2003;45:405-413. ArticlePubMed
- 8. Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 1997;336:1276-1282. ArticlePubMed
- 9. Virdis A, Giannarelli C, Neves MF, Taddei S, Ghiadoni L. Cigarette smoking and hypertension. Curr Pharm Des 2010;16:2518-2525. ArticlePubMed
- 10. Slone D, Shapiro S, Rosenberg L, Kaufman DW, Hartz SC, Rossi AC, Stolley PD, Miettinen OS. Relation of cigarette smoking to myocardial infarction in young women. N Engl J Med 1978;298:1273-1276. ArticlePubMed
- 11. Colditz GA, Bonita R, Stampfer MJ, Willett WC, Rosner B, Speizer FE, Hennekens CH. Cigarette smoking and risk of stroke in middle-aged women. N Engl J Med 1988;318:937-941. ArticlePubMed
- 12. Pan A, Wang Y, Talaei M, Hu FB. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation 2015;132:1795-1804. ArticlePubMedPMC
- 13. Kim JH, Kim BJ, Kang JG, Kim BS, Kang JH. Association between cigarette smoking and diabetes mellitus using two different smoking stratifications in 145 040 Korean individuals: self-reported questionnaire and urine cotinine concentrations. J Diabetes 2019;11:232-241. ArticlePubMedPDF
- 14. Kim JH, Noh J, Choi JW, Park EC. Association of education and smoking status on risk of diabetes mellitus: a population-based nationwide cross-sectional study. Int J Environ Res Public Health 2017;14:E655. Article
- 15. Foy CG, Bell RA, Farmer DF, Goff DC Jr, Wagenknecht LE. Smoking and incidence of diabetes among U.S. adults: findings from the Insulin Resistance Atherosclerosis Study. Diabetes Care 2005;28:2501-2507. PubMed
- 16. Kowall B, Rathmann W, Strassburger K, Heier M, Holle R, Thorand B, Giani G, Peters A, Meisinger C. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: the KORA S4/F4 cohort study. Eur J Epidemiol 2010;25:393-402. ArticlePubMedPDF
- 17. Connor Gorber S, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res 2009;11:12-24. ArticlePubMed
- 18. Perez-Stable EJ, Marin G, Marin BV, Benowitz NL. Misclassification of smoking status by self-reported cigarette consumption. Am Rev Respir Dis 1992;145:53-57. ArticlePubMed
- 19. Parker DR, Lasater TM, Windsor R, Wilkins J, Upegui DI, Heimdal J. The accuracy of self-reported smoking status assessed by cotinine test strips. Nicotine Tob Res 2002;4:305-309. ArticlePubMed
- 20. Lee A, Gin T, Chui PT, Tan PE, Chiu CH, Tam TP, Samy W. The accuracy of urinary cotinine immunoassay test strip as an add-on test to self-reported smoking before major elective surgery. Nicotine Tob Res 2013;15:1690-1695. ArticlePubMed
- 21. Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health 1987;77:1435-1438. ArticlePubMedPMC
- 22. Alshaarawy O, Elbaz HA. Serum cotinine levels and diabetes mellitus in never smokers. J Diabetes Complications 2015;29:1032-1036. ArticlePubMedPMC
- 23. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4:149-159. ArticlePubMed
- 24. Kim BJ, Seo DC, Kim BS, Kang JH. Relationship between cotinine-verified smoking status and incidence of hypertension in 74,743 Korean adults. Circ J 2018;82:1659-1665. ArticlePubMed
- 25. Chiu YL, Huang SJ, Lai CH, Huang CC, Jiang SH, Li SR, Hwang SL, Lin FG, Tzeng YM, Kao S. Validation of self-reported smoking with urinary cotinine levels and influence of second-hand smoke among conscripts. Sci Rep 2017;7:15462. ArticlePubMedPMCPDF
- 26. Aurrekoetxea JJ, Murcia M, Rebagliato M, Lopez MJ, Castilla AM, Santa-Marina L, Guxens M, Fernandez-Somoano A, Espada M, Lertxundi A, Tardon A, Ballester F. Determinants of self-reported smoking and misclassification during pregnancy, and analysis of optimal cut-off points for urinary cotinine: a cross-sectional study. BMJ Open 2013;3:e002034.ArticlePubMedPMC
- 27. Ohkuma T, Iwase M, Fujii H, Kaizu S, Ide H, Jodai T, Kikuchi Y, Idewaki Y, Hirakawa Y, Nakamura U, Kitazono T. Dose- and time-dependent association of smoking and its cessation with glycemic control and insulin resistance in male patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. PLoS One 2015;10:e0122023. ArticlePubMedPMC
- 28. Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654-2664. ArticlePubMed
- 29. Zhang L, Curhan GC, Hu FB, Rimm EB, Forman JP. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care 2011;34:892-897. ArticlePubMedPMCPDF
- 30. Wannamethee SG, Shaper AG, Perry IJ. British Regional Heart Study. Smoking as a modifiable risk factor for type 2 diabetes in middle-aged men. Diabetes Care 2001;24:1590-1595. ArticlePubMedPDF
- 31. Beziaud F, Halimi JM, Lecomte P, Vol S, Tichet J. Cigarette smoking and diabetes mellitus. Diabetes Metab 2004;30:161-166. ArticlePubMed
- 32. Korea Centers for Disease Control and Prevention. Trend of current smoking rates among Korean adults aged 19 and over, 2007–2016 cited 2019 Sep 29. Available from: http://www.cdc.go.kr/CDC/main.jsp.
- 33. Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. Baltimore Longitudinal Study of Aging. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 2003;52:1475-1484. ArticlePubMedPDF
- 34. Korean Diabetes Association. Diabetes fact sheet in Korea 2018 cited 2019 Sep 29. Available from: http://www.diabetes.or.kr/pro/news/admin.php?code=admin&category=A&number=1615&mode=view.
- 35. Deeb SS, Fajas L, Nemoto M, Pihlajamaki J, Mykkanen L, Kuusisto J, Laakso M, Fujimoto W, Auwerx J. A Pro12Ala substitution in PPARgamma2 associated with decreased receptor activity, lower body mass index and improved insulin sensitivity. Nat Genet 1998;20:284-287. ArticlePubMedPDF
- 36. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014;383:1068-1083. ArticlePubMed
REFERENCES
(A) New-onset diabetes mellitus (NODM) incidence in baseline self-reported smoking groups. (B) NODM incidence in baseline cotinine-verified smoking groups.
Baseline characteristics according to cotinine-verified smoking status
Values are presented as mean±standard deviation, number (%), or median (interquartile range). Triglyceride, hs-CRP, cotinine, daily alcohol amount, and HOMA-IR were log-transformed for this analysis. P values were based on Student's t-test or chi-square test. P value for all variables are <0.001.
LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; HbA1c, glycosylated hemoglobin; HOMA-IR, homeostasis model assessment of insulin resistance.
Baseline characteristics according to NODM status
Values are presented as mean±standard deviation, number (%), or median (interquartile range). Triglyceride, hs-CRP, cotinine, daily alcohol amount, and HOMA-IR were log-transformed for this analysis. P values were based on Student's t-test or chi-square test.
NODM, new-onset diabetes mellitus; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; HbA1c, glycosylated hemoglobin; HOMA-IR, homeostasis model assessment of insulin resistance.
Multivariate Cox-hazard regression analyses for association of NODM with baseline cotinine-verified and self-reported smoking status, smoking amount, and smoking duration
Multivariate model was adjusted for age, sex, waist circumference, body mass index, vigorous exercise (≥5 times/week), daily alcohol consumption, systolic blood pressure, blood urea nitrogen, creatinine, glucose, uric acid, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglyceride, and high-sensitivity C-reactive protein.
NODM, new-onset diabetes mellitus; RR, relative risk; CI, confidence interval.
aThis value was log-transformed for the analysis, bReference group is baseline cotinine-verified current non-smoking, cReference group is baseline self-reported never smoking.
Figure & Data
References
Citations

- Variability in the association of smoking status with the prevalence of diabetes mellitus in the Korean population according to different definitions of smoking status: analysis based on the Korea National Health and Nutrition Examination Survey (2014-202
Yechan Kyung, Young Sook Park, Mi Hyeon Jin, Hae Jeong Lee
International Journal of Environmental Health Research.2024; : 1. CrossRef - Determination of Diabetes-associated Cardiovascular Autonomic Neuropathy Risk Factors among Insulin and Non-insulin Dependent Diabetics
Ibrahim Abdulsada, Zain Alabdeen Obaid, Farah Almerza, Mays Alwaeli, Anmar Al-Elayawi, Taha Al-Dayyeni, Harir Al-Tuhafy
The Journal of Medical Research.2023; 9(6): 141. CrossRef - Trends and Risk Factors of Metabolic Syndrome among Korean Adolescents, 2007 to 2018 (Diabetes Metab J 2021;45:880-9)
Jiun Chae, Moon Young Seo, Shin-Hye Kim, Mi Jung Park
Diabetes & Metabolism Journal.2022; 46(2): 351. CrossRef - Xenobiotics Delivered by Electronic Nicotine Delivery Systems: Potential Cellular and Molecular Mechanisms on the Pathogenesis of Chronic Kidney Disease
Pablo Scharf, Felipe Rizzetto, Luana Filippi Xavier, Sandra Helena Poliselli Farsky
International Journal of Molecular Sciences.2022; 23(18): 10293. CrossRef - Cigarette Smoking Increases the Risk of Type 2 Diabetes Mellitus in
Patients with Non-Alcoholic Fatty Liver Disease: A Population-Based Cohort
Study
Chan Liu, Yanqin Wu, Wenjuan Duan, Wenming Xu
Experimental and Clinical Endocrinology & Diabetes.2022; 130(12): 793. CrossRef - Current status of health promotion in Korea
Soo Young Kim
Journal of the Korean Medical Association.2022; 65(12): 776. CrossRef - Association between secondhand smoke exposure and diabetes mellitus in 131 724 Korean never smokers using self‐reported questionnaires and cotinine levels: Gender differences
Byung Jin Kim, Ji Hye Kim, Jeong Gyu Kang, Bum Soo Kim, Jin Ho Kang
Journal of Diabetes.2021; 13(1): 43. CrossRef - Changes in creatinine‐to‐cystatin C ratio over 4 years, risk of diabetes, and cardiometabolic control: The China Health and Retirement Longitudinal Study
Shanhu Qiu, Xue Cai, Yang Yuan, Bo Xie, Zilin Sun, Tongzhi Wu
Journal of Diabetes.2021; 13(12): 1025. CrossRef - Trends in the Socioeconomic Inequalities Related to Second-Hand Smoke Exposure as Verified by Urine Cotinine Levels Among Nonsmoking Adults: Korea National Health and Nutrition Examination Survey 2008–2018
Seo Young Kang, Min Kyung Lim, Hong-Jun Cho
Nicotine & Tobacco Research.2021; 23(9): 1518. CrossRef - Letter: Association between Cigarette Smoking and New-Onset Diabetes Mellitus in 78,212 Koreans Using Self-Reported Questionnaire and Urine Cotinine (Diabetes Metab J 2020;44:426–35)
Bo-Yeon Kim
Diabetes & Metabolism Journal.2020; 44(4): 619. CrossRef - Response: Association between Cigarette Smoking and New-Onset Diabetes Mellitus in 78,212 Koreans Using Self-Reported Questionnaire and Urine Cotinine (Diabetes Metab J 2020;44:426–35)
Ji Hye Kim, Byung Jin Kim
Diabetes & Metabolism Journal.2020; 44(4): 623. CrossRef - Smoking as a Target for Prevention of Diabetes
Ye Seul Yang, Tae Seo Sohn
Diabetes & Metabolism Journal.2020; 44(3): 402. CrossRef

 KDA
KDA PubReader
PubReader Cite
Cite




