- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 42(6); 2018 > Article
-
Original ArticleComplications Association between Serum Cystatin C and Vascular Complications in Type 2 Diabetes Mellitus without Nephropathy
-
Hye Jeong Kim, Dong Won Byun, Kyoil Suh, Myung Hi Yoo, Hyeong Kyu Park

-
Diabetes & Metabolism Journal 2018;42(6):513-518.
DOI: https://doi.org/10.4093/dmj.2018.0006
Published online: October 15, 2018
Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Hospital, Soonchunhyang University College of Medicine, Seoul, Korea.
- Corresponding author: Hyeong Kyu Park. Division of Endocrinology and Metabolism, Department of Internal Medicine, Soonchunhyang University Hospital, Soonchunhyang University College of Medicine, 59 Daesagwan-ro, Yongsan-gu, Seoul 04401, Korea. hkpark@schmc.ac.kr
Copyright © 2018 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Recent studies have correlated serum cystatin C (CysC) with vascular complications, but few studies have investigated this correlation in diabetes patients without nephropathy. This study aimed to evaluate if higher serum CysC levels increase the risk for vascular complications in type 2 diabetes mellitus patients with normal renal function or mild renal impairment.
-
Methods
- A total of 806 consecutive patients with type 2 diabetes mellitus who were admitted to the diabetes center of Soonchunhyang University Hospital for blood glucose control were retrospectively reviewed. Patients with nephropathy were excluded. Subjects were categorized into quartiles of serum CysC levels (Q1, ≤0.65 mg/L; Q2, 0.66 to 0.79 mg/L; Q3, 0.80 to 0.94 mg/L; and Q4, ≥0.95 mg/L).
-
Results
- The proportion of patients with diabetic retinopathy (DR) (P for trend <0.001), coronary heart disease (CHD) (P for trend <0.001), and stroke (P for trend <0.001) increased across the serum CysC quartiles. After adjustment for confounding factors, the highest serum CysC level remained a significant risk factor for DR (odds ratio [OR], 1.929; 95% confidence interval [CI], 1.007 to 4.144; P=0.040). Compared with Q1, a significant positive association was observed between serum CysC and CHD in Q2 (OR, 7.321; 95% CI, 1.114 to 48.114; P=0.012), Q3 (OR, 6.027; 95% CI, 0.952 to 38.161; P=0.020), and Q4 (OR, 8.122; 95% CI, 1.258 to 52.453; P=0.007). No associations were observed between CysC and stroke after additional adjustment for confounding variables.
-
Conclusion
- Serum CysC levels are independently associated with DR and CHD, suggesting that CysC may be useful for identifying type 2 diabetes mellitus patients without nephropathy who are at high risk for vascular complications.
- Due to the increasing incidence of aging and obesity, diabetes incidence has been steadily increasing for the past three decades [1]. Diabetes is a leading cause of mortality and morbidity from vascular complications worldwide. The risk for vascular complications may be reduced with early detection, when irreversible changes are not present and a suitable treatment can be effective. Identification of patients at greatest risk for diabetes complications may prevent or slow down progression more effectively.
- Cystatin C (CysC), a potent inhibitor of lysosomal and extracellular cysteine proteinases [2], is suggested to be a more reliable surrogate marker than serum creatinine for detecting early decrease in renal function in diabetes patients [3]. Increased cysteine protease expression and decreased CysC expression in human atherosclerotic lesions suggest the involvement of cysteine proteases in atherogenesis [4]. Thus, increased CysC concentrations may promote inhibition of cysteine proteases that contribute to the development of atherosclerosis and vascular complications of diabetes [5].
- Recent studies have reported that CysC is independently associated with microvascular complications such as diabetic retinopathy (DR) in type 2 diabetes mellitus patients [67]. Moreover, CysC has been recognized as an independent predictor of all-cause mortality, cardiovascular events, incident heart failure, and future secondary cardiovascular events in patients with coronary heart disease (CHD) [89]. A recent study has shown that high CysC levels are associated with CHD in type 2 diabetes mellitus patients [10]. Serum CysC is also reported to be independently associated with cerebral artery stenosis in ischemic stroke patients [11]. However, few studies have evaluated the relationship between CysC and vascular complications in type 2 diabetes mellitus patients. Furthermore, previous studies enrolled subjects with a broad range of renal function; thus, the association between CysC and vascular complications might have been affected by confounding related to the presence of nephropathy.
- This study aimed to evaluate if higher serum CysC levels increase the risk for vascular complications in type 2 diabetes mellitus patients with normal renal function or mild renal impairment.
INTRODUCTION
- Study population
- This study retrospectively reviewed 806 consecutive patients with type 2 diabetes mellitus who were admitted for blood glucose control at the diabetes center of Soonchunhyang University Hospital between March 2010 and February 2016. Patients with an estimated glomerular filtration rate using the chronic kidney disease-epidemiology collaboration (CKD-EPI) equation <60 mL/min/1.73 m2, 24-hour urine albumin excretion rate ≥30 mg/day and/or serum creatinine >1.3 mg/dL were excluded. Patients who had no data for body mass index (BMI), and/or serum CysC levels were also excluded. Finally, 601 subjects were eligible for analysis in this study. All subjects were categorized into quartiles of serum CysC levels (Q1, ≤0.65 mg/L; Q2, 0.66 to 0.79 mg/L; Q3, 0.80 to 0.94 mg/L; and Q4, ≥0.95 mg/L).
- The patients' anthropometric data, laboratory test results, and coded answers to self-reported questionnaires were stored in electronic medical records. Informed consent for this study was waived by the Institutional Review Board because we only accessed the database for analysis purposes and did not access personal identifying information. The study protocol was approved by the Institutional Review Board of Soonchunhyang University Hospital (IRB No 2017-09-001).
- Clinical and laboratory measurements
- Clinical variables for each patient were obtained from medical records: gender, age, smoking, BMI, systolic blood pressure (BP), diastolic BP, presence of DR, duration of diabetes, use of medications (anti-hypertensive drugs and lipid-lowering agents), hypertension (BP ≥140/90 mm Hg and/or on anti-hypertensive drugs), history of CHD, and previous stroke.
- Smoking status was evaluated using a questionnaire completed during an interview and patients were defined as never smokers, former smokers, or current smokers.
- Height and weight were measured while subjects were wearing light clothing without shoes. BMI was calculated as weight in kilograms divided by square of height in meters (kg/m2). BP was measured using an automatic manometer with participants in a seated position after 5 minutes of quiet rest.
- After overnight fasting, blood samples were drawn from the antecubital vein into vacuum tubes and subsequently analyzed at a central, certified laboratory in Soonchunhyang University Hospital. Serum CysC level was assessed using a particle-enhanced immunoturbidimetric assay with a Cobas 8000 c702 analyzer (Roche Diagnostics, Basel, Switzerland). Blood glucose concentration was determined using a non-enzymatic method. Glycosylated hemoglobin (HbA1c) was measured using an immunoturbidimetric assay with a Cobas Integra 800 automatic analyzer (Roche Diagnostics) with a reference value range of 4.4% to 6.4%. HbA1c measurements were standardized to the reference method in the Diabetes Control and Complications Trial (DCCT) and according to the National Glycohemoglobin Standardization Program (NGSP) standards.
- Diabetic retinopathy
- Presence of retinopathy was evaluated by ophthalmoscopic examination through fundoscopic examination and slit-lamp microscopic examination and patients were classified as having no DR, nonproliferative diabetic retinopathy (NPDR), and proliferative diabetic retinopathy (PDR) [12].
- Definition of CHD
- CHD was defined as a history of myocardial infarction or angina based on physician-entered data in electronic medical records, or a history of inpatient treatment in our hospital.
- Definition of stroke
- Stroke was defined as the presence of neurologic deficits due to ischemic stroke not known to be secondary to brain trauma, tumor, infection, or other cause, based on physician-entered data in electronic medical records.
- Statistical analysis
- The patients' age, BMI, systolic BP, diastolic BP, fasting blood glucose, HbA1c, and duration of diabetes were expressed as medians (interquartile range), and differences between groups were compared using the Kruskal-Wallis test. Group comparisons of categorical variables were performed using the chi-square test or, for small cell values, Fisher's exact test. Results of categorical data were summarized using frequencies and percent values. We used P for trend to explore the differences among the patient proportion with DR, CHD, and stroke according to CysC category. Multivariate logistic regression was conducted to estimate odds ratios (OR) with 95% confidence intervals (CI) for the risk of vascular complications. All P values and 95% CI for OR were corrected by Bonferroni's method due to multiple testing. All statistical analyses were performed using SPSS Statistics version 14.0 (SPSS Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
METHODS
- Comparison of type 2 diabetes mellitus characteristics according to serum CysC levels
- Patient characteristics according to serum CysC quartiles are presented in Table 1. As the serum CysC quartile increased, the proportion of males decreased and patients were more likely to be former or never smokers. Positive associations between serum CysC quartiles and age, systolic BP, frequency of DR, duration of diabetes, use of antihypertensive drug, history of CHD, and previous stroke were observed. Diastolic BP, fasting glucose, and HbA1c levels were significantly different among the groups, but we could not find linear relationships in an increase or a decrease. No significant differences existed be tween serum CysC quartiles and BMI, and use of lipid-lowering medication.
- Risk for vascular complications in type 2 diabetes mellitus patients
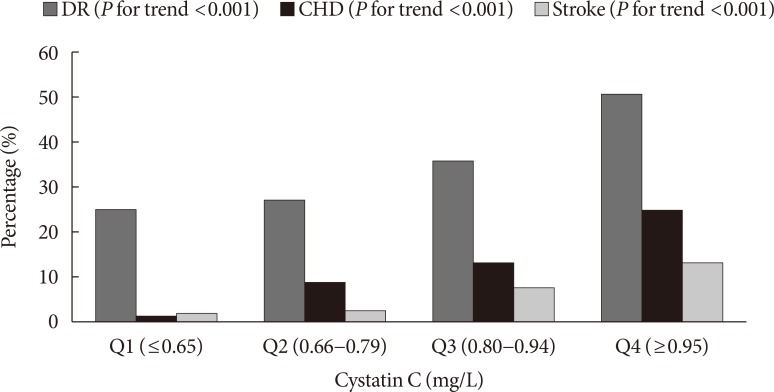
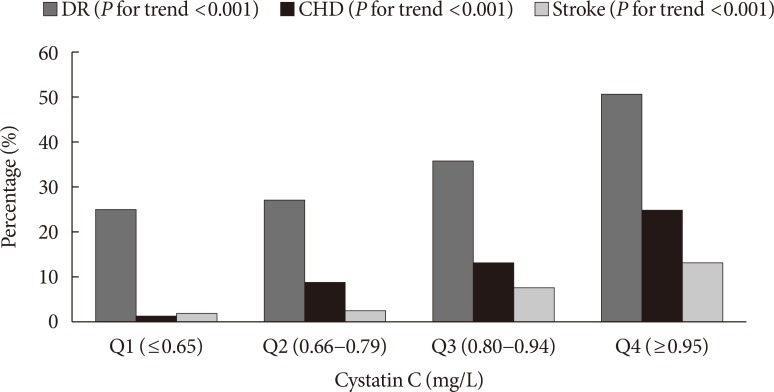
- The proportion of patients with DR (P for trend <0.001), CHD (P for trend <0.001), and stroke (P for trend <0.001) increased across the serum CysC quartiles (Fig. 1). After adjustment for gender, age, smoking, BMI, HbA1c, duration of diabetes, hypertension, history of CHD, and previous stroke (Table 2), the highest serum CysC level remained a significant risk factor for DR (OR, 1.929; 95% CI, 1.030 to 3.614; P=0.040). Compared with Q1, a significant positive association was observed between serum CysC and CHD in Q2 (OR, 7.321; 95% CI, 1.560 to 34.361; P=0.012), Q3 (OR, 6.027; 95% CI, 1.324 to 27.435; P=0.020), and Q4 (OR, 8.122; 95% CI, 1.755 to 37.577; P=0.007) in model 3. The OR of stroke significantly increased as the CysC quartile increased. However, no associations were observed after additional adjustment for confounding variables including gender, age, smoking, BMI, HbA1c, duration of diabetes, hypertension, DR and history of CHD.
RESULTS
- This study demonstrated that higher serum CysC levels were positively associated with the frequency of DR, CHD and stroke in type 2 diabetes mellitus patients with normal renal function or mild renal impairment. Moreover, serum CysC was independently predictive of DR and CHD even after adjustment for potential confounders.
- Recent cross-sectional studies have reported that high serum CysC levels correlated with microvascular complications in type 2 diabetes mellitus patients [67]. He et al. [6] demonstrated that serum CysC levels were associated with the severity of DR and could predict sight-threatening DR. Wong et al. [7] revealed that serum CysC in type 2 diabetes mellitus correlated positively with moderate DR. Our observations of positive associations between serum CysC and DR in type 2 diabetes mellitus are also consistent with the results of previous studies [67]. Although the exact mechanisms underlying the association between CysC and DR are not clear, several mechanisms have been suggested. CysC is secreted by the retinal pigment epithelium and may contribute to the development of macular degeneration [13], which could explain the association between serum CysC and DR. CysC may also play a direct role in DR by promoting vascular endothelial growth factor driven angiogenesis [14]. Additionally, CysC was assumed to be involved in arterial wall remodeling, vessel integrity, neovascularization, inflammation, and neuronal degeneration [6].
- In patients with CHD, CysC has been recognized as an independent predictor of all-cause mortality, cardiovascular events, incident heart failure, and future secondary cardiovascular events [89]. More recently, Triki et al. [10] reported that serum CysC level above 1.10 mg/L was associated with an increased risk for CHD in diabetes patients. However, they enrolled subjects with a broad range of renal function; hence, the association between CysC and CHD might have been affected by confounding related to the presence of renal impairment. In our study, we investigated whether serum CysC is associated with CHD in type 2 diabetes mellitus patients without nephropathy. The results showed that the proportion of patients with CHD increased across the CysC quartile categories and higher serum CysC levels were strongly correlated with CHD independent of traditional risk factors. Several underlying mechanisms may be involved in the probability that CysC predict macrovascular complications. An imbalance between cysteine protease and CysC in atherosclerotic lesions occurs during atherogenesis [4]. Increased CysC concentrations may contribute to plaque vulnerability and progression of atherosclerosis by regulating inflammation [515]. Regarding stroke in macrovascular complications, a recent study reported that increased CysC levels were associated with cerebral artery stenosis in ischemic stroke patients [11]. In our study, we found that higher CysC levels were significantly associated with stroke in type 2 diabetes mellitus patients with normal renal function or mild renal impairment. However, no significant differences were observed in the risk for stroke according to CysC levels after additional adjustment for confounding factors. Therefore, future investigations on the relationships between CysC and stroke are necessary
- This study has some limitations. First, we used a cross-sectional design and therefore could not determine a causal link between CysC and the risk for diabetic complications. Second, history of CHD and previous stroke were defined based on physician-entered data in electronic medical record. Therefore, we could not rule out the possibility that the actual prevalence of CHD or stroke was underestimated.
- In conclusion, our study revealed relationships between serum CysC levels and DR, CHD, and stroke in type 2 diabetes mellitus patients with normal renal function or mild renal impairment. More importantly, a higher serum CysC level indicated an increased risk for DR and CHD. These findings suggest that CysC might be useful for identifying individuals at high risk for vascular complications among type 2 diabetes mellitus patients without nephropathy. Further research on the potential mechanisms underlying the relationships between CysC and vascular complications may help to prevent or slow down progression and to explore new treatment strategies for diabetes complications.
DISCUSSION
-
Acknowledgements
- This work was supported by the Soonchunhyang University Research Fund.
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
NOTES
- 1. World Health Organization: 10 Facts on diabetes updated 2016 Apr. Available from: http://www.who.int/features/factfiles/diabetes/en/.
- 2. Mussap M, Plebani M. Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci 2004;41:467-550. ArticlePubMed
- 3. Pucci L, Triscornia S, Lucchesi D, Fotino C, Pellegrini G, Pardini E, Miccoli R, Del Prato S, Penno G. Cystatin C and estimates of renal function: searching for a better measure of kidney function in diabetic patients. Clin Chem 2007;53:480-488. ArticlePubMedPDF
- 4. Liu J, Sukhova GK, Sun JS, Xu WH, Libby P, Shi GP. Lysosomal cysteine proteases in atherosclerosis. Arterioscler Thromb Vasc Biol 2004;24:1359-1366. ArticlePubMed
- 5. Domingueti CP, Fuzatto JA, Foscolo RB, Reis JS, Dusse LM, Carvalho MDG, Gomes KB, Fernandes AP. Association between Von Willebrand factor, disintegrin and metalloproteinase with thrombospondin type 1 motif member 13, d-Dimer and cystatin C levels with retinopathy in type 1 diabetes mellitus. Clin Chim Acta 2016;459:1-4. ArticlePubMed
- 6. He R, Shen J, Zhao J, Zeng H, Li L, Zhao J, Liu F, Jia W. High cystatin C levels predict severe retinopathy in type 2 diabetes patients. Eur J Epidemiol 2013;28:775-778. ArticlePubMedPDF
- 7. Wong CW, Teo BW, Lamoureux E, Ikram MK, Wang JJ, Tai ES, Sethi S, Wong TY, Sabanayagam C. Serum cystatin C, markers of chronic kidney disease, and retinopathy in persons with diabetes. J Diabetes Res 2015;2015:404280. ArticlePubMedPMCPDF
- 8. Koenig W, Twardella D, Brenner H, Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem 2005;51:321-327. ArticlePubMedPDF
- 9. Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation 2007;115:173-179. ArticlePubMed
- 10. Triki S, Fekih O, Hellara I, Neffati F, Douki W, Hamda KB, Maatouk F, Najjar MF. Association between serum cystatin C levels and cardiovascular disease in type 2 diabetic patients. Ann Biol Clin (Paris) 2013;71:438-442. ArticlePubMed
- 11. Xu Z, Leng C, Yang B, Wang H, Sun J, Liu Z, Yang L, Ge W, Zhu J. Serum cystatin C is associated with large cerebral artery stenosis in acute ischemic stroke. Oncotarget 2017;8:67181-67188. ArticlePubMedPMC
- 12. Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R. Diabetic retinopathy. Diabetes Care 1998;21:143-156. ArticlePubMedPDF
- 13. Paraoan L, Hiscott P, Gosden C, Grierson I. Cystatin C in macular and neuronal degenerations: implications for mechanism(s) of age-related macular degeneration. Vision Res 2010;50:737-742. ArticlePubMed
- 14. Im E, Venkatakrishnan A, Kazlauskas A. Cathepsin B regulates the intrinsic angiogenic threshold of endothelial cells. Mol Biol Cell 2005;16:3488-3500. ArticlePubMedPMC
- 15. Yang S, Song L, Zhao L, Dong P, Lai L, Wang H. Predictive value of cystatin C in people with suspected or established coronary artery disease: a meta-analysis. Atherosclerosis 2017;263:60-67. ArticlePubMed
REFERENCES
Proportion of patients with vascular complications according to cystatin C quartiles. DR, diabetic retinopathy; CHD, coronary heart disease.
Clinical and biochemical characteristics of type 2 diabetes mellitus patients with normal renal function or mild renal impairment according to serum cystatin C quartiles
Values are presented as number (%) or median (interquartile range). Demographic and biochemical characteristics were compared using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables.
BMI, body mass index; BP, blood pressure; HbA1c, glycosylated hemoglobin; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
Odds ratio and 95% confidence intervals for the risk of diabetes complications based on serum cystatin C quartiles
Values are presented as number (%). Model 1, adjusted for gender (male, female) and age (years); Model 2, adjusted for gender (male, female), age (years), smoking (current, former, never), body mass index (BMI, kg/m2), glycosylated hemoglobin (HbA1c, %), duration of diabetes (years), hypertension (no, yes), history of coronary heart disease (no, yes), and previous stroke (no, yes); Model 3, adjusted for gender (male, female), age (years), smoking (current, former, never), BMI (kg/m2), HbA1c (%), duration of diabetes (years), hypertension (no, yes), diabetic retinopathy (no, yes), and previous stroke (no, yes); Model 4, adjusted for gender (male, female), age (years), smoking (current, former, never), BMI (kg/m2), HbA1c (%), duration of diabetes (years), hypertension (no, yes), diabetic retinopathy (no, yes), and history of coronary heart disease (no, yes). Odds ratio (OR) and 95% confidence interval (CI) for development of metabolic syndrome were estimated using logistic regression models. All P values and 95% CI for OR were corrected by Bonferroni's method due to multiple testing.
aP<0.001, b0.001≤P<0.01, c0.01≤P<0.05.
Figure & Data
References
Citations

- A systematic literature review of machine learning based risk prediction models for diabetic retinopathy progression
Tiwalade Modupe Usman, Yakub Kayode Saheed, Augustine Nsang, Abel Ajibesin, Sandip Rakshit
Artificial Intelligence in Medicine.2023; 143: 102617. CrossRef - Serum cystatin C for risk stratification of prediabetes and diabetes populations
Kun Xiong, Shiran Zhang, Pingting Zhong, Zhuoting Zhu, Yanping Chen, Wenyong Huang, Wei Wang
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2023; 17(11): 102882. CrossRef - Serum VEGF, high-sensitivity CRP, and cystatin-C assist in the diagnosis of type 2 diabetic retinopathy complicated with hyperuricemia
Jing Wei, Jincheng Zhang, Yanan Shi, Huiqin Zhang, Yan Wu
Open Medicine.2023;[Epub] CrossRef - Diagnostic circulating biomarkers to detect vision‐threatening diabetic retinopathy: Potential screening tool of the future?
Karen Frudd, Sobha Sivaprasad, Rajiv Raman, Subramanian Krishnakumar, Yeddula Rebecca Revathy, Patric Turowski
Acta Ophthalmologica.2022;[Epub] CrossRef - Association between circulating cystatin C and hyperuricemia: a cross-sectional study
Yanjun Guo, Hangkai Huang, Yishu Chen, Chao Shen, Chengfu Xu
Clinical Rheumatology.2022; 41(7): 2143. CrossRef - Multicenter Evaluation of Diagnostic Circulating Biomarkers to Detect Sight-Threatening Diabetic Retinopathy
Sarega Gurudas, Karen Frudd, Jayapal Jeya Maheshwari, Yeddula Rebecca Revathy, Sobha Sivaprasad, Shruthi Mahalakshmi Ramanathan, Vignesh Pooleeswaran, A. Toby Prevost, Eleni Karatsai, Sandra Halim, Shruti Chandra, Paul Nderitu, Dolores Conroy, Subramanian
JAMA Ophthalmology.2022; 140(6): 587. CrossRef - A Cross-Sectional Study of Serum and Urine Fluoride in Diabetes in Fluoride Exposed Population
Sai Deepika Ram Mohan, Shashidhar Kurpad Nagaraj, Raveesha Anjanappa, Muninarayana Chandrappa
Journal of Evolution of Medical and Dental Sciences.2021; 10(11): 798. CrossRef - Cystatin C predicts the risk of incident cerebrovascular disease in the elderly
Xin Zheng, Hong-da She, Qiao-xin Zhang, Tong Si, Ku-sheng Wu, Ying-xiu Xiao
Medicine.2021; 100(28): e26617. CrossRef - Proteinuria Is Associated with Carotid Artery Atherosclerosis in Non-Albuminuric Type 2 Diabetes: A Cross-Sectional Study
Jaehyun Bae, Yong-ho Lee, Eun Seok Kang, Bong-Soo Cha, Byung-Wan Lee
Journal of Clinical Medicine.2020; 9(1): 136. CrossRef
- Figure
- Related articles
-
- Combining Ezetimibe and Rosuvastatin: Impacts on Insulin Sensitivity and Vascular Inflammation in Patients with Type 2 Diabetes Mellitus
- Association between Metabolic Syndrome and Microvascular Complications in Chinese Adults with Type 1 Diabetes Mellitus (Diabetes Metab J 2022;46:93-103)
- Association between Metabolic Syndrome and Microvascular Complications in Chinese Adults with Type 1 Diabetes Mellitus (Diabetes Metab J 2022;46:93-103)
- Association between Type 2 Diabetes Mellitus and Brain Atrophy: A Meta-Analysis
- Association between Metabolic Syndrome and Microvascular Complications in Chinese Adults with Type 1 Diabetes Mellitus

 KDA
KDA PubReader
PubReader Cite
Cite