
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 36(1); 2012 > Article
-
ReviewAnti-Obesity Drugs: A Review about Their Effects and Safety
- Jun Goo Kang1, Cheol-Young Park2
-
Diabetes & Metabolism Journal 2012;36(1):13-25.
DOI: https://doi.org/10.4093/dmj.2012.36.1.13
Published online: February 17, 2012
1Department of Endocrinology and Metabolism, Hallym University Sacred Heart Hospital, Hallym University School of Medicine, Anyang, Korea.
2Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- Corresponding author: Cheol-Young Park. Department of Endocrinology and Metabolism, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 110-746, Korea. cydoctor@chol.com
Copyright © 2012 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
- The current recommendations for the treatment of obese people include increased physical activity and reduced calories intake. When the behavioral approach is not sufficient, a pharmacologic treatment is recommended. In past years, numerous drugs have been approved for the treatment of obesity; however, most of them have been withdrawn from the market because of their adverse effects. In fact, amphetamine, rimonabant and sibutramine licenses have been withdrawn due to an increased risk of psychiatric disorders and non-fatal myocardial infarction or stroke. Even if orlistat is not as effective as other drugs in reducing body weight, orlistat is presently the only available choice for the treatment of obesity because of its safety for cardiovascular events and positive effects on diabetic control. Hopefully, more effective and better tolerated anti-obesity drugs will be developed through an improved understanding of the multiple mechanisms and complex physiological systems targeting appetite.
- Obesity is now a global problem [1] and is associated with a number of chronic conditions including osteoarthritis, obstructive sleep apnea, gallstones, fatty liver disease, reproductive and gastrointestinal cancers, dyslipidemia, hypertension, type 2 diabetes, heart failure, coronary artery disease, and stroke [2,3]. Lifestyle modifications such as diet and exercise intervention are essential for both prevention and management of obesity, and pharmacotherapy may be considered if the interventions are ineffective for individuals with a body mass index [BMI] ≥30 kg/m2 or for those with a BMI ≥27 kg/m2 when co-morbidities, such as hypertension or type 2 diabetes mellitus are present [4]. However, anti-obesity drugs are a frequent adjunct, because these interventions have limited long-term success [5] and the weight is regained when treatment is discontinued.
- Many medications have been used to manage obesity over the years. However, most of the anti-obesity drugs that were approved and marketed have now been withdrawn due to serious adverse effects. In the 1990s, fenfluramine and dexfenfluramine were withdrawn from the market because of heart valve damage [6]. In 2000, the European Medicines Agency (EMA) recommended the market withdrawal of several anti-obesity drugs, including phentermine, diethylpropion, and mazindol, due to an unfavorable risk to benefit ratio [7]. The first selective CB1 receptor blocker, rimonabant, was available in 56 countries from 2006 but was never approved by the U.S. Food and Drug Administration (FDA) due to an increased risk of psychiatric adverse events, including depression, anxiety, and suicidal ideation [8]. Subsequently, rimonabant was withdrawn from the European market in 2009 (Table 1).
- Recently, many newer agents have been tried, though only orlistat and sibutramine have been approved for long-term use. In October 2010, sibutramine, widely used after approval by the U.S. FDA in 1997, was withdrawn from the market because of an association with increased cardiovascular events and strokes [11], leaving only orlistat (Table 2). More recently, in February 2011, the U.S. FDA rejected approval of the bupropion/ naltrexone combination marketed as Contrave due to concerns over potential cardiovascular risks. The long-term safety and efficacy of newly-developed drugs should also be evaluated in the management of obesity, which often requires continuous treatment to achieve and maintain weight loss, though the rigidity of a regulatory committee for the approval of novel anti-obesity drugs and the regulatory guidelines for anti-obesity therapy represent a significant limitation to developing drugs. The present paper reviews the effects and safety of the medications which are available for the treatment of obesity including many recently withdrawn from the market, and discusses several newer treatments currently being investigated.
INTRODUCTION
- Phentermine
- Phentermine is one of the centrally acting appetite-suppressant drugs of the β-phenethylamine family, which was approved for short-term (up to 3 months) use in the treatment of obesity by the U.S. FDA in 1959 and remains available today. There is little data from large randomized controlled trials (RCTs) relating to the long-term efficacy or safety of phentermine, especially when used as monotherapy. The β-phenethylamine family drugs have limited use in the routine management of obesity and are not currently approved for long-term use. A study in 1968 was the only longer-term (36 weeks) controlled trial of phentermine and demonstrated a mean weight loss of 12.2 kg with phentermine compared to 4.8 kg with a placebo (P<0.001). A meta-analysis, which included 6 randomized trials lasting from 2 to 24 weeks, reported patients treated with phentermine lost an average of 3.6 kg of additional weight compared with a placebo [14]. There have been no new RCTs of phentermine since 1999, and our research team recently reported the efficacy and safety of a newly developed formulation of phentermine diffuse-controlled release (DCR) in obese Korean patients [15]. This was a randomized, double-blind, placebo-controlled trial of 12 weeks treatment with phentermine DCR 30 mg (n=37) or placebo (n=37) once daily in obese patients with controlled diabetes, hypertension, or dyslipidemia. The participants in the phentermine DCR group showed significant reductions in body weight (-9.3±3.4 kg vs. -1.8±3.1 kg, P<0.001) and waist circumference (7.2±0.5 cm vs. 2.1±0.6 cm, P<0.001) compared with the placebo group. Weight reductions of 5% or greater from the baseline (95.8% vs. 20.8%, P<0.001) and 10% or more (62.5% vs. 4.7%, P<0.001) were shown in the phentermine DCR group. Total cholesterol and low density lipoprotein cholesterol (LDL-C) levels were significantly improved in the phentermine DCR group. However, there were no significant differences in systolic and diastolic blood pressure between the groups, and pulse rate in the phentermine DCR group significantly increased compared with the placebo group (P=0.02). Dry mouth and insomnia were the most common adverse events, but were mild to moderate and transient. Although the long-term effects and safety of the new formulation of phentermine DCR cannot be evaluated because of Korea FDA regulations, short-term phentermine DCR treatment resulted in significant reduction of weight and improvement of metabolic parameters, including waist circumference and several lipid profiles, without clinically severe adverse events.
- The authors of the present study did not identify any systematic reports of adverse events with phentermine [15]. However, because phentermine has sympathomimetic properties, possible side effects such as insomnia, dry mouth, dizziness, palpitation, hand tremor, and elevation in blood pressure and pulse rate should be considered. Although no serious adverse events were reported in the meta-analysis of RCTs on the use of phentermine for weight loss [14], the upper limit of the 1-sided 95% confidence interval [CI] given the number of patients studied who received phentermine was 1.5%, meaning the rate of serious adverse events could be as high as 15 per 1,000 [13]. Sympathomimetic drugs are also scheduled by the U.S. Drug Enforcement Agency, suggesting the government's view the drugs may be abused. If health professionals decide to use any of these drugs, physicians should monitor blood pressure and heart rate regularly to ensure acceptable levels are maintained in patients receiving phentermine.
- Phentermine had been used in combination with fenfluramine. The combination therapy demonstrated significantly more weight loss than a placebo in a 28-week RCT (15.5% vs. 4.9%, P<0.001). However, fenfluramine was withdrawn from the market by the U.S. FDA in 1997 [10]. A preliminary report identified heart valve damage and pulmonary arterial hypertension in association with the use of fenfluramine [6]. Phentermine is currently under evaluation in combination with topiramate (see section below entitled "Combination treatments being developed").
- Diethylpropion
- Another amphetamine-like analogue, diethylpropion, is a phenylethylamine ring compound with minor sympathomimetic properties and with fewer stimulant effects than amphetamine. Although diethylpropion has been approved by the U.S. FDA for treatment of obesity since 1959, diethylpropion has been used off-label to achieve modest weight loss, and few studies have evaluated the long-term use of diethylpropion [7]. A meta-analysis that assessed the use of diethylpropion for weight loss in obese individuals identified 13 studies published between 1965 and 1983. Although most studies were less than 20 weeks, the duration of treatment with diethylpropion varied from 6 to 52 weeks, and obese patients treated with diethylpropion lost an average of 3.0 kg of additional weight compared with a placebo [14].
- No new phentermine's RCTs have been conducted since 1983, but a recent report evaluates the efficacy of diethylpropion on a long-term basis, with an emphasis on cardiovascular and psychiatric safety aspects [16]. Sixty-nine obese healthy adults received a hypocaloric diet and were randomized to diethylpropion 50 mg twice daily or placebo for 6 months. After this period, all participants received diethylpropion in an open-label extension for an additional 6 months. After the initial 6 months, the diethylpropion group lost an average of 9.8% of initial body weight vs. 3.2% in the placebo group (P<0.0001). From baseline to month 12, the mean weight loss produced by diethylpropion was 10.6%. Participants in the placebo group who were switched to diethylpropion after 6 months lost an average of 7.0% of initial body weight. No differences in blood pressure, pulse rate, electrocardiogram, and psychiatric evaluation were observed. Dry mouth and insomnia were the most frequent adverse events, which were experienced in the first 3 months and became less apparent with continued treatment.
- Common side effects of diethylpropion included insomnia, dry mouth, dizziness, headache, mild increases in blood pressure, palpitations and rash, which are similar to the pharmacologic effect of amphetamines. Diethylpropion and phentermine are classified by the U.S. Drug Enforcement Agency as schedule IV drugs, meaning they have a very low potential for drug abuse. Although no serious adverse events were reported in the RCTs of diethylpropion, the upper limit of the 1-sided 95% CI given the number of patients studied who received diethylpropion was 1.5%, meaning the rate of serious adverse events could be as high as 15 per 1,000 [13].
SYMPATHOMIMETIC DRUGS
- The endocannabinoid system has been identified as playing a significant role in the control of appetite as well as glucose metabolism [17]. Rimonabant was the first in a new class of agents that appears to work by selectively blocking the cannabinoid-1 receptors in the endocannabinoid system and has been extensively investigated in the Rimonabant in Obesity (RIO) program, comprised of 4 randomized, double-blinded, placebo-controlled phase 3 clinical trials recruiting more than 6,000 overweight or obese patients whose weight at the start of the studies was on average 94 to 104 kg [18-21]. Each of the 4 studies on rimonabant showed significant reductions in body weight and waist circumference over a 1 to 2-year period. Rimonabant also improved cardiometabolic risk factors, including triglycerides, blood pressure, insulin resistance, C-reactive protein levels, and high density lipoprotein cholesterol concentrations in both non-diabetic and type 2 diabetic overweight/obese patients. Rimonabant was generally well-tolerated. However, later reports showed the use of rimonabant was associated with psychiatric side effects, including anxiety, depression, and suicidal ideation. The adverse psychiatric events were observed in 26% of the participants in the rimonabant group compared with 14% in the placebo group in the same 4 studies [22], and the risk of depressive symptoms was estimated at 2.5 fold higher than in placebo-treated patients [8]. In spite of the extensive favorable clinical data, the U.S. FDA refused marketing authorization for rimonabant as a consequence of the risks. Rimonabant was marketed in 18 EU member states. However, in November 2008, the EMA subsequently withdrew authorization for rimonabant in Europe.
- Taranabant, a second CB1 antagonist, has also been assessed in large scale clinical trials over a 52-week period and showed 4 kg placebo-adjusted significant weight reduction, similar to rimonabant [23]. However, psychiatric side effects were observed in all studies with taranabant using both high [23,24] and low doses [25]. For this reason, psychiatric side effects are considered a class issue for first generation CB1 antagonists. As a consequence, the development of other first generation CB1 antagonists, including the Pfizer CB1 antagonist, otenabant (CP-945,598), and the Bristol Myers Squibb compound, SLV-319, have also been halted.
CANNABINOID-1 RECEPTORS ANTAGONISTS (RIMONABANT AND TARANABANT)
- Sibutramine, a selective noradrenaline/serotonin reuptake inhibitor, was widely used after approval by the U.S FDA in 1997. In meta-analyses that included obese participants treated with sibutramine for at least 12 months, the mean placebo subtracted weight loss was 4.2 to 4.45 kg [26]. Among Korean obese patients receiving sibutramine, 68.2% of patients lost 5% or more of body weight compared with 13.0% in the placebo group with the mean absolute weight change of -5.9±3.8 kg in the sibutramine group and -1.6±2.6 kg in the placebo group after 12 weeks of treatment [27].
- Because sibutramine led to a 4.5% body weight loss for long-term treatment (over 24 to 52 weeks) [13] and also showed potential benefits by improving cardiometabolic factors including plasma glucose, insulin, and lipid-profiles [28], sibutramine was the most effective anti-obesity drug marketed, though in a meta-analysis which included 10 studies of approximately 1,213 participants who were treated with sibutramine or placebo for at least 6 to 12 months, treatment was not associated with a significant decrease in total cholesterol after adjustment for weight loss [29].
- Sibutramine was relatively well tolerated because common side effects included only constipation, headache, dry mouth, and insomnia. However, sibutramine was originally reviewed by the EMA in 1999 and 2002, following concerns over its safety, particularly cardiovascular side effects (increased blood pressure and heart rate), and was temporarily withdrawn from the Italian market on the basis of 47 adverse event reports (arrhythmias, primarily tachycardia, and hypertension) and 2 deaths from cardiovascular disease causes in that country [30]. At that time, the Agency's Committee for Medicinal Products for Human Use (CHMP) concluded the benefits of sibutramine for the management of obese and overweight patients outweighed the risks. However, the CHMP also requested Abbott Laboratories start a long-term study of sibutramine in patients with cardiovascular risk factors, with particular focus on the medicine's safety.
- The 5-year Sibutramine Cardiovascular Outcomes (SCOUT) trial was a randomized, double-blind, placebo-controlled study involving 10,742 overweight or obese patients with cardiovascular disease, hypertension, or type 2 diabetes [31]. After a 6-week lead-in period, patients who received single-blind sibutramine had on average a 2.2 kg reduction of body weight, a 2.0 cm reduction of waist circumference, a 3.0 mm Hg decrease in systolic blood pressure, a 1.0 mm Hg decrease in diastolic blood pressure, and a 1.5 mm Hg decrease in pulse rate. In addition, sibutramine was found to be efficacious, tolerable, and safe in the 6-week single-blind period. In January 2010, a preliminary report of the SCOUT study, which showed sibutrasibutramine was associated with an increased risk of serious, nonfatal cardiovascular events such as myocardial infarction or stroke as compared with a placebo (11.4% vs. 10%; hazard ratio [HR], 1.16; 95% CI, 1.03 to 1.31), led to the recommendation to suspend the use of sibutramine by the CHMP of the EMA. Sibutramine has been subsequently withdrawn from the European market. The U.S. FDA requested healthcare professionals be notified sibutramine should not be used in patients with known cardiovascular disease. The full results of the SCOUT study were published in September 2010 [11]. Long-term sibutramine treatment was shown to increase the risk of nonfatal myocardial infarction and nonfatal stroke, but not of cardiovascular death or death from any cause in overweight or obese patients with pre-existing cardiovascular diseases. The U.S. FDA initially allowed sibutramine to be available and reviewed its potential benefits and risks [32], but asked for stronger warnings on the product labels. The warning recommended sibutramine not be used by people with a history of stroke or heart attacks and uncontrolled high blood pressure. A 3-year prospective observational study of 15,686 patients who were prescribed sibutramine in New Zealand has not demonstrated a higher risk of death from a cardiovascular event [33]. However, the U.S. FDA decided the drug may pose unnecessary cardiovascular risks to patients, and thus sibutramine was withdrawn on October 8, 2010.
SIBUTRAMINE
- Orlistat is a potent and reversible gastrointestinal lipase inhibitor preventing dietary fat absorption by 30% by inhibiting pancreatic and gastric lipase. Orlistat was approved in 1998 and is currently the only available drug for the long-term management of obesity. The prescribed dose is 120 mg capsule 3 times daily, and a half dose (60 mg) is available over-the-counter in some countries, including the U.S. The efficacy of orlistat for weight loss has been reported in several RCTs for the long-term management of obesity (approximately 4 years) [34,35]. In meta-analyses of 12 and 15 trials, the mean difference in weight loss due to orlistat was -2.59 kg (95% CI, -3.46 to -1.74 kg) at 6 months and -2.9 kg (-3.2 to -2.5 kg) at 12 months [13], which was more than the placebo. The beneficial effect on body weight is sufficient to improve several cardiometabolic parameters, including waist circumference, blood pressure, blood glucose levels, and lipid profiles [35,36]. In a meta-analysis which included 15 studies of approximately 10,995 participants who were treated with orlistat or placebo for at least 6 to 12 months, treatment with orlistat was associated with a significant decrease in total cholesterol after adjustment for weight loss [29], which indicates orlistat is a useful adjunctive tool for improving cardiovascular risk factor profiles in obese patients. Orlistat also reduced the incidence of type 2 diabetes from 9.0% to 6.2% (HR, 0.63; 95% CI, 0.46 to 0.86) in a longer 4-year trial [35].
- Among Korean obese patients receiving orlistat for 24 weeks, the mean weight change from baseline was -2.8 kg. Treatment with orlistat also improved several metabolic parameters. There were significant improvements of glycemic control (HbA1c, -0.87%; P<0.01), fasting insulin, total cholesterol, LDL-C (P<0.001), waist circumference (-5.48±0.54 cm, P<0.001), systolic blood pressure and diastolic blood pressure (P=0.000), without serious side effects [37].
- The most common side effects of orlistat are gastrointestinal and include diarrhea, fecal incontinence, oily spotting, flatulence, bloating, and dyspepsia [10,26,38]. As a result of the adverse effects, orlistat may not be well tolerated. However, the side effects tend to occur early and can be reduced as patients learn how to avoid fat-rich diets.
- Recently, serious liver injury has been reported over the past 10 years. Between 1999 and 2008, the U.S. FDA received 32 reports of severe liver injury, including 6 cases of liver failure in patients using orlistat, which prompted the U.S. FDA to undertake a review of orlistat's treatment safety. The review identified a total of 13 cases of severe liver injury, reported between April 1999 and August 2009 out of an estimated 40 million people worldwide who had used Xenical or Alli. The U.S. FDA advised healthcare professionals to continue prescription of orlistat in August 2009, because severe liver injury was rare. However, a review in May 2010 led to a label revision and the addition of a warning of severe liver injury to educate the public regarding the signs and symptoms of liver injury.
ORLISTAT
- Lorcaserin
- Lorcaserin is a selective serotonin 2C (5-HT2C) receptor agonist that reduces body weight by reducing food intake and is not thought to stimulate the 5-HT2B receptor associated with cardiac valvulopathy. Nonselective serotoninergic agents, including fenfluramine and dexfenfluramine, were withdrawn form the market in 1997 after they were reported to be associated with valvular heart disease [6].
- Two phase 3 studies have now been reported (Behavioral Modification and Lorcaserin for Overweight and Obesity Management [BLOOM] [39] and BLOSSOM [40]) and have shown a modest but significant placebo-adjusted weight reduction. In a 2-year, double-blind, placebo-controlled study, 3,182 obese and overweight patients who were treated with placebo or lorcaserin 10 mg/kg twice daily, obese patients lost 3.6 kg (3.6% body weight) more than controls at the end of the first year. Additionally, the weight reduction was maintained in more patients who continued to receive lorcaserin during year 2 (67.9%) than in patients who received a placebo during year 2 (50.3%, P<0.001), among the patients who received lorcaserin during year 1 and who had lost 5% or more of their baseline weight at 1 year [39]. A second trial of similar design over 1 year, termed "BLOSSOM" in which 4,008 patients were treated with lorcaserin 10 mg every day or twice daily, was designed to assess the efficacy and safety of a dose range of lorcaserin when administered in conjunction with a nutritional and physical exercise program to promote weight loss in obese patients and at-risk overweight patients. The participants who took lorcaserin 10 mg twice daily achieved an average weight loss of 5.9% of their body weight, compared to 2.8% with a placebo (P<0.0001). Similarly, patients who took lorcaserin 10 mg once daily achieved an average weight loss of 4.8% of their body weight at the end of 12 months (P<0.0001) [40]. The most frequent adverse events in the 2 phase 3 studies were fatigue, headache, dizziness, dry mouth, and nausea, which were not significantly different between treatment groups. In addition, there was no increase in the rate of cardiac valve disease after a 2-year treatment with lorcaserin [39]. As a result of the trials, a new drug application (NDA) for lorcaserin was filed with the U.S. FDA in December 2009. However, the advisors recommended against approval in September 2010, as they did not conclude the potential benefits of the drug outweighed the risks [41]. Arena Pharmaceuticals Inc. (San Diego, CA, USA) reported results from a third phase 3 clinical trial for lorcaserin, Behavioral Modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus (BLOOM-DM), which evaluated the safety and efficacy of lorcaserin for weight management in obese and overweight patients with type 2 diabetes and planned to submit the final study report from the BLOOM-DM trial as a supplement to the NDA for lorcaserin to the U.S. FDA [42]. The BLOOM-DM study evaluated 604 obese and overweight patients with type 2 diabetes. Patients were randomized to lorcaserin 10 mg twice daily (n=256), lorcaserin 10 mg dosed once daily (QD) (n=95) or placebo (n=253). To expedite enrollment, randomization to the lorcaserin 10 mg once daily dose was discontinued after approximately 300 patients were enrolled in the trial. The 3 primary efficacy endpoints at week 52 were as follows: the proportion of patients who lost at least 5% of their baseline body weight, change from baseline in body weight, and the proportion of patients who lost at least 10% of their baseline body weight. Using Modified Intent-to-Treat Last Observation Carried Forward (MITT-LOCF) analysis, lorcaserin 10 mg twice daily met the 3 primary efficacy endpoints by producing statistically significant weight loss compared to placebo (P<0.0001). At week 52, 37.5% of patients treated with lorcaserin 10 mg twice daily achieved at least 5% weight loss, more than double the 16.1% of patients taking a placebo. Patients treated with lorcaserin 10 mg twice daily achieved a mean weight loss of 4.5% (4.7 kg), compared to 1.5% (1.6 kg) with the placebo. Additionally, at week 52, 16.3% of lorcaserin 10 mg twice daily patients achieved at least 10% weight loss compared to 4.4% of patients taking a placebo. BLOOM-DM also evaluated multiple secondary endpoints at week 52. Five families of endpoints have been or are being evaluated: glycemic, lipid, blood pressure, body composition, and quality of life (QOL). Data from the first 3 families are available and analysis of body composition and QOL are pending. Within the glycemic, lipid and blood pressure families, lorcaserin patients achieved statistically significant improvements relative to placebo in HbA1c and fasting glucose. Lorcaserin 10 mg twice daily patients achieved a 0.9% reduction in HbA1c, compared to a 0.4% reduction for the placebo group (P<0.0001). However, the FDA did not approve the application for lorcaserin due to unexplained preclinical carcinogenicity signals in rats, specifically, an increase in breast tumors. In January 2012, Arena Pharmaceuticals submitted a response to the Complete Response Letter issued by the FDA following review of the lorcaserin NDA and the company expects by the end of January, the FDA will confirm acceptance of the response and assign a new Prescription Drug User Fee Act (PDUFA) date.
- Glucagon-like peptide-1 (GLP-1) analogues
- Anorexigenic GLP-1 are gut hormones that increase secretion of insulin in pancreatic β-cells. GPL-1 analogues such as exenatide and liraglutide are approved for the treatment of type 2 diabetes, and the long-term use of the GLP-1 analogues leads to a decrease in HbA1c level and blood pressure [43,44]. Meta- analysis of clinical trials also revealed an average weight loss of 2.13 kg in exenatide-treated groups more than the placebo, and a 4.76 kg weight loss compared with insulin [45]. Exenatide is currently only in phase 2 trials for obesity [46]. A recent 32-week open-label extension study of liraglutide in 564 non-diabetic obese patients following the 20 week dose-ranging placebo-controlled phase 2 study of liraglutide in comparison with orlistat treatment (a mean weight loss of approximately 6.0 kg with liraglutide compared with 2.8 kg with a placebo and 4.1 kg with orlistat) [47] demonstrated that high-dose liraglutide led to a 5.5 to 6.0 kg placebo-adjusted weight loss at 52 weeks [48]. Novo Nordisk plans to initiate additional phase 3 trials of liraglutide for anti-obesity treatment in 2011.
- Tesofensine
- Tesofensine is an inhibitor of noradrenaline, dopamine and serotonin reuptake that reportedly also indirectly stimulates the cholinergic system and a sympathomimetic in the family of sibutramine. The drug was initially developed for the treatment of Parkinson's disease or Alzheimer's disease. Although its efficacy was limited for this application, significant weight loss was evident [49]. A proof of concept phase 2 study, a 24-week randomized double-blind placebo-controlled study, showed the proportion of patients achieving ≥5 kg (4.9%) was 59%, 87%, and 91% for 0.25, 0.5, and 1 mg groups, respectively, compared with 29% of controls. However, heart rate was significantly elevated in all tesofensine groups, and the highest dose of tesofensine (1 mg daily) showed a significant increase in blood pressure and the highest frequency of mood change [50]. Reportedly, the phase 3 program agreed with the FDA which assessed the 0.5 and 0.25 mg dose levels only [51]. Given the results, further phase 3 trials may limit the dose to 0.25 and 0.5 mg in order to reduce the impact on heart rate and blood pressure. NeuroSearch plans to initiate a clinical phase 3 program endorsed by the FDA and EMA. The trial consists of 3 trials: 2 traditional 1-year obesity trials with a mixed population with and without co-morbidities such as type 2 diabetes and dyslipidemia and 1 cardiovascular outcome study with more than 2 years of treatment in patients with a history of cardiovascular disease. The cardiovascular study is planned to enroll approximately 6,000 patients.
- Cetilistat
- Cetilistat is an inhibitor of pancreatic lipase, an enzyme that breaks down triglycerides in the intestine. Without this enzyme, triglycerides from the diet are prevented from being hydrolyzed into absorbable free fatty acids and are left to be excreted undigested. This drug, while similar to the currently FDA-approved drug orlistat, may have a more tolerable side-effect profile due to a different molecular structure. A phase 2 trial studied 612 obese, diabetic subjects with a BMI of 28 to 45 kg/m2 over a 12-week treatment period. Cetilistat 80 and 120 mg promoted significant weight loss compared with placebo (3.85 kg and 4.32 kg vs. 2.86 kg, respectively). Cetilistat-induced weight loss was similar to the weight loss achieved with orlistat (3.78 kg). Cetilistat was well tolerated and showed fewer discontinuations due to adverse events than in the placebo and orlistat groups. Given that discontinuation in the orlistat group was significantly worse than in the 120 mg cetilistat and placebo groups, and was entirely due to gastrointestinal adverse events, cetilistat may become a preferred lipase inhibitor for achieving weight loss [52]. Phase 3 trials of cetilistat are currently in progress in Japan.
- Combination treatments being developed
- Combination treatments of anti-obesity drugs showed disappointing results. The first important clinical study for weight reduction combining drugs used phentermine and fenfluramine as previously mentioned. The trial showed a highly significant weight reduction. However, fenfluramine was withdrawn from the market worldwide on September 15, 1997, because of heart valve damage [6]. Combination treatment of orlistat and sibutramine, which had been approved only for long-term use, did not induce any further weight loss [53]. Because none of the single-agent drugs that have been approved or appear close to approval have consistently been able to achieve a weight loss of more than approximately 10% of body weight [54], several other combinations of existing drugs are now under development and may be the next therapeutic option for treatment of obesity.
- Qnexa (Vivus Pharmaceuticals, Mountain View, CA, USA), a combination of low-dose phentermine and the antiepileptic agent topiramate, has recently been shown to be effective for the long-term treatment of obesity, though topiramate remains unlicensed for obesity. The efficacy and safety of this combination drug as a treatment for obesity was recently evaluated in 3 phase 3 trials (EQUATE, EQUIP, CONQUER) [55,56] in over 4,500 obese patients and the U.S FDA acknowledged the effect of weight reduction. However, In October 2010, the FDA did not approve Qnexa and requested more evidence that the elevated heart rate does not increase cardiovascular risk. In addition, Vivus Pharmaceuticals had concerns regarding the drug's teratogenic potential. In January 2011, the agency announced the FDA had requested additional information regarding teratogenicity and the FDA's endocrinologic and metabolic drugs advisory committee is scheduled to review the NDA for Qnexa for the treatment of obesity in February 2012. The company resubmitted the NDA on October 2011 seeking approval to market Qnexa in the U.S. and the FDA accepted the NDA for review in November 2011. The target date for the FDA to complete review of the Qnexa NDA is April 2012.
- Contrave (Orexigen Pharmaceuticals, La Jolla, CA, USA) is a fixed-dose combination of naltrexone sustained-release (SR) and bupropion SR. Bupropion, a dopamine and norepinephrine reuptake inhibitor was approved for depression and smoking cessation and showed modest weight loss. Naltrexone, approved for the treatment of opioid addiction and alcoholism, is not associated with weight reduction. However, the combination with bupropion and naltrexone leads to a synergistic effect on weight control, although the mechanism by which the naltrexone/bupropion combination induces weight loss is not entirely understood. Several phase 3 trials (COR, COR-BMOD, and COR-Diabetes) have been completed to evaluate this combination in both diabetic and non-diabetic obese patients [57-59].
- The Contrave group showed significant weight reduction and improvement in cardiometabolic markers compared to the placebo group in all studies. The COR-Diabetes trial showed overweight or obese patients with type 2 diabetes lost significantly more weight and achieved greater improvement in glycemic control than patients treated with a placebo after 56 weeks. The Contrave group lost significantly more weight (5.0% vs. 1.8%, P<0.001) at 56 weeks in 44.5% of patients (≥5% loss of body weight) compared to 18.9% using the placebo. Baseline A1C, the standard test for monitoring glycemic control, was significantly reduced by 0.6% with Contrave compared to 0.1% with the placebo [57]. A specific 56-week, randomized, placebo-controlled trial examined the efficacy and safety of naltrexone plus bupropion as an adjunct to intensive behavior modification (BMOD) in obese and overweight patients with major depressive illness because of recent concerns regarding psychiatric side effect issues and the known potential for bupropion to induce a depressive mood. The study concluded Contrave significantly improved body weight and ameliorated depressive symptoms as scored by the MADRS [58]. Contrave was submitted for U.S. regulatory approval in March 2010. The original submission was based on multiple clinical trials that evaluated Contrave in more than 4,500 patients. However, the U.S. FDA rejected Contrave in January 31, 2011 due to concerns regarding the cardiovascular safety profile of naltrexone/bupropion when used long-term in a population of overweight and obese subjects [8].
MONOTHERAPY AND COMBINATION THERAPIES CURRENTLY UNDER INVESTIGATION
- Obesity can be an incurable chronic disease that increases the risk for cardiovascular diseases such as diabetes and hypertension. Because lifestyle interventions alone rarely result in long-term weight loss and the large proportion of patients return to baseline weight within 3 to 5 years, pharmacotherapy as a lifestyle modification adjunct to improve the induction and maintenance of weight loss may be considered for individuals with a BMI ≥30 kg/m2 or for individuals with a BMI ≥27 kg/m2 and co-morbidities [4]. Early administration of anti-obesity pharmacotherapy could provide significant benefit in the reduction of both weight and the risk for the development of comorbidities. However, despite promising results on reduction of body weight and improvement of several cardio-metabolic factors, many drugs that have been effective weight loss medications were withdrawn from the market in the last few years due to serious adverse effects. The positive data observed for surrogate markers was found not necessarily corresponding to positive clinical outcomes, though the anti-obesity drugs were expected to reduce cardiovascular outcome, beyond weight control. Treatments that reduce weight but do not improve cardiovascular outcome are thought to be of cosmetic benefit only and would thereby be less likely to gain approval for clinical use. Because orlistat is currently the only anti-obesity drug approved for long-term use, the development of new anti-obesity drugs is therefore urgently needed. This reasoning has led to the development of a number of new treatments for obesity in which multiple mechanisms are targeted, either by a single drug, such as tesofensine, or through drug combinations such as Qnexa, Contrave and Empatic (Table 3). However, the previous withdrawals also suggest regulatory committees are increasingly reluctant to approve a recommendation for the treatment of obesity using novel drugs, including lorcaserin, Qnexa, and Contrave without data that clearly supports long-term safety. The emphasis that both the U.S. FDA and EMA have placed on metabolic indices is based on recognition the drugs impact the onset of cardiovascular outcome more directly than weight loss alone. Although the guidelines for approval and market withdrawal are considerable barriers to the development of new anti-obesity drugs, the long-term safety and efficacy of newly developed drugs should also be evaluated in the management of obesity, which often requires ongoing therapy to achieve and maintain weight loss. Finally, government policy should support social intervention as a means of managing obesity because obese patients often return to toxic environments and behaviors. When considering anti-obesity therapy, in addition to scientific and regulatory considerations, patients' financial barriers also need to be taken into account.
- In conclusion, it is expected that more effective and better tolerated anti-obesity drugs will be developed through a better understanding of the multiple mechanisms and complex physiological systems targeting appetite. Additionally, a lifestyle modification will hopefully be supported alongside all other treatments for obesity, because it remains the cornerstone of the medical management of obesity.
SUMMARY
- 1. James WP. The epidemiology of obesity: the size of the problem. J Intern Med 2008;263:336-352. ArticlePubMed
- 2. Park HS, Park CY, Oh SW, Yoo HJ. Prevalence of obesity and metabolic syndrome in Korean adults. Obes Rev 2008;9:104-107. Article
- 3. National Task Force on the Prevention and Treatment of Obesity. Overweight, obesity, and health risk. Arch Intern Med 2000;160:898-904. ArticlePubMed
- 4. National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res 1998;6(Suppl 2):51S-209S. ArticlePubMed
- 5. Kaukua J, Pekkarinen T, Sane T, Mustajoki P. Health-related quality of life in obese outpatients losing weight with very-low-energy diet and behaviour modification: a 2-y follow-up study. Int J Obes Relat Metab Disord 2003;27:1072-1080. ArticlePubMedPDF
- 6. Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med 1997;337:581-588. ArticlePubMed
- 7. Glazer G. Long-term pharmacotherapy of obesity 2000: a review of efficacy and safety. Arch Intern Med 2001;161:1814-1824. ArticlePubMed
- 8. Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 2007;370:1706-1713. ArticlePubMed
- 9. Powell AG, Apovian CM, Aronne LJ. New drug targets for the treatment of obesity. Clin Pharmacol Ther 2011;90:40-51. ArticlePubMed
- 10. Ioannides-Demos LL, Proietto J, Tonkin AM, McNeil JJ. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf 2006;29:277-302. ArticlePubMed
- 11. James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA, Renz CL. SCOUT Investigators. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 2010;363:905-917. ArticlePubMed
- 12. Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol 2010;6:578-588. ArticlePubMedPMCPDF
- 13. Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, Hilton L, Suttorp M, Solomon V, Shekelle PG, Morton SC. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med 2005;142:532-546. ArticlePubMed
- 14. Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metab Disord 2002;26:262-273. ArticlePubMedPDF
- 15. Kang JG, Park CY, Kang JH, Park YW, Park SW. Randomized controlled trial to investigate the effects of a newly developed formulation of phentermine diffuse-controlled release for obesity. Diabetes Obes Metab 2010;12:876-882. ArticlePubMed
- 16. Cercato C, Roizenblatt VA, Leanca CC, Segal A, Lopes Filho AP, Mancini MC, Halpern A. A randomized double-blind placebo-controlled study of the long-term efficacy and safety of diethylpropion in the treatment of obese subjects. Int J Obes (Lond) 2009;33:857-865. ArticlePubMedPDF
- 17. Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov 2004;3:771-784. ArticlePubMedPDF
- 18. Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. RIO-Europe Study Group. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005;365:1389-1397. ArticlePubMed
- 19. Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. RIO-North America Study Group. Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 2006;295:761-775. ArticlePubMed
- 20. Despres JP, Golay A, Sjostrom L. Rimonabant in Obesity-Lipids Study Group. Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005;353:2121-2134. ArticlePubMed
- 21. Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. RIO-Diabetes Study Group. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 2006;368:1660-1672. ArticlePubMed
- 22. Samat A, Tomlinson B, Taheri S, Thomas GN. Rimonabant for the treatment of obesity. Recent Pat Cardiovasc Drug Discov 2008;3:187-193. ArticlePubMed
- 23. Aronne LJ, Tonstad S, Moreno M, Gantz I, Erondu N, Suryawanshi S, Molony C, Sieberts S, Nayee J, Meehan AG, Shapiro D, Heymsfield SB, Kaufman KD, Amatruda JM. A clinical trial assessing the safety and efficacy of taranabant, a CB1R inverse agonist, in obese and overweight patients: a high-dose study. Int J Obes (Lond) 2010;34:919-935. ArticlePubMedPDF
- 24. Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohorquez SM, Stoch A, Stevens C, Fong TM, De Lepeleire I, Cilissen C, Cote J, Rosko K, Gendrano IN 3rd, Nguyen AM, Gumbiner B, Rothenberg P, de Hoon J, Bormans G, Depre M, Eng WS, Ravussin E, Klein S, Blundell J, Herman GA, Burns HD, Hargreaves RJ, Wagner J, Gottesdiener K, Amatruda JM, Heymsfield SB. The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab 2008;7:68-78. ArticlePubMed
- 25. Proietto J, Rissanen A, Harp JB, Erondu N, Yu Q, Suryawanshi S, Jones ME, Johnson-Levonas AO, Heymsfield SB, Kaufman KD, Amatruda JM. A clinical trial assessing the safety and efficacy of the CB1R inverse agonist taranabant in obese and overweight patients: low-dose study. Int J Obes (Lond) 2010;34:1243-1254. ArticlePubMedPDF
- 26. Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ 2007;335:1194-1199. ArticlePubMedPMC
- 27. Park CY, Kim YS, Ryu MS, Nam SY, Park HS, Kim SM. A phase 3 double-blind, parallel-group, placebo-controlled trial of the efficacy and safety of sibutramine (Reductil) in the treatment of obese patients. J Korean Soc Study Obes 2001;10:336-347.
- 28. Nisoli E, Carruba MO. An assessment of the safety and efficacy of sibutramine, an anti-obesity drug with a novel mechanism of action. Obes Rev 2000;1:127-139. ArticlePubMed
- 29. Mannucci E, Dicembrini I, Rotella F, Rotella CM. Orlistat and sibutramine beyond weight loss. Nutr Metab Cardiovasc Dis 2008;18:342-348. ArticlePubMed
- 30. Bosello O, Carruba MO, Ferrannini E, Rotella CM. Sibutramine lost and found. Eat Weight Disord 2002;7:161-167. ArticlePubMedPDF
- 31. Torp-Pedersen C, Caterson I, Coutinho W, Finer N, Van Gaal L, Maggioni A, Sharma A, Brisco W, Deaton R, Shepherd G, James P. SCOUT Investigators. Cardiovascular responses to weight management and sibutramine in high-risk subjects: an analysis from the SCOUT trial. Eur Heart J 2007;28:2915-2923. ArticlePubMed
- 32. Astrup A. Drug management of obesity: efficacy versus safety. N Engl J Med 2010;363:288-290. ArticlePubMed
- 33. Harrison-Woolrych M, Ashton J, Herbison P. Fatal and non-fatal cardiovascular events in a general population prescribed sibutramine in New Zealand: a prospective cohort study. Drug Saf 2010;33:605-613. PubMed
- 34. Rossner S, Sjostrom L, Noack R, Meinders AE, Noseda G. European Orlistat Obesity Study Group. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. Obes Res 2000;8:49-61. ArticlePubMed
- 35. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155-161. PubMed
- 36. Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, Heimburger DC, Lucas CP, Robbins DC, Chung J, Heymsfield SB. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA 1999;281:235-242. ArticlePubMed
- 37. Chon S, Park C, Koh G, Oh S, Woo JT, Kim SW, Kim JW, Kim YS, Son HY, Cha BY, Yoon KH, Kwon HS, Cha BS, Lee HC. The effect of orlistat in obese patients with type 2 diabetes: benefit on abdominal obesity and glycemic control. J Korean Soc Study Obes 2004;13:281-292.
- 38. Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev 2004;CD004094ArticlePubMed
- 39. Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 2010;363:245-256. ArticlePubMed
- 40. Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, Anderson CM. BLOSSOM Clinical Trial Group. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab 2011;96:3067-3077. ArticlePubMedPDF
- 41. Briefing information for the September 16, 2010 meeting of the Endocrinologic and Metabolic Drugs Advisory Committee. U.S. Food and Drug Administration updated 2010 Sep 14. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm225628.htm.
- 42. Arena and Eisai complete end-of-review meeting with FDA for Lorcaserin new drug application. Arena Pharmaceuticals updated 2010 Dec 22. Available from: http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=538430.
- 43. Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006;8:436-447. ArticlePubMed
- 44. Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, Zdravkovic M, During M, Matthews DR. LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009;32:84-90. PubMedPMC
- 45. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194-206. ArticlePubMed
- 46. Meade LT, Tackett KL, McKeever AL. Practical use of exenatide and pramlintide for the treatment of type 2 diabetes. J Pharm Pract 2009;22:540-545.ArticlePDF
- 47. Astrup A, Rossner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME. NN8022-1807 Study Group. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 2009;374:1606-1616. ArticlePubMed
- 48. Significant weight loss sustained in obese people treated with liraglutide for one year. Novo Nordisk updated 2008 Jun 16. Available from: http://www.novonordisk.com/investors/sea/sea.asp?sShowNewsItemGuID=34d7e084-1d6b-4b78-bb9e-f0bf70920b0a&sShowLanguageCode=en-GB&sSearchText=Liraglutide+AND+obesity.
- 49. Astrup A, Meier DH, Mikkelsen BO, Villumsen JS, Larsen TM. Weight loss produced by tesofensine in patients with Parkinson's or Alzheimer's disease. Obesity (Silver Spring) 2008;16:1363-1369. ArticlePubMedPDF
- 50. Astrup A, Madsbad S, Breum L, Jensen TJ, Kroustrup JP, Larsen TM. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1906-1913. ArticlePubMed
- 51. NeuroSearch successfully completes End of Phase II meeting with the FDA for tesofensine, a treatment for obesity. NeuroSearch updated 2009 Jun 8. Available from: https://newsclient.omxgroup.com/cdsPublic/viewDisclosure.action?disclosureId=329924&messageId=396203.
- 52. Kopelman P, Groot Gde H, Rissanen A, Rossner S, Toubro S, Palmer R, Hallam R, Bryson A, Hickling RI. Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical). Obesity (Silver Spring) 2010;18:108-115. ArticlePubMedPDF
- 53. Glandt M, Raz I. Present and future: pharmacologic treatment of obesity. J Obes 2011;2011:636181ArticlePubMedPMCPDF
- 54. Kaya A, Aydin N, Topsever P, Filiz M, Ozturk A, Dagar A, Kilinc E, Ekmekcioglu C. Efficacy of sibutramine, orlistat and combination therapy on short-term weight management in obese patients. Biomed Pharmacother 2004;58:582-587. ArticlePubMed
- 55. Qnexa meets primary endpoint by demonstrating superior weight loss over components and placebo in the 28-week equate study (OB-301). Vivus updated 2008 Dec 11. Available from: http://ir.vivus.com/releasedetail.cfm?ReleaseID=353965.
- 56. Vivus announces positive results from two phase 3 studies; obese patients on Qnexa achieve average weight loss up to 14.7% and significant improvements in co-morbidities. Vivus cited 2009 Sep 9. Available from: http://ir.vivus.com/releasedetail.cfm?ReleaseID=407933.
- 57. Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E. COR-I Study Group. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010;376:595-605. ArticlePubMed
- 58. Orexigen (R) therapeutics presents new data showing contrave (R) significantly lowers weight, improves blood glucose control in obese patients with type 2 diabetes. Orexigen Therapeutics Inc cited 2010 Jun 25. Available from: http://ir.orexigen.com/phoenix.zhtml?c=207034&p=irol-newsArticle_print&ID=1441827&highlight=.
- 59. Wadden TA, Foreyt JP, Foster GD, Hill JO, Klein S, O'Neil PM, Perri MG, Pi-Sunyer FX, Rock CL, Erickson JS, Maier HN, Kim DD, Dunayevich E. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19:110-120. ArticlePubMedPDF
REFERENCES
Figure & Data
References
Citations

- Design, synthesis and anticholinergic properties of novel α-benzyl dopamine, tyramine, and phenethylamine derivatives
Ali Naderi, Akın Akıncıoğlu, Ahmet Çağan, Hilal Çelikkaleli, Hülya Akıncıoğlu, Süleyman Göksu
Bioorganic Chemistry.2024; 144: 107146. CrossRef - Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: a pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database
Mansour Tobaiqy, Hajer Elkout
International Journal of Clinical Pharmacy.2024; 46(2): 488. CrossRef - Ilex paraguariensis A.St.-Hil. improves lipid metabolism in high-fat diet-fed obese rats and suppresses intracellular lipid accumulation in 3T3-L1 adipocytes via the AMPK-dependent and insulin signaling pathways
Maya Kudo, Ming Gao, Misa Hayashi, Yukiko Kobayashi, Jinwei Yang, Tonghua Liu
Food & Nutrition Research.2024;[Epub] CrossRef -
Discovery of thiazolidinedione-based pancreatic lipase inhibitors as anti-obesity agents: synthesis,
in silico
studies and pharmacological investigations
Prashant Dhiman, Nisha Yadav, Prashant S. Auti, Shalini Jaswal, Gurpreet Singh, Sidharth Mehan, Balaram Ghosh, Atish T. Paul, Vikramdeep Monga
Journal of Biomolecular Structure and Dynamics.2024; : 1. CrossRef - Glucagon-like Peptide-1 Receptor Agonists and Suicidal Ideation: Analysis of Real-Word Data Collected in the European Pharmacovigilance Database
Rosanna Ruggiero, Annamaria Mascolo, Angela Spezzaferri, Claudia Carpentieri, Daniele Torella, Liberata Sportiello, Francesco Rossi, Giuseppe Paolisso, Annalisa Capuano
Pharmaceuticals.2024; 17(2): 147. CrossRef - Electro-acupuncture for central obesity: a patient-assessor blinded, randomized sham-controlled clinical trial
Tsz Fung Lam, Zipan Lyu, Xingyao Wu, Yi Ping Wong, Peihua Cao, Emily Yen Wong, Hung Bun Hung, Shiping Zhang, Zhaoxiang Bian, Linda L. D. Zhong
BMC Complementary Medicine and Therapies.2024;[Epub] CrossRef - Synthesis and Characterization of Short α and β-Mixed Peptides with Excellent Anti-Lipase Activities
Naeem Ahmed, Sabahat Asif, Muhammad Arfan, Qaiser Mahmood, Amjad Islam, Mansour K. Gatasheh, Muhammad Zia
Molecules.2024; 29(4): 765. CrossRef - Polyphenols: Role in Modulating Immune Function and Obesity
Md Abdullah Al Mamun, Ahmed Rakib, Mousumi Mandal, Santosh Kumar, Bhupesh Singla, Udai P. Singh
Biomolecules.2024; 14(2): 221. CrossRef - Anti-Obesity Effect and Signaling Mechanism of Potassium Poly-γ-Glutamate Produced by Bacillus subtilis Chungkookjang in High-Fat Diet-Induced Obese Mice
Seung-Hyeon Lee, Jiwon Choi, Jae Young Park, Ha-Rim Kim, Myeongkuk Shim, Kyunghyun Im, Hyeonjeong Choe, Jae-Chul Choi, Young-Chul Park, Tae-Gyu Lim, Hyangyim Seo, Hansu Jang, Boung-Jun Oh, Seon-Young Kim, Mi Hee Park
Nutrients.2024; 16(6): 809. CrossRef - Revealing Molecular Mechanisms of the Bioactive Saponins from Edible Root of Platycodon grandiflorum in Combating Obesity
Bincheng Han, Jinhai Luo, Baojun Xu
Plants.2024; 13(8): 1123. CrossRef - Analysis of 15 anti-obesity drugs in urine using thermal-assisted paper spray mass spectrometry
Shijia Jiang, Junbo Zhao, Hui Yan, Ping Xiang, Min Shen
Analytical Methods.2023; 15(35): 4434. CrossRef - Effect of Abutilon indicum (L) Extract on Adipogenesis, Lipolysis and Cholesterol Esterase in 3T3-L1 Adipocyte Cell Lines
Lavanya Lakshminarayana, V. Veeraraghavan, Kuruvalli Gouthami, Renuka Srihari, Prashantha Chowdadenahalli Nagaraja
Indian Journal of Clinical Biochemistry.2023; 38(1): 22. CrossRef - Nutritional and bioactive composition, nutraceutical potential, food and packaging applications of Cydonia oblonga and its byproducts: A review
Jahangir A. Rather, Sabreena Yousuf, Qazi Showkat Ashraf, Shabir A. Mir, Hilal A. Makroo, Darakshan Majid, Francisco J. Barba, B.N. Dar
Journal of Food Composition and Analysis.2023; 115: 105000. CrossRef - Bifidobacterium lactis IDCC 4301 Exerts Anti‐Obesity Effects in High‐Fat Diet‐Fed Mice Model by Regulating Lipid Metabolism
O‐Hyun Ban, Minjee Lee, Won Yeong Bang, Eoun Ho Nam, Hyeon Ji Jeon, Minhye Shin, Jungwoo Yang, Young Hoon Jung
Molecular Nutrition & Food Research.2023;[Epub] CrossRef - In Vivo Pharmacodynamics of Calophyllum soulattri as Antiobesity with In Silico Molecular Docking and ADME/Pharmacokinetic Prediction Studies
Inarah Fajriaty, Hariyanto Ih, Irda Fidrianny, Neng Fisheri Kurniati, Muhammad Andre Reynaldi, I Ketut Adnyana, Rommy Rommy, Fransiska Kurniawan, Daryono Hadi Tjahjono
Pharmaceuticals.2023; 16(2): 191. CrossRef - Pharmacological Treatments and Natural Biocompounds in Weight Management
Amin Gasmi, Pavan Kumar Mujawdiya, Amine Nehaoua, Mariia Shanaida, Yuliya Semenova, Salva Piscopo, Alain Menzel, Volodymyr Voloshyn, Olena Voloshyn, Volodymyr Shanaida, Geir Bjørklund
Pharmaceuticals.2023; 16(2): 212. CrossRef - A Multi-Ingredient Supplement Protects against Obesity and Infertility in Western Diet-Fed Mice
Mats I. Nilsson, Linda May, Liza J. Roik, Matthew R. Fuda, Ashely Luo, Bart P. Hettinga, Adam L. Bujak, Mark A. Tarnopolsky
Nutrients.2023; 15(3): 611. CrossRef - The efficacy of topical aminophylline in local fat reduction: A systematic review
Ramin Abdi Dezfouli, Ali Hosseinpour, Mostafa Qorbani, Elnaz Daneshzad
Frontiers in Endocrinology.2023;[Epub] CrossRef - Pharmacologic Treatment of Obesity in Reproductive Aged Women
Akua Nuako, Lucy Tu, Karen J. Campoverde Reyes, Shradha M. Chhabria, Fatima Cody Stanford
Current Obstetrics and Gynecology Reports.2023; 12(2): 138. CrossRef - Obesity and renal disease: Benefits of bariatric surgery
Leopoldo G. Ardiles
Frontiers in Medicine.2023;[Epub] CrossRef - A green protocol ball milling synthesis of dihydropyrano[2,3-c]pyrazole using nano-silica/aminoethylpiperazine as a metal-free catalyst
Dina Mallah, Bi Bi Fatemeh Mirjalili
BMC Chemistry.2023;[Epub] CrossRef - The Beneficial Effect of Salicornia herbacea Extract and Isorhamnetin-3-O-Glucoside on Obesity
Ji Hwan Lee, Sanghyun Lee, Jun Yeon Park, Il-Ho Park, Ki Sung Kang, Myoung-Sook Shin
Processes.2023; 11(4): 977. CrossRef - Encapsulated Peptides and Proteins with an Effect on Satiety
Rafael O. de A. Costa, Thaís S. Passos, Eloyse Mikaelly de S. Silva, Nicolle Caroline S. dos Santos, Ana Heloneida de A. Morais
Nanomaterials.2023; 13(7): 1166. CrossRef - Chinese chestnut shell polyphenol extract regulates the JAK2/STAT3 pathway to alleviate high-fat diet-induced, leptin-resistant obesity in mice
Suwen Liu, Wenhong Jiang, Chang Liu, Shuo Guo, Hao Wang, Xuedong Chang
Food & Function.2023; 14(10): 4807. CrossRef - Investigation of the Therapeutic Value of Verbascum pyramidatum Bieb. for Obesity
Sıla SENER, Merve BADEM, Mehmet ÇATALBAŞ, Şeyda KANBOLAT, Ufuk ÖZGEN, Nevin ULAŞ ÇOLAK
Hacettepe Journal of Biology and Chemistry.2023; 51(3): 251. CrossRef - Obesity as a Neurobiologic Disorder: A Heavyweight Contender
Mervin Chávez-Castillo, Pablo Duran, Bermary Garrido, Andrea Díaz, Daniel Escalona, Clímaco Cano
Current Psychiatry Research and Reviews.2023; 19(2): 109. CrossRef - The Effect of Guisangyou Tea on Abnormal Lipid Metabolism in Mice Induced by High-Fat Diet
Yan Zhu, Xianghui Zhou, Nan Ling, Qiming Yu, Huijuan Wang, Qizhen Du
Foods.2023; 12(11): 2171. CrossRef - The anti-obesity effects of resveratrol on the 3T3-L1 adipocytes
Roghayeh Molani-Gol, Maryam Rafraf
International Journal for Vitamin and Nutrition Research.2023;[Epub] CrossRef - Novel Lipids to Regulate Obesity and Brain Function: Comparing Available Evidence and Insights from QSAR In Silico Models
Francisca S. Teixeira, Paula T. Costa, Ana M. S. Soares, Ana Luiza Fontes, Manuela E. Pintado, Susana S. M. P. Vidigal, Lígia L. Pimentel, Luís M. Rodríguez-Alcalá
Foods.2023; 12(13): 2576. CrossRef - Newly Synthesized N-Glycosidic Halochalcones Reveal Inhibitory Activity on Pancreatic Triacylglycerol Lipase
M. Yikilmaz, S. Fandakli, S. O. Sener, M. Badem, S. Kanbolat, N. Yayli, U. Uzuner, R. Aliyazicioglu
Chemistry of Natural Compounds.2023; 59(4): 629. CrossRef - Effects of Ocimum basilicum mucilage on hyperlipidemia and gut microbiota on mice fed a high-fat diet
Duy Nguyen-Le, Cao-Tri Nguyen, Minh-Vu Ngo-Phan, Thuoc Linh Tran, Minh-Duy Phan, Tatsuya Unno, Hieu Tran-Van
Bioactive Carbohydrates and Dietary Fibre.2023; 30: 100384. CrossRef - Improvement of blood lipid metabolism and obesity through the administration of mixed lactic acid bacteria including Lactobacillus plantarum K-1 in mice fed a high-fat diet
Hyeon Ju Lim, Young Geol Yoon
Journal of Applied Biological Chemistry.2023;[Epub] CrossRef - Knowledge and Attitude of the General Population in Saudi Arabia Toward Weight Management Medications (WMMs): A Cross-Sectional Study
Malak A Algarni , Ameera Ali M Algarni, Waleed A Alqarni, Ahmad Y Alqassim
Cureus.2023;[Epub] CrossRef - Paeonia lactifloraroot decreases lipid accumulation through the induction of lipolysis and thermogenesis via AMPK activation in 3T3‑L1 cells
Jeong Choi, Hyeok Choi, Gwang Ryu, Jae Lee, Jueng Beak, Eun Koh, Jin Jeong
International Journal of Molecular Medicine.2023;[Epub] CrossRef - Micro-Executor of Natural Products in Metabolic Diseases
Jinxin Liu, Huanwen Chen, Xiaoli Li, Chunmei Song, Li Wang, Deguo Wang
Molecules.2023; 28(17): 6202. CrossRef - A nationwide pharmacovigilance investigation on trends and seriousness of adverse events induced by anti-obesity medication
Yeo Jin Choi, Chang-Young Choi, Choong Ui Kim, Sooyoung Shin
Journal of Global Health.2023;[Epub] CrossRef - The Effect of Baekhogainsam-tang on Metabolism through Modulation of the Gut Microbiota and Gene Expression in High-Fat Diet Induced Metabolic Syndrome Animal Model
Min-Jin Cho, Song-Yi Han, Soo Kyoung Lim, Eun-Ji Song, Young-Do Nam, Hojun Kim
Journal of Korean Medicine Rehabilitation.2023; 33(3): 1. CrossRef - Exploring the anti-obesity bioactive compounds of Thymelaea hirsuta and Ziziphus spina-christi through integration of lipase inhibition screening and molecular docking analysis
Rokia M. Abdallah, Hala M. Hammoda, Nahla S. El-Gazzar, Reham S. Ibrahim, Shaimaa M. Sallam
RSC Advances.2023; 13(39): 27167. CrossRef - Cold Exposure and Oral Delivery of GLP-1R Agonists by an Engineered Probiotic Yeast Strain Have Antiobesity Effects in Mice
Karl Alex Hedin, Hongbin Zhang, Vibeke Kruse, Vanessa Emily Rees, Fredrik Bäckhed, Thomas U. Greiner, Ruben Vazquez-Uribe, Morten Otto Alexander Sommer
ACS Synthetic Biology.2023; 12(11): 3433. CrossRef - Advances in oligosaccharides production from brown seaweeds: extraction, characterization, antimetabolic syndrome, and other potential applications
Pitchurajan Krishna Perumal, Chun-Yung Huang, Chiu-Wen Chen, Grace Sathyanesan Anisha, Reeta Rani Singhania, Cheng-Di Dong, Anil Kumar Patel
Bioengineered.2023;[Epub] CrossRef - The Use of Phentermine for Obesity in Psychiatric Patients With Antipsychotics
Eunju Kim, Daniel Rim, Jeong-Hun Shin, Denise Wong, Dong Wook Kim
Psychiatry Investigation.2023; 20(9): 799. CrossRef - Pharmacotherapy before and after bariatric surgery
Khaled Alabduljabbar, Carel W. le Roux
Metabolism.2023; 148: 155692. CrossRef - Sublethal toxicities of 2,4-dinitrophenol as inferred from online self-reports
Ali Abdelati, Michele M. Burns, Michael Chary, Heather M. Barkholtz
PLOS ONE.2023; 18(9): e0290630. CrossRef - Anti-obesity effects of Glycyrrhiza uralensis
ethanol extract on the inhibition of 3T3-L1 adipocyte differentiation in
high-fat diet-induced C57BL/6J mice
Seon Kyeong Park, Jangho Lee, Soo Hyun Park, Yu Geon Lee
Korean Journal of Food Preservation.2023; 30(4): 716. CrossRef - Cydonia oblonga Miller fruit extract exerts an anti-obesity effect in 3T3-L1 adipocytes by activating the AMPK signaling pathway
Hyun Sook Lee, Jae In Jung, Jung Soon Hwang, Myeong Oh Hwang, Eun Ji Kim
Nutrition Research and Practice.2023; 17(6): 1043. CrossRef - Effect of Plant Extracts on Protein Changes During Adipogenesis: A Scoping Review
Nur Dayana Hassan Cheong, Emida Mohamed, Norhisham Haron, Siti Nazrina Camalxaman
Malaysian Journal of Medicine and Health Sciences.2023; 19(5): 331. CrossRef - An Investigative Study of Medicinal Herbs for Anti-obesity Potential: (A-Review)
Roma Ghai, Sneha Chaudhary, Kandasamy Nagarajan, Richa Goel, Shardendu Kumar Mishra, Naveen Kumar Tholia, Nazakat Ali, Monika Kaurav
Oriental Journal Of Chemistry.2023; 39(6): 1437. CrossRef - Anti-Lipase and Antioxidant Activities of the Selected Plant Materials
Vidhi Khatlawala, Viraj Roghelia
The Indian Journal of Nutrition and Dietetics.2023; : 389. CrossRef - A review on the genus Populus: a potential source of biologically active compounds
Ishita Guleria, Amita Kumari, Marie-Aleth Lacaille-Dubois, Nishant, Vikas Kumar, Adesh K. Saini, Jyoti Dhatwalia, Sohan Lal
Phytochemistry Reviews.2022; 21(4): 987. CrossRef - Myrianthus arboreus P. Beauv improves insulin sensitivity in high fat diet-induced obese mice by reducing inflammatory pathways activation
Rasidat O. Tijani, Jose Alberto Molina-Tijeras, Teresa Vezza, Antonio Jesús Ruiz-Malagón, María de la Luz Cádiz-Gurrea, Antonio Segura-Carretero, Oyindamola O. Abiodun, Julio Galvez
Journal of Ethnopharmacology.2022; 282: 114651. CrossRef - β-Carotene stimulates browning of 3T3-L1 white adipocytes by enhancing thermogenesis via the β3-AR/p38 MAPK/SIRT signaling pathway
Sulagna Mukherjee, Jong Won Yun
Phytomedicine.2022; 96: 153857. CrossRef - Anti-Obesity Potential of Ponciri Fructus: Effects of Extracts, Fractions and Compounds on Adipogenesis in 3T3-L1 Preadipocytes
Gopal Lamichhane, Prakash Raj Pandeya, Ramakanta Lamichhane, Su-jin Rhee, Hari Prasad Devkota, Hyun-Ju Jung
Molecules.2022; 27(3): 676. CrossRef - Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review
Azrina Azlan, Sharmin Sultana, Chan Suk Huei, Muhammad Rizal Razman
Molecules.2022; 27(3): 898. CrossRef - Rapid Screening of Lipase Inhibitors from Ophiopogonis Radix Using High-Performance Thin Layer Chromatography by Two Step Gradient Elution Combined with Bioautographic Method
Xue Hua, Hui-Jie Hong, Dai-Yan Zhang, Qiao Liu, Fong Leong, Qi Yang, Yuan-Jia Hu, Xiao-Jia Chen
Molecules.2022; 27(4): 1155. CrossRef - α-Glucosidase Inhibitory Activity and Anti-Adipogenic Effect of Compounds from Dendrobium delacourii
May Thazin Thant, Hnin Ei Ei Khine, Justin Quiel Lasam Nealiga, Nutputsorn Chatsumpun, Chatchai Chaotham, Boonchoo Sritularak, Kittisak Likhitwitayawuid
Molecules.2022; 27(4): 1156. CrossRef - Zebrafish obesogenic test identifies anti‐adipogenic fraction in Moringa oreifera leaf extracts
Izumi Matsuoka, Kanae Hata, Hirotaka Katsuzaki, Hiroko Nakayama, Liqing Zang, Mizuho Ota, Youngil Kim, Djong‐Chi Chu, Lekh Raj Juneja, Norihiro Nishimura, Yasuhito Shimada
Food Science & Nutrition.2022; 10(4): 1248. CrossRef - Abuse Potential of Cathinones in Humans: A Systematic Review
Lourdes Poyatos, Adrián Torres, Esther Papaseit, Clara Pérez-Mañá, Olga Hladun, Melani Núñez-Montero, Georgina de la Rosa, Marta Torrens, Daniel Fuster, Robert Muga, Magí Farré
Journal of Clinical Medicine.2022; 11(4): 1004. CrossRef - Current Therapy and Therapeutic Targets for Microsporidiosis
Junhong Wei, Zhihui Fei, Guoqing Pan, Louis M. Weiss, Zeyang Zhou
Frontiers in Microbiology.2022;[Epub] CrossRef - Shinorine and porphyra-334 isolated from laver (Porphyra dentata) inhibit adipogenesis in 3T3-L1 cells
Su-Young Choi, Su Yeon Lee, Hyung Gyun Kim, Jae Cheon Jeong, Don Carlo Batara, Sung-Hak Kim, Jeong-Yong Cho
Food Science and Biotechnology.2022; 31(5): 617. CrossRef - Sargassum horneri inhibits fat accumulation via up-regulation of thermogenesis in obese mice
Min-Cheol Kang, Hyo Geun Lee, Sang Hoon Lee, Kyung-Mo Song, Hyun-Soo Kim, Sera Kim, Yun-Sang Choi, You-Jin Jeon
Journal of Functional Foods.2022; 92: 105022. CrossRef - Anti-Obesity and Anti-Hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice
Min Ju Kim, Ramakrishna Chilakala, Hee Geun Jo, Seung-Jae Lee, Dong-Sung Lee, Sun Hee Cheong
International Journal of Molecular Sciences.2022; 23(7): 4015. CrossRef - Effects of the Antiobesity Drugs Aplex and Venera on Certain Biochemical and Physiological Indices in Obese Adult Male Albino Rats
Suzan S. A. Elpasty, Eman G. E. Helal, Ashraf M. M. Algendy, Hany N. Yousef, Benedetto Natalini
Advances in Pharmacological and Pharmaceutical Sciences.2022; 2022: 1. CrossRef - Fenugreek (Trigonella foenum-graecum): Nutraceutical values, phytochemical, ethnomedicinal and pharmacological overview
Pushpa Ruwali, Niharika Pandey, Khusboo Jindal, Rahul Vikram Singh
South African Journal of Botany.2022; 151: 423. CrossRef - Untapped Pharmaceutical Potential of 4,5,4′-Trihydroxy-3,3′-dimethoxybibenzyl for Regulating Obesity: A Cell-Based Study with a Focus on Terminal Differentiation in Adipogenesis
Hnin Ei Ei Khine, Rungroch Sungthong, Boonchoo Sritularak, Eakachai Prompetchara, Chatchai Chaotham
Journal of Natural Products.2022; 85(6): 1591. CrossRef - Efficacy of subcutaneous semaglutide compared to placebo for weight loss in obese, non-diabetic adults: a systematic review & meta-analysis
Naazneen Arastu, Olivia Cummins, Wanda Uribe, Eric C. Nemec
International Journal of Clinical Pharmacy.2022; 44(4): 852. CrossRef - Anti-Obesity Effect of Porcine Collagen Peptide in 3T3-L1 Adipocytes and High-Fat Diet-Fed Mice by Regulating Adipogenesis
Eunji Lee, Jiyoung Bang, Jeong Yoon Lee, Woojin Jun, Yoo-Hyun Lee
Journal of Medicinal Food.2022; 25(7): 732. CrossRef - FTO gene expression in diet-induced obesity is downregulated by Solanum fruit supplementation
Edeke Affiong Asuquo, Okwesilieze Fred Chiletugo Nwodo, Anosike Chioma Assumpta, Uchendu Nene Orizu, Okoro Nkwachukwu Oziamara, Odiba Arome Solomon
Open Life Sciences.2022; 17(1): 641. CrossRef - The Benefits of Anthocyanins against Obesity-Induced Inflammation
Chanya Ngamsamer, Jintana Sirivarasai, Nareerat Sutjarit
Biomolecules.2022; 12(6): 852. CrossRef - Methanol leaf extracts of Chrysophyllum albidum and Irvingia gabonensis protected against dyslipidaemia and oxidative stress induced by high-fat diet in Wistar rats
Osebhahiemen Ibukun, Ehimwenma S. Omoregie
Bulletin of the National Research Centre.2022;[Epub] CrossRef - Biomaterial-Based Therapeutic Strategies for Obesity and Its Comorbidities
Jing Li, Hongli Duan, Yan Liu, Lu Wang, Xing Zhou
Pharmaceutics.2022; 14(7): 1445. CrossRef - Computational Methods in Cooperation with Experimental Approaches to Design Protein Tyrosine Phosphatase 1B Inhibitors in Type 2 Diabetes Drug Design: A Review of the Achievements of This Century
Mara Ibeth Campos-Almazán, Alicia Hernández-Campos, Rafael Castillo, Erick Sierra-Campos, Mónica Valdez-Solana, Claudia Avitia-Domínguez, Alfredo Téllez-Valencia
Pharmaceuticals.2022; 15(7): 866. CrossRef - Drivers of medicalization in the Canadian Adult Obesity Clinical Practice Guidelines
Andrea E. Bombak, Louise Adams, Patricia Thille
Canadian Journal of Public Health.2022; 113(5): 743. CrossRef - PRO: Should patients with nonalcoholic steatohepatitis fibrosis undergo bariatric surgery as primary treatment?
Nayantara Orekondy, David Lee, Raza Malik
Clinical Liver Disease.2022; 20(1): 5. CrossRef - Effects of Coix Seed Extract, Bifidobacterium BPL1, and Their Combination on the Glycolipid Metabolism in Obese Mice
Wei Zhang, Xiuzhen Jia, Yuhan Xu, Qiaoling Xie, Meizhen Zhu, Hesong Zhang, Zifu Zhao, Jingyu Hao, Haoqiu Li, Jinrui Du, Yan Liu, Wei-Hsien Liu, Xia Ma, Weilian Hung, Haotian Feng, Hongwei Li
Frontiers in Nutrition.2022;[Epub] CrossRef - Anti-obesity weight loss medications: Short-term and long-term use
Dagam Jeong, Ronny Priefer
Life Sciences.2022; 306: 120825. CrossRef - Total saponins from quinoa bran alleviate high‐fat diet‐induced obesity and systemic inflammation via regulation of gut microbiota in rats
Wei Li, Yu Song, Ya‐Nan Cao, Le‐Le Zhang, Gang Zhao, Ding‐Tao Wu, Liang Zou
Food Science & Nutrition.2022; 10(11): 3876. CrossRef - White to brown adipocyte transition mediated by Apigenin via VEGF‐PRDM16 signaling
Sreelekshmi Sreekumar, Vinu Vijayan, Fathe Singh, Manu Sudhakar, Rachita Lakra, Purna Sai Korrapati, Manikantan Syamala Kiran
Journal of Cellular Biochemistry.2022; 123(11): 1793. CrossRef - A Critical Review on Obesity: Herbal Approach, Bioactive Compounds, and Their Mechanism
Mukul Kumar, Deepika Kaushik, Jasjit Kaur, Charalampos Proestos, Fatih Oz, Emel Oz, Prerna Gupta, Priyanka Kundu, Anmol Kaur, Anisha Anisha, Ritika Ritika
Applied Sciences.2022; 12(16): 8342. CrossRef - Inhibition of enzymes associated with obesity by the polyphenol-rich extracts of Hibiscus sabdariffa
Manisha Singh, Thilini Thrimawithana, Ravi Shukla, Benu Adhikari
Food Bioscience.2022; 50: 101992. CrossRef - Cross-Talk between Obesity and Diabetes: Introducing Polyphenols as an Effective Phytomedicine to Combat the Dual Sword Diabesity
Muhammad Ajmal Shah, Muhammad Haris, Hafiza Ishmal Faheem, Ayesha Hamid, Rimsha Yousaf, Azhar Rasul, Ghulam Mujtaba Shah, Atif Ali Khan Khalil, Abdul Wahab, Haroon Khan, Reem Hasaballah Alhasani, Norah A. Althobaiti
Current Pharmaceutical Design.2022; 28(19): 1523. CrossRef - Morchella esculenta polysaccharide attenuate obesity, inflammation and modulate gut microbiota
Ata Ur Rehman, Asif Iqbal Khan, Yi Xin, Wang Liang
AMB Express.2022;[Epub] CrossRef - A Botanical Mixture Consisting of Inula japonica and Potentilla chinensis Relieves Obesity via the AMPK Signaling Pathway in 3T3-L1 Adipocytes and HFD-Fed Obese Mice
Su-Yeon Lee, Kyung-Sook Chung, So-Ri Son, So Young Lee, Dae Sik Jang, Jong-Kil Lee, Hyun-Jae Kim, Chang-Seon Na, Sun-Hee Lee, Kyung-Tae Lee
Nutrients.2022; 14(18): 3685. CrossRef - Drug therapy for obesity in the Russian Federation: pharmacoepidemiological study
V. V. Strizheletsky, Yu. М. Gomon, Е. А. Spichakova, А. S. Kolbin, А. А. Kalyapin, S. А. Makarov, А. B. Lomiya, F. М. Sultanova
FARMAKOEKONOMIKA. Modern Pharmacoeconomics and Pharmacoepidemiology.2022; 15(3): 320. CrossRef - Critical review on anti-obesity effects of phytochemicals through Wnt/β-catenin signaling pathway
Jinhai Luo, Zhiling Yu, Juscelino Tovar, Anne Nilsson, Baojun Xu
Pharmacological Research.2022; 184: 106461. CrossRef - Anti-obesity effect of the combination of fermented extracts from Momordica charanatia and Withania somnifera in mice fed a high-fat diet
Seung Yeon Choi, Hyun A Park, Young Geol Yoon
Journal of Applied Biological Chemistry.2022; 65(3): 143. CrossRef - Microalgae oil fromSchizochytriumsp. alleviates obesity and modulates gut microbiota in high-fat diet-fed mice
Liyuan Ran, Jinhui Yu, Rui Ma, Qing Yao, Mingjie Wang, Yuping Bi, Zichao Yu, Yingjie Wu
Food & Function.2022; 13(24): 12799. CrossRef - Tea Plant (Camellia sinensis): A Current Update on Use in Diabetes, Obesity, and Cardiovascular Disease
James Michael Brimson, Mani Iyer Prasanth, Kishoree Krishna Kumaree, Premrutai Thitilertdecha, Dicson Sheeja Malar, Tewin Tencomnao, Anchalee Prasansuklab
Nutrients.2022; 15(1): 37. CrossRef - Effects of black onion vinegar on high fat diet-induced obese C57BL/6 mice model
Mi Suk Kim, Ji Yun Baek, Ye Jung Choi, Ki Sung Kang, Weon Taek Seo, Ji Hyun Kim, Hyun Young Kim
Journal of Applied Biological Chemistry.2022; 65(4): 313. CrossRef - A Review of Genetic Understanding and Amelioration of EdibleAlliumSpecies
Geetika Malik, Ajmer Singh Dhatt, Ajaz Ahmed Malik
Food Reviews International.2021; 37(4): 415. CrossRef - Toxicity from illegitimate slimming agents – a 10-year case series at a tertiary toxicology laboratory in Hong Kong
Nike Kwai Cheung Lau, Magdalene Huen Yin Tang, Sau Wah Ng, Yeow Kuan Chong, Sammy Pak Lam Chen, Hencher Han Chih Lee, Chor Kwan Ching, Tony Wing Lai Mak
Clinical Toxicology.2021; 59(5): 426. CrossRef - The effect of magnesium supplementation on anthropometric indices: a systematic review and dose–response meta-analysis of clinical trials
Masoumeh Rafiee, Abed Ghavami, Ali Rashidian, Amir Hadi, Gholamreza Askari
British Journal of Nutrition.2021; 125(6): 644. CrossRef - Screening of Weight-Loss Herbal Products for Synthetic Anti-Obesity Adulterants: A Target-Oriented Analysis by Liquid Chromatography–Tandem Mass Spectrometry
P. Girish, M. Jayanthi, B. Gitanjali, S. Manikandan, S. Rajan
Journal of Dietary Supplements.2021; 18(1): 92. CrossRef - Transcriptional analyses of the effects of Catharanthus roseus L. medicinal plant extracts on some markers related to obesity and inflammation in 3T3-L1 mouse cell lines
Gülben Uytan, Hilal Büşra Tokgöz, Reşat Ünal, Filiz Altan
Biologia.2021; 76(1): 297. CrossRef - Implementation andin vitrocharacterization of calcium-freein situgelling oral reconstituted suspension for potential overweight treatment
Fouzia Gherrak, Abdelkader Hadjsadok, Sonia Lefnaoui
Drug Development and Industrial Pharmacy.2021; 47(1): 36. CrossRef - Interrelated In Vitro Mechanisms of Sibutramine-Induced Cardiotoxicity
Feyza Alyu, Yusuf Olgar, Sinan Degirmenci, Belma Turan, Yusuf Ozturk
Cardiovascular Toxicology.2021; 21(4): 322. CrossRef - The Beneficial Effects of Red Sun‐Dried Capsicum annuum L. Cv Senise Extract with Antioxidant Properties in Experimental Obesity are Associated with Modulation of the Intestinal Microbiota
Chiara Sinisgalli, Teresa Vezza, Patricia Diez‐Echave, Angela Ostuni, Immacolata Faraone, Laura Hidalgo‐Garcia, Daniela Russo, Maria Francesca Armentano, José Garrido‐Mesa, Maria Elena Rodriguez‐Cabezas, Alba Rodríguez‐Nogales, Luigi Milella, Julio Galvez
Molecular Nutrition & Food Research.2021;[Epub] CrossRef - FoxO1 signaling as a therapeutic target for type 2 diabetes and obesity
Khaled Benchoula, Aditya Arya, Ishwar S. Parhar, Wong Eng Hwa
European Journal of Pharmacology.2021; 891: 173758. CrossRef - Herbal nanotherapy: A new paradigm over conventional obesity treatment
Pravin Shende, Roma Narvenker
Journal of Drug Delivery Science and Technology.2021; 61: 102291. CrossRef - Design of oxa-spirocyclic PHOX ligands for the asymmetric synthesis of lorcaserin via iridium-catalyzed asymmetric hydrogenation
Xiang-Yu Ye, Zhi-Qin Liang, Cong Jin, Qi-Wei Lang, Gen-Qiang Chen, Xumu Zhang
Chemical Communications.2021; 57(2): 195. CrossRef - Metabolic Profile of Four Selected Cathinones in Microsome Incubations: Identification of Phase I and II Metabolites by Liquid Chromatography High Resolution Mass Spectrometry
Beatriz T. Lopes, Maria João Caldeira, Helena Gaspar, Alexandra M. M. Antunes
Frontiers in Chemistry.2021;[Epub] CrossRef - Garcinia cambogia and Glucomannan reduce weight, change body composition and ameliorate lipid and glucose blood profiles in overweight/obese patients
Andrea Maia-Landim, Carolina Lancho, María S. Poblador, José L. Lancho, Juan M. Ramírez
Journal of Herbal Medicine.2021; 26: 100424. CrossRef - Screening for pancreatic lipase natural modulators by capillary electrophoresis hyphenated to spectrophotometric and conductometric dual detection
Ghassan Al Hamoui Dit Banni, Rouba Nasreddine, Syntia Fayad, Phu Cao-Ngoc, Jean-Christophe Rossi, Laurent Leclercq, Hervé Cottet, Axel Marchal, Reine Nehmé
The Analyst.2021; 146(4): 1386. CrossRef - Anti-diabesity potential of various multifunctional natural molecules
Priyanka Rathod, Raman P. Yadav
Journal of Herbal Medicine.2021; 27: 100430. CrossRef - Antiobesity and antidiabetic effects of the dairy bacterium Propionibacterium freudenreichii MJ2 in high-fat diet-induced obese mice by modulating lipid metabolism
Mirae An, Yeon-Hee Park, Young-Hee Lim
Scientific Reports.2021;[Epub] CrossRef - Efficacy of a Novel Herbal Formulation (F2) on the Management of Obesity: In Vitro and In Vivo Study
Prakash Raj Pandeya, Ramakanta Lamichhane, Kyung-Hee Lee, Gopal Lamichhane, Se-Gun Kim, Hyun-Ju Jung, Weicheng Hu
Evidence-Based Complementary and Alternative Medicine.2021; 2021: 1. CrossRef - Emblica officinalisandHordeum vulgareL. Mixture Regulates Lipolytic Activity in Differentiated 3T3-L1 Cells
Soo-Jeung Park, Minhee Lee, Dong Hwan Oh, Jong-Lae Kim, Mi-Ryeong Park, Tae Gi Kim, Ok-Kyung Kim, Jeongmin Lee
Journal of Medicinal Food.2021; 24(2): 172. CrossRef - Anti-Atherogenic Effects of Orlistat on Obesity-Induced Vascular Oxidative Stress Rat Model
Zaidatul Akmal Othman, Zaida Zakaria, Joseph Bagi Suleiman, Wan Syaheedah Wan Ghazali, Mahaneem Mohamed
Antioxidants.2021; 10(2): 251. CrossRef - Standardized hot water extract from the leaves of Hydrangea serrata (Thunb.) Ser. alleviates obesity via the AMPK pathway and modulation of the gut microbiota composition in high fat diet-induced obese mice
Hee-Soo Han, Hwi-Ho Lee, Hyo-Sun Gil, Kyung-Sook Chung, Jeon-Kyung Kim, Dong-Hyun Kim, Jimin Yoon, Eun Kyoung Chung, Jong Kil Lee, Woong Mo Yang, Yu-Kyong Shin, Hye Shin Ahn, Sun Hee Lee, Kyung-Tae Lee
Food & Function.2021; 12(6): 2672. CrossRef - What are the mechanisms of action of anti-inflammatory agents in adipose tissue?
Sara Sayonara da Cruz Nascimento, Jaluza Luana Carvalho de Queiroz, Amanda Fernandes de Medeiros, Ana Clara de França Nunes, Grasiela Piuvezam, Bruna Leal Lima Maciel, Thaís Souza Passos, Ana Heloneida de Araújo Morais
Medicine.2021; 100(8): e24677. CrossRef - Moringa peregrina leaf extracts produce anti-obesity, hypoglycemic, anti-hyperlipidemic, and hepatoprotective effects on high-fat diet fed rats
Dalal A. Alkhudhayri, Magdi A. Osman, Ghedeir M. Alshammari, Salah A. Al Maiman, Mohammed Abdo Yahya
Saudi Journal of Biological Sciences.2021; 28(6): 3333. CrossRef - An edible bioactive fraction from Rosa multiflora regulates adipogenesis in 3T3-L1 adipocytes and high-fat diet-induced C57Bl/6 mice models of obesity
HV Sudeep, K Gouthamchandra, I Ramanaiah, Amritha Raj, K Shyamprasad
Pharmacognosy Magazine.2021; 17(73): 84. CrossRef - Alleviation of Liver Dysfunction, Oxidative Stress, and Inflammation Underlines the Protective Effects of Polysaccharides from Cordyceps cicadae on High Sugar/High Fat Diet‐Induced Metabolic Syndrome in Rats
Xiao Zhang, Jinpeng Li, Bo Yang, Qina Leng, Ji Li, Xintuan Wang, Junyao Lu, Opeyemi Joshua Olatunji, Jian Tang
Chemistry & Biodiversity.2021;[Epub] CrossRef - Pharmacotherapy of obesity: An update
Andrea Cignarella, Luca Busetto, Roberto Vettor
Pharmacological Research.2021; 169: 105649. CrossRef - Anacardic Acid Suppresses Adipogenesis Through Inhibition of the Hsp90/Akt Signaling Pathway in 3T3-L1 Preadipocytes
Jangho Lee, Min-Yu Chung, Sangwon Chung, Hyo-Kyoung Choi
Journal of Medicinal Food.2021; 24(5): 487. CrossRef - Prolonged Glucagon Infusion Does Not Affect Energy Expenditure in Individuals with Overweight/Obesity: A Randomized Trial
Katie L. Whytock, Elvis A. Carnero, Rick B. Vega, Joachim Tillner, Christopher Bock, Karthik Chivukula, Fanchao Yi, Christian Meyer, Steven R. Smith, Lauren M. Sparks
Obesity.2021; 29(6): 1003. CrossRef - Anti-Obesity Effect of Erigeron annuus (L.) Pers. Extract Containing Phenolic Acids
Yulong Zheng, Yoon-Hee Choi, Ji-Hyun Lee, So-Yeon Lee, Il-Jun Kang
Foods.2021; 10(6): 1266. CrossRef - Investigation of therapeutic potential of three endemic Cirsium species for global health problem obesity
Sila Ozlem Sener, Ufuk Ozgen, Seyda Kanbolat, Nuriye Korkmaz, Merve Badem, Hatice Hanci, Tuncay Dirmenci, Turan Arabaci, Rezzan Aliyazicioglu, Engin Yenilmez, Gulcin Saltan Iscan
South African Journal of Botany.2021; 141: 243. CrossRef - Standardization of marketed anti‐obesity nutraceuticals containing amla and ginseng
Omkar Sawant, Tabassum Khan
Journal of Food Processing and Preservation.2021;[Epub] CrossRef - Network Pharmacology Study to Interpret Signaling Pathways of Ilex cornuta Leaves against Obesity
Ki-Kwang Oh, Md. Adnan, Dong-Ha Cho
Processes.2021; 9(7): 1106. CrossRef - Susceptible genetic polymorphisms and their association with adverse effects of orlistat therapy
Logesh Rajan, Arun Radhakrishnan, Gobi Selleppan, Suresh Kumar Mohankumar
Obesity Medicine.2021; 25: 100360. CrossRef - The Demethoxy Derivatives of Curcumin Exhibit Greater Differentiation Suppression in 3T3-L1 Adipocytes Than Curcumin: A Mechanistic Study of Adipogenesis and Molecular Docking
Ahmed Alalaiwe, Jia-You Fang, Hsien-Ju Lee, Chun-Hui Chiu, Ching-Yun Hsu
Biomolecules.2021; 11(7): 1025. CrossRef - The effects of ginseng on the metabolic syndrome: An updated review
Tahereh Aminifard, Bibi Marjan Razavi, Hossein Hosseinzadeh
Food Science & Nutrition.2021; 9(9): 5293. CrossRef - Glycyrrhizic acid suppresses early stage of adipogenesis through repression of MEK/ERK-mediated C/EBPβ and C/EBPδ expression in 3T3-L1 cells
Masayuki Yamamoto, Yasuna Nagasawa, Ko Fujimori
Chemico-Biological Interactions.2021; 346: 109595. CrossRef - Ginsenoside F2 Suppresses Adipogenesis in 3T3-L1 Cells and Obesity in Mice via the AMPK Pathway
Jing Zhou, Ji Zhang, Jiayi Li, Yiqiu Guan, Ting Shen, Fu Li, Xueqin Li, Xiaojun Yang, Weicheng Hu
Journal of Agricultural and Food Chemistry.2021; 69(32): 9299. CrossRef - Evaluation of Anti-Obesity Activity of an Herbal Formulation (F2) in DIO Mice Model and Validation of UPLC-DAD Method for Quality Control
Prakash Raj Pandeya, Kyung-Hee Lee, Ramakanta Lamichhane, Gopal Lamichhane, Amrit Poudel, Hyun-Ju Jung
Applied Sciences.2021; 11(16): 7404. CrossRef - New Incretin Combination Treatments under Investigation in Obesity and Metabolism: A Systematic Review
Agni Kakouri, Georgia Kanti, Efthymios Kapantais, Alexandros Kokkinos, Leonidas Lanaras, Paul Farajian, Christos Galanakis, Georgios Georgantopoulos, Nikos F. Vlahos, George Mastorakos, Alexandra Bargiota, Georgios Valsamakis
Pharmaceuticals.2021; 14(9): 869. CrossRef - Genes in human obesity loci are causal obesity genes in C. elegans
Wenfan Ke, Jordan N. Reed, Chenyu Yang, Noel Higgason, Leila Rayyan, Carolina Wählby, Anne E. Carpenter, Mete Civelek, Eyleen J. O’Rourke, Gregory P. Copenhaver
PLOS Genetics.2021; 17(9): e1009736. CrossRef - The use of drug based on technologically processed antibodies to endocannabinoid receptor type 1 in the treatment of obesity in adults: results of a multicenter double blind placebo controlled randomized clinical trial
Tatiana Y. Demidova, Elena I. Krasil’nikova, Sergey V. Vorob’ev, Tatiana V. Morugova, Tatiana V. Adasheva
Terapevticheskii arkhiv.2021; 93(8): 904. CrossRef - Anti-Obesity Effect of Hot Water Extract of Barley Sprout through the Inhibition of Adipocyte Differentiation and Growth
Myeong-Jin Kim, Hye-Won Kawk, Sang-Hyeon Kim, Hyo-Jae Lee, Ji-Won Seo, Jong-Tae Kim, Seung-Hee Jang, Min-Jeong Kim, Young-Min Kim
Metabolites.2021; 11(9): 610. CrossRef - Papain Ameliorates Lipid Accumulation and Inflammation in High-Fat Diet-Induced Obesity Mice and 3T3-L1 Adipocytes via AMPK Activation
Yun-Mi Kang, Hyun-Ae Kang, Divina C. Cominguez, Su-Hyun Kim, Hyo-Jin An
International Journal of Molecular Sciences.2021; 22(18): 9885. CrossRef - Cannabinoid Receptor 1 and 2 Signaling Pathways Involved in Sepsis
Mariane C.G. Leite-Avalca, Aleksander Zampronio, Christian Lehmann
Shock.2021; 56(5): 673. CrossRef - Sulforaphane Regulates Glucose and Lipid Metabolisms in Obese Mice by Restraining JNK and Activating Insulin and FGF21 Signal Pathways
Shuhua Tian, Yunfan Wang, Xiangfei Li, Jie Liu, Jing Wang, Yingjian Lu
Journal of Agricultural and Food Chemistry.2021; 69(44): 13066. CrossRef - Synbiotic (Lactiplantibacillus pentosus GSSK2 and isomalto-oligosaccharides) supplementation modulates pathophysiology and gut dysbiosis in experimental metabolic syndrome
Sakshi Khanna, Mahendra Bishnoi, Kanthi Kiran Kondepudi, Geeta Shukla
Scientific Reports.2021;[Epub] CrossRef - Combined Anti-Adipogenic Effects of Hispidulin and p-Synephrine on 3T3-L1 Adipocytes
Dahae Lee, Hee Jae Kwak, Byoung Ha Kim, Seung Hyun Kim, Dong-Wook Kim, Ki Sung Kang
Biomolecules.2021; 11(12): 1764. CrossRef - Fatty Acid Metabolism and Derived-Mediators Distinctive of PPAR-α Activation in Obese Subjects Post Bariatric Surgery
Claudia Manca, Stefano Pintus, Elisabetta Murru, Giovanni Fantola, Michela Vincis, Barbara Batetta, Enrico Moroni, Gianfranca Carta, Sebastiano Banni
Nutrients.2021; 13(12): 4340. CrossRef - Theoretical and Experimental Insights into the Possible Interfacial Interactions between β-Glucan and Fat Molecules in Aqueous Media
Tamanna Islam, Md. Nurul Huda, Md Ariful Ahsan, Humayra Afrin, Christiancel Joseph J Salazar, Md Nurunnabi
The Journal of Physical Chemistry B.2021; 125(50): 13730. CrossRef - Inhibitory Effects of Pinostilbene on Adipogenesis in 3T3-L1 Adipocytes: A Study of Possible Mechanisms
You Chul Chung, Chang-Gu Hyun
International Journal of Molecular Sciences.2021; 22(24): 13446. CrossRef - 18KHT01, a Potent Anti-Obesity Polyherbal Formulation
Prakash Raj Pandeya, Ramakanta Lamichhane, Gopal Lamichhane, Kyung-Hee Lee, Hyeong Kyu Lee, Su-jin Rhee, Hyun-Ju Jung
Frontiers in Pharmacology.2021;[Epub] CrossRef - Potential Benefits of Acupuncture and Herbs for Obesity-Related Chronic Inflammation by Adipokines
Ji-Youn Kim, Seon-Eun Baek, Rehna Paula Ginting, Min-Woo Lee, Jeong-Eun Yoo
Evidence-Based Complementary and Alternative Medicine.2020; 2020: 1. CrossRef - Beneficial effect of phospholipase A2 group IIA inhibitors from Acacia suma in obesity: an in silico and in vitro study
Nikita Kanbarkar, Sanjay Mishra, Pukar Khanal
Advances in Traditional Medicine.2020; 20(4): 599. CrossRef - The metabolic footprint during adipocyte commitment highlights ceramide modulation as an adequate approach for obesity treatment
Weilong Hou, Qiang Chen, Haitao Wang, Pengxiang Qiu, Xueying Lyu, Weiping Chen, Melvin L.K. Chua, Y. Eugene Chinn, Chu-Xia Deng, Ruihong Wang
EBioMedicine.2020; 51: 102605. CrossRef - Computational approaches for the discovery of natural pancreatic lipase inhibitors as antiobesity agents
Ihab M Almasri
Future Medicinal Chemistry.2020; 12(8): 741. CrossRef - Managing obesity through natural polyphenols: A review
Manisha Singh, Thilini Thrimawithana, Ravi Shukla, Benu Adhikari
Future Foods.2020; 1-2: 100002. CrossRef - Downregulation of adipogenic genes in 3T3-L1 Pre adipocytes- a possible mechanism of anti-obesity activity of herbal decoction Varanadi Kashayam
Chinchu J.U, Mohind C. Mohan, Prakash Kumar B
Journal of Herbal Medicine.2020; 19: 100309. CrossRef - Gut and Metabolic Hormones Changes After Endoscopic Sleeve Gastroplasty (ESG) Vs. Laparoscopic Sleeve Gastrectomy (LSG)
Gontrand Lopez-Nava, Anuradha Negi, Inmaculada Bautista-Castaño, Miguel Angel Rubio, Ravishankar Asokkumar
Obesity Surgery.2020; 30(7): 2642. CrossRef - Anti-obesity and lipid lowering effects of Varanadi kashayam (decoction) on high fat diet induced obese rats
J.U. Chinchu, Mohind C. Mohan, B. Prakash Kumar
Obesity Medicine.2020; 17: 100170. CrossRef - Lipase inhibitory activity assay for fermented milk
Ana María Gil-Rodríguez, Thomas P. Beresford
MethodsX.2020; 7: 100999. CrossRef - Khat (Catha edulis) upregulates lipolytic genes in white adipose tissue of male obese mice (C57BL/6J)
Mustafa Ahmed Alshagga, Zahurin Mohamed, Atefehalsadat Seyedan, Francis J.P. Ebling, Mohammed Abdullah Alshawsh
Journal of Ethnopharmacology.2020; 262: 113187. CrossRef - Biomaterials that regulate fat digestion for the treatment of obesity
Paul Joyce, Tahlia R. Meola, Hayley B. Schultz, Clive A. Prestidge
Trends in Food Science & Technology.2020; 100: 235. CrossRef - Bergenia pacumbis from Nepal, an astonishing enzymes inhibitor
Bishnu Prasad Pandey, Suman Prakash Pradhan, Kapil Adhikari, Saroj Nepal
BMC Complementary Medicine and Therapies.2020;[Epub] CrossRef - Genetically Engineered Escherichia coli Nissle 1917 Secreting GLP‐1 Analog Exhibits Potential Antiobesity Effect in High‐Fat Diet‐Induced Obesity Mice
Jie Ma, Cuiying Li, Junrui Wang, Jianwen Gu
Obesity.2020; 28(2): 315. CrossRef - Preclinical Research on a Mixture of Red Ginseng and Licorice Extracts in the Treatment and Prevention of Obesity
Yulong Zheng, Eun-Hye Lee, Ji-Hyun Lee, Gyo In, JongHan Kim, Mi-Hyang Lee, Ok-Hwan Lee, Il-Jun Kang
Nutrients.2020; 12(9): 2744. CrossRef - Dietary polyphenols turn fat “brown”: A narrative review of the possible mechanisms
Jiamiao Hu, Zhenyu Wang, Bee K. Tan, Mark Christian
Trends in Food Science & Technology.2020; 97: 221. CrossRef - Sinensol-C Isolated from Spiranthes sinensis Inhibits Adipogenesis in 3T3-L1 Cells through the Regulation of Adipogenic Transcription Factors and AMPK Activation
Pei-Hsin Shie, Chung-Ping Yang, Guan-Jhong Huang, Sheng-Yang Wang, Yueh-Hsiung Kuo
Molecules.2020; 25(18): 4204. CrossRef - Lemon Extract Reduces Angiotensin Converting Enzyme (ACE) Expression and Activity and Increases Insulin Sensitivity and Lipolysis in Mouse Adipocytes
Shilpa Tejpal, Alan M. Wemyss, Claire C. Bastie, Judith Klein-Seetharaman
Nutrients.2020; 12(8): 2348. CrossRef - A review of saponin intervention in metabolic syndrome suggests further study on intestinal microbiota
Zichen Luo, Weichen Xu, Ying Zhang, Liuqing Di, Jinjun Shan
Pharmacological Research.2020; 160: 105088. CrossRef - Statistical Design of Sustained-Release Tablet Garcinia cambogia Extract and Bioconverted Mulberry Leaf Extract for Anti-Obesity
Hye-Jin Lee, Young-Guk Na, Mingu Han, Thi Mai Anh Pham, Hyeonmin Lee, Hong-Ki Lee, Chang-Seon Myung, Joo-Hui Han, Jong-Seong Kang, Kyung-Tae Kim, Cheong-Weon Cho
Pharmaceutics.2020; 12(10): 932. CrossRef - The effects of supplementation with L-arginine on anthropometric indices and body composition in overweight or obese subjects: A systematic review and meta-analysis
Mohammad Zeinali Khosroshahi, Omid Asbaghi, Sajjad Moradi, Mahnaz Rezaei kelishadi, Mojtaba Kaviani, Mahnaz Mardani, Cyrus Jalili
Journal of Functional Foods.2020; 71: 104022. CrossRef - Polyphenol-enriched fraction and the compounds isolated from Garcinia indica fruits ameliorate obesity through suppression of digestive enzymes and oxidative stress
Kavita Munjal, Sheeraz Ahmad, Apeksha Gupta, Abdul Haye, Saima Amin, ShowkatR Mir
Pharmacognosy Magazine.2020; 16(70): 236. CrossRef - Comparative Studies of Fraxinus Species from Korea Using Microscopic Characterization, Phytochemical Analysis, and Anti-Lipase Enzyme Activity
Kazi-Marjahan Akter, Woo Sung Park, Hye-Jin Kim, Atif Ali Khan Khalil, Mi-Jeong Ahn
Plants.2020; 9(4): 534. CrossRef - Comparative evaluation of the pharmacological value of virgin coconut oil, omega 3 fatty acids, and orlistat in experimental study on obesity with normo/hyper-lipidaemic diet
Wale Johnson Adeyemi, Luqman Aribidesi Olayaki, Tahir Ahmad Abdussalam, Serah Funke Ige, Bidemi Kazeem Okesina, Patrick Oluwole Abolarin, Hidayah Usman, Aishat Oluwatofunmi Tiamiyu, Maryam Oluremi Seidu, Abdmukit Olalekan Opabode
PharmaNutrition.2020; 13: 100192. CrossRef - The dorsomedial hypothalamus and nucleus of the solitary tract as key regulators in a rat model of chronic obesity
Chen Zhang, Pernille Barkholt, Jens Christian Nielsen, Ditte Dencker Thorbek, Kristoffer Rigbolt, Niels Vrang, David Paul Drucker Woldbye, Jacob Jelsing
Brain Research.2020; 1727: 146538. CrossRef - Bioactivity-Guided Identification of Anti-Adipogenic Isothiocyanates in the Moringa (Moringa oleifera) Seed and Investigation of the Structure-Activity Relationship
Linhua Huang, Chunmao Yuan, Yu Wang
Molecules.2020; 25(11): 2504. CrossRef - Urolithin A Induces Brown-like Phenotype in 3T3-L1 White Adipocytes via β3-adrenergic Receptor-p38 MAPK Signaling Pathway
Subramani Manigandan, Jong Won Yun
Biotechnology and Bioprocess Engineering.2020; 25(3): 345. CrossRef - It’s No Has Bean: A Review of the Effects of White Kidney Bean Extract on Body Composition and Metabolic Health
Ruth Nolan, Oliver M. Shannon, Natassia Robinson, Abraham Joel, David Houghton, Fiona C. Malcomson
Nutrients.2020; 12(5): 1398. CrossRef Kukoamine B Ameliorate Insulin Resistance, Oxidative Stress, Inflammation and Other Metabolic Abnormalities in High-Fat/High-Fructose-Fed Rats
Quan Zhao, Linhai Li, Yu Zhu, Dezhi Hou, Yuejin Li, Xiaodong Guo, Yongzhi Wang, Opeyemi Joshua Olatunji, Ping Wan, Kunmei Gong
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2020; Volume 13: 1843. CrossRef- Effect of red edible seaweed Eucheuma denticulatum on diet-induced obesity in vivo
V Balasubramaniam, N Aznyda, M Hussin, L Faradianna, AR Aswir, MN Mohd Fairulnizal
Journal of Applied Phycology.2020; 32(4): 2407. CrossRef - Anti-obesity effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on high-fat and high-fructose diet mice base on inflammatory response alleviation and gut microbiota regulation
Tao Wang, Hong Yan, Yingying Lu, Xin Li, Xin Wang, Yuanyuan Shan, Yanglei Yi, Bianfang Liu, Yuan Zhou, Xin Lü
European Journal of Nutrition.2020; 59(6): 2709. CrossRef - Novel insights in health-promoting properties of sweet cherries
Maria Felicia Faienza, Filomena Corbo, Alessia Carocci, Alessia Catalano, Maria Lisa Clodoveo, Maria Grano, David Q.-H. Wang, Gabriele D'Amato, Marilena Muraglia, Carlo Franchini, Giacomina Brunetti, Piero Portincasa
Journal of Functional Foods.2020; 69: 103945. CrossRef - Antioxidant and anti-obesity properties of local chilies varieties in Malaysia
Chan Suk Huei, Azrina Azlan, Amin Ismail, Nurul Husna Shafie, Sharmin Sultana
Journal of Food Science and Technology.2020; 57(10): 3677. CrossRef - Virgin Coconut Oil Associated with High-Fat Diet Induces Metabolic Dysfunctions, Adipose Inflammation, and Hepatic Lipid Accumulation
Deise Jaqueline Ströher, Micaela Federizzi de Oliveira, Patrícia Martinez-Oliveira, Bruna Cocco Pilar, Márcia Denise Pavanelo Cattelan, Eliseu Rodrigues, Kalyne Bertolin, Paulo Bayard Dias Gonçalves, Jacqueline da Costa Escobar Piccoli, Vanusa Manfredini
Journal of Medicinal Food.2020; 23(7): 689. CrossRef - A Review of Biologically Active Natural Products from Mediterranean Wild Edible Plants: Benefits in the Treatment of Obesity and Its Related Disorders
Mariangela Marrelli, Giancarlo Statti, Filomena Conforti
Molecules.2020; 25(3): 649. CrossRef - Availability and composition of weight‐loss supplements in Sri Lanka
Ranil Jayawardena, Piumika Sooriyaarachchi, Priyanga Ranasinghe, Amila Perera, Andrew P. Hills
Nutrition & Dietetics.2020; 77(2): 247. CrossRef - Lancing Drug Reservoirs into Subcutaneous Fat to Combat Obesity and Associated Metabolic Diseases
Aung Than, Phan Khanh Duong, Ping Zan, Junjie Liu, Melvin Khee‐Shing Leow, Peng Chen
Small.2020;[Epub] CrossRef - Engineered butyrate-producing bacteria prevents high fat diet-induced obesity in mice
Liang Bai, Mengxue Gao, Xiaoming Cheng, Guangbo Kang, Xiaocang Cao, He Huang
Microbial Cell Factories.2020;[Epub] CrossRef - Mechanisms of Action of Cassiae Semen for Weight Management: A Computational Molecular Docking Study of Serotonin Receptor 5-HT2C
Heidi Yuen, Andrew Hung, Angela Wei Hong Yang, George Binh Lenon
International Journal of Molecular Sciences.2020; 21(4): 1326. CrossRef - Chemistry, Analysis, Pharmacokinetics and Pharmacodynamics Aspects of Lorcaserin, a Selective Serotonin 5-HT2C Receptor Agonist: An Update
Sanjay Sharma, Komal S. Aware, Ketan Hatware, Kiran Patil
Mini-Reviews in Medicinal Chemistry.2020; 20(9): 768. CrossRef - Caffeic acid reduces body weight by regulating gut microbiota in diet-induced-obese mice
Jia Xu, Jianbing Ge, Xiaoyun He, Yao Sheng, Shujuan Zheng, Chuanhai Zhang, Wentao Xu, Kunlun Huang
Journal of Functional Foods.2020; 74: 104061. CrossRef - The Societal Value of Broader Access to Antiobesity Medications
Mina Kabiri, Alison Sexton Ward, Abhilasha Ramasamy, Emma van Eijndhoven, Rahul Ganguly, B. Gabriel Smolarz, Tracy Zvenyach, Dana P. Goldman, James R. Baumgardner
Obesity.2020; 28(2): 429. CrossRef - Sesaminol induces brown and beige adipocyte formation through suppression of myogenic program
Soumya Jaya Divakaran, Simran Srivastava, Anusha Jahagirdar, Rajprabu Rajendran, Shinde Vijay Sukhdeo, Sona Rajakumari
The FASEB Journal.2020; 34(5): 6854. CrossRef - A Mix of Natural Bioactive Compounds Reduces Fat Accumulation and Modulates Gene Expression in the Adipose Tissue of Obese Rats Fed a Cafeteria Diet
Albert Gibert-Ramos, Miguel Z. Martín-González, Anna Crescenti, M. Josepa Salvadó
Nutrients.2020; 12(11): 3251. CrossRef - Anti obesity prospective of Dalbergia latifolia (Roxb.) hydroalcoholic bark extract in high fat diet induced obese rats
Mohammad Khalid, Ambreen Shoaib, Juber Akhtar, Mohammed H. Alqarni, Badruddeen, Hefazat H. Siddiqui, Ahmed I. Foudah
3 Biotech.2020;[Epub] CrossRef - Anti-Obesity Effect of an Ethanol Extract of Cheongchunchal In Vitro and In Vivo
Hye Won Kawk, Gun-He Nam, Myeong Jin Kim, Sang-Yong Kim, Gi No Kim, Young-Min Kim
Nutrients.2020; 12(11): 3453. CrossRef - Efficacy and Safety of Combined Extracts of Cornus officinalis and Ribes fasciculatum for Body Fat Reduction in Overweight Women
Eunkuk Park, Chang Gun Lee, Jeonghyun Kim, Jae-Heon Kang, Young Gyu Cho, Seon-Yong Jeong
Journal of Clinical Medicine.2020; 9(11): 3629. CrossRef - Effectiveness and Safety of Liraglutide Treatment in Patients with a Psychiatric Disorder
Young Jae Choi, Jungsun Lee, Soyeon Park, Yuree Kang, Sung Woo Joo
Journal of Korean Neuropsychiatric Association.2020; 59(4): 325. CrossRef - Delayed Exercise Training Improves Obesity-Induced Chronic Kidney Disease by Activating AMPK Pathway in High-Fat Diet-Fed Mice
Florian Juszczak, Maud Vlassembrouck, Olivia Botton, Thomas Zwakhals, Morgane Decarnoncle, Alexandra Tassin, Nathalie Caron, Anne-Emilie Declèves
International Journal of Molecular Sciences.2020; 22(1): 350. CrossRef - Anti-Obesity Effect of Dictyophora indusiata Mushroom Polysaccharide (DIP) in High Fat Diet-Induced Obesity via Regulating Inflammatory Cascades and Intestinal Microbiome
Sadia Kanwal, Shams Aliya, Yi Xin
Frontiers in Endocrinology.2020;[Epub] CrossRef - Anti-Obesity Natural Products Tested in Juvenile Zebrafish Obesogenic Tests and Mouse 3T3-L1 Adipogenesis Assays
Hiroko Nakayama, Kanae Hata, Izumi Matsuoka, Liqing Zang, Youngil Kim, Djongchi Chu, Lekh Raj Juneja, Norihiro Nishimura, Yasuhito Shimada
Molecules.2020; 25(24): 5840. CrossRef - Views and Experiences of Adults who are Overweight and Obese on the Barriers and Facilitators to Weight Loss in Southeast Brazil: A Qualitative Study
Caroline Morgan, Gilles de Wildt, Renata Billion Ruiz Prado, Nisha Thanikachalam, Marcos Virmond, Ruth Riley
International Journal of Qualitative Studies on Health and Well-being.2020; 15(1): 1852705. CrossRef - Exploring the Potential Inhibition of Candidate Drug Molecules for Clinical Investigation Based on their Docking or Crystallographic Analyses against M. tuberculosis Enzyme Targets
Rishita Dey, Sisir Nandi, Asmita Samadder, Aaruni Saxena, Anil Kumar Saxena
Current Topics in Medicinal Chemistry.2020; 20(29): 2662. CrossRef - PREVENTION OF APPARENT ADIPOSITY BY FRACTIONS OF DISTILLED COW URINE: A NON-INVASIVE APPROACH
Ketan Hatware, Sanjay Sharma, Ashwini Deshpande, Kiran Patil, Sravani Karri, Rupesh Gautam
INDIAN DRUGS.2020; 57(03): 73. CrossRef - Energy imbalance: obesity, associated comorbidities, prevention, management and public health implications
Shazia Jehan, Ferdinand Zizi, Seithikurippu R Pandi-Perumal, Samy I McFarlane, Girardin Jean-Louis, Alyson K Myers
Advances in Obesity, Weight Management & Control.2020; 10(5): 146. CrossRef - The effects of supplementation with conjugated linoleic acid on anthropometric indices and body composition in overweight and obese subjects: A systematic review and meta-analysis
Nazli Namazi, Pardis Irandoost, Bagher Larijani, Leila Azadbakht
Critical Reviews in Food Science and Nutrition.2019; 59(17): 2720. CrossRef - Acceptability of alginate enriched bread and its effect on fat digestion in humans
David Houghton, Matthew D. Wilcox, Iain A. Brownlee, Peter I. Chater, Chris J. Seal, Jeffrey P. Pearson
Food Hydrocolloids.2019; 93: 395. CrossRef - Identification of Cyanobacterial Strains with Potential for the Treatment of Obesity-Related Co-Morbidities by Bioactivity, Toxicity Evaluation and Metabolite Profiling
Margarida Costa, Filipa Rosa, Tiago Ribeiro, Rene Hernandez-Bautista, Marco Bonaldo, Natália Gonçalves Silva, Finnur Eiríksson, Margrét Thorsteinsdóttir, Siegfried Ussar, Ralph Urbatzka
Marine Drugs.2019; 17(5): 280. CrossRef - A novel peptide RIFV suppresses human adipocyte differentiation through the inhibition of C/EBP-β expression
Wen Zhang, Dan Shen, Yun Li, Hong Zhong, Xing Wang, Xian-wei Cui, Chun-Mei Shi, Chen-Bo Ji, Xi-Rong Guo, Ling Chen
Nutrition & Metabolism.2019;[Epub] CrossRef - Regulatory and therapeutic potential for obesity
O. E. Poluliakh, E. I. Kalinovskaya, A. A. Basalai
Proceedings of the National Academy of Sciences of Belarus, Medical series.2019; 15(4): 483. CrossRef - Virgin coconut oil is effective to treat metabolic and inflammatory dysfunction induced by high refined carbohydrate-containing diet in mice
Marina Campos Zicker, Ana Letícia Malheiros Silveira, Débora Romualdo Lacerda, Débora Fernandes Rodrigues, Cíntia Tarabal Oliveira, Letícia Maria de Souza Cordeiro, Leandro Ceotto Freitas Lima, Sérgio Henrique Sousa Santos, Mauro Martins Teixeira, Adalien
The Journal of Nutritional Biochemistry.2019; 63: 117. CrossRef - Ameliorative effect of fermentable fibres on adiposity and insulin resistance in C57BL/6 mice fed a high-fat and sucrose diet
Surender Jangra, Raja Shekar K., Raj Kumar Sharma, Ramesh Pothuraju, A. K. Mohanty
Food & Function.2019; 10(6): 3696. CrossRef - An Approach to Obesity Management for Gastroenterologists and Hepatologists
Jessica Briscoe, Monica Saumoy, Octavia Pickett-Blakely
Current Treatment Options in Gastroenterology.2019; 17(4): 587. CrossRef - Possible anti‐obesity effects of phytosterols and phytostanols supplementation in humans: A systematic review and dose–response meta‐analysis of randomized controlled trials
Ehsan Ghaedi, Hamed Kord Varkaneh, Jamal Rahmani, Seyed Mohammad Mousavi, Hamed Mohammadi, Somaye Fatahi, Ana Pantovic, Manije Darooghegi Mofrad, Yong Zhang
Phytotherapy Research.2019; 33(5): 1246. CrossRef - Anti-obesity effect of Yangkyuksanwha-tang in high-fat diet-induced obese mice
Young-Mee Koh, Soon-Woo Jang, Taek-Won Ahn
BMC Complementary and Alternative Medicine.2019;[Epub] CrossRef - Glycoprotein from Mytilus edulis extract inhibits lipid accumulation and improves male reproductive dysfunction in high-fat diet-induced obese rats
Yi-Cheng Lu, Sabri Sudirman, Chien-Feng Mao, Zwe-Ling Kong
Biomedicine & Pharmacotherapy.2019; 109: 369. CrossRef - Isolation, identification and characterization of pancreatic lipase inhibitors from Trigonella foenum-graecum seeds
W.I.T. Fernando, A.M.K.C. Attanayake, H.K.I. Perera, R. Sivakanesan, L. Jayasinghe, H. Araya, Y. Fujimoto
South African Journal of Botany.2019; 121: 418. CrossRef - Neuropeptide receptors as potential pharmacological targets for obesity
Beatriz T. Meneguetti, Marlon H. Cardoso, Camila F.A. Ribeiro, Mário R. Felício, Ingrid B. Pinto, Nuno C. Santos, Cristiano M.E. Carvalho, Octávio L. Franco
Pharmacology & Therapeutics.2019; 196: 59. CrossRef - Improved anti-obesity effect of herbal active and endogenous lipids co-loaded lipid nanocarriers: Preparation, in vitro and in vivo evaluation
I. Lacatusu, N. Badea, D. Udeanu, L. Coc, A. Pop, C. Cioates Negut, C. Tanase, R. Stan, A. Meghea
Materials Science and Engineering: C.2019; 99: 12. CrossRef - Identification of Novel Pancreatic Lipase Inhibitors UsingIn SilicoStudies
Umesh Panwar, Sanjeev Kumar Singh
Endocrine, Metabolic & Immune Disorders - Drug Targets.2019; 19(4): 449. CrossRef - Hypolipidemic effects of herbal extracts by reduction of adipocyte differentiation, intracellular neutral lipid content, lipolysis, fatty acid exchange and lipid droplet motility
Renate Haselgrübler, Peter Lanzerstorfer, Clemens Röhrl, Flora Stübl, Jonas Schurr, Bettina Schwarzinger, Clemens Schwarzinger, Mario Brameshuber, Stefan Wieser, Stephan M. Winkler, Julian Weghuber
Scientific Reports.2019;[Epub] CrossRef - Beneficial Effects of an Aged Black Garlic Extract in the Metabolic and Vascular Alterations Induced by a High Fat/Sucrose Diet in Male Rats
Sara Amor, Daniel González-Hedström, Beatriz Martín-Carro, Antonio Inarejos-García, Paula Almodóvar, Marin Prodanov, Angel García-Villalón, Miriam Granado
Nutrients.2019; 11(1): 153. CrossRef - Limonoid 7-Deacetoxy-7-oxogedunin from Andiroba, Carapa guianensis, Meliaceae, Decreased Body Weight Gain, Improved Insulin Sensitivity, and Activated Brown Adipose Tissue in High-Fat-Diet-Fed Mice
Chihiro Matsumoto, Toko Maehara, Reiko Tanaka, Ko Fujimori
Journal of Agricultural and Food Chemistry.2019; 67(36): 10107. CrossRef - Inhibition of Key Enzymes Linked to Obesity and Cytotoxic Activities of Whole Plant Extracts of Vernonia mesplilfolia Less
Jeremiah Oshiomame Unuofin, Gloria Aderonke Otunola, Anthony Jide Afolayan
Processes.2019; 7(11): 841. CrossRef - Carboxylesterases: Pharmacological Inhibition Regulated Expression and Transcriptional Involvement of Nuclear Receptors and other Transcription Factors
Yuanjun Shen, Zhanquan Shi, Bingfang Yan
Nuclear Receptor Research.2019;[Epub] CrossRef - Rice Bran Reduces Weight Gain and Modulates Lipid Metabolism in Rats with High-Energy-Diet-Induced Obesity
Yang, Huang, Ng, Lee, Hsu, Huang, Wu, Yeh, Shirakawa, Budijanto, Tung, Tung
Nutrients.2019; 11(9): 2033. CrossRef - Peptides obtained from fermented faba bean seeds (Vicia faba) as potential inhibitors of an enzyme involved in the pathogenesis of metabolic syndrome
Anna Jakubczyk, Monika Karaś, Urszula Złotek, Urszula Szymanowska, Barbara Baraniak, Justyna Bochnak
LWT.2019; 105: 306. CrossRef - Nanostructured clay particles supplement orlistat action in inhibiting lipid digestion: An in vitro evaluation for the treatment of obesity
Paul Joyce, Tahnee J. Dening, Tahlia R. Meola, Hanna Gustafsson, Miia Kovalainen, Clive A. Prestidge
European Journal of Pharmaceutical Sciences.2019; 135: 1. CrossRef - Possible Mechanisms Involved in the Vasorelaxant Effect Produced by Anorexigenic Drugs in Rat Aortic Rings
Daniela García-Alonso, Dan Morgenstern-Kaplan, Ariel Cohen-Welch, Jair Lozano-Cuenca, Jorge López-Canales
Medical Sciences.2019; 7(3): 39. CrossRef - Characterization of Lipstatin and the Minor Components from Streptomyces toxytricini Fermentation Broth by HPLC–ESI–Q-TOF–MS
Huang-jian Yang, Zhu-lan Zhang, Ling-bin Yan, Xian Cheng, Zhou-qin Chen, De-sen Wang, Yun-yang Lian
Chromatographia.2019; 82(12): 1791. CrossRef - Chemical constituents, antioxidant, antimicrobial and anti-lipase activities of composites derived from green tea, lemon peels and red wine lees
Levan Gulua, Tamar Turmanidze, Merab Jgenti, Manana Gurielidze
Brazilian Journal of Food Technology.2019;[Epub] CrossRef - Phytanic acid activates PPARα to promote beige adipogenic differentiation of preadipocytes
Hanning Wang, Xueying Mao, Min Du
The Journal of Nutritional Biochemistry.2019; 67: 201. CrossRef - Bioassay-guided isolation of active anti-adipogenic compound from royal jelly and the study of possible mechanisms
Prakash Raj Pandeya, Ramakanta Lamichhane, Kyung-Hee Lee, Se-Gun Kim, Dae-Ho Lee, Hyeong-Kyu Lee, Hyun-Ju Jung
BMC Complementary and Alternative Medicine.2019;[Epub] CrossRef - A pharmacoinformatic approach on Cannabinoid receptor 2 (CB2) and different small molecules: Homology modelling, molecular docking, MD simulations, drug designing and ADME analysis
S. Vijayakumar, P. Manogar, S. Prabhu, M. Pugazhenthi, P.K. Praseetha
Computational Biology and Chemistry.2019; 78: 95. CrossRef - Lipase inhibitory activity of skim milk fermented with different strains of lactic acid bacteria
Ana María Gil-Rodríguez, Thomas P. Beresford
Journal of Functional Foods.2019; 60: 103413. CrossRef - Preventive Cardiology as a Subspecialty of Cardiovascular Medicine
Michael D. Shapiro, David J. Maron, Pamela B. Morris, Mikhail Kosiborod, Pratik B. Sandesara, Salim S. Virani, Amit Khera, Christie M. Ballantyne, Seth J. Baum, Laurence S. Sperling, Deepak L. Bhatt, Sergio Fazio
Journal of the American College of Cardiology.2019; 74(15): 1926. CrossRef - Effects of Polyphenol-Rich Fruit Extracts on Diet-Induced Obesity in Rodents: Systematic Review and Meta-Analysis
Cíntia R. Ballard, Tais F. Galvão, Cinthia B.B. Cazarin, Mário R. Maróstica
Current Pharmaceutical Design.2019; 25(32): 3484. CrossRef - Serum asprosin levels and bariatric surgery outcomes in obese adults
Chao-Yung Wang, Tien-An Lin, Keng-Hau Liu, Chien-Hung Liao, Yu-Yin Liu, Victor Chien-Chia Wu, Ming-Shien Wen, Ta-Sen Yeh
International Journal of Obesity.2019; 43(5): 1019. CrossRef - Polymeric Carriers for Controlled Drug Delivery in Obesity Treatment
Di Huang, Meng Deng, Shihuan Kuang
Trends in Endocrinology & Metabolism.2019; 30(12): 974. CrossRef - Rubrofusarin-6-β-gentiobioside inhibits lipid accumulation and weight gain by regulating AMPK/mTOR signaling
Yo-Han Han, Ji-Ye Kee, Seong-Hwan Park, Jeong-Geon Mun, Hee-Dong Jeon, Jinbong Park, Qin-Peng Zou, Xiang-Qian Liu, Seung-Heon Hong
Phytomedicine.2019; 62: 152952. CrossRef - Effects of Spirulina supplementation on obesity: A systematic review and meta-analysis of randomized clinical trials
Sajjad Moradi, Rahele Ziaei, Sahar Foshati, Hamed Mohammadi, Seyed Mostafa Nachvak, Mohammad Hossein Rouhani
Complementary Therapies in Medicine.2019; 47: 102211. CrossRef - Morus alba (mulberry), a natural potent compound in management of obesity
Mohaddese Mahboubi
Pharmacological Research.2019; 146: 104341. CrossRef - Fermented Castanea crenata Inner Shell Extract Increases Fat Metabolism and Decreases Obesity in High-Fat Diet-Induced Obese Mice
Kyung Min Kim, Hee-Seop Lee, Min-Kyu Yun, Hong-Yon Cho, Heui-Jong Yu, Johann Sohn, Sung-Jin Lee
Journal of Medicinal Food.2019; 22(3): 264. CrossRef - Chlorophyll Derivatives from Marine Cyanobacteria with Lipid-Reducing Activities
Sara Freitas, Natália Gonçalves Silva, Maria Lígia Sousa, Tiago Ribeiro, Filipa Rosa, Pedro N. Leão, Vitor Vasconcelos, Mariana Alves Reis, Ralph Urbatzka
Marine Drugs.2019; 17(4): 229. CrossRef - Effects of Gambisan in overweight adults and adults with obesity
Dae-Hyun Jo, Seunghoon Lee, Jae-Dong Lee
Medicine.2019; 98(47): e18060. CrossRef - Trypsin inhibitors: promising candidate satietogenic proteins as complementary treatment for obesity and metabolic disorders?
Vanessa Cristina Oliveira de Lima, Grasiela Piuvezam, Bruna Leal Lima Maciel, Ana Heloneida de Araújo Morais
Journal of Enzyme Inhibition and Medicinal Chemistry.2019; 34(1): 405. CrossRef - A new enantioselective synthesis of antiobesity drug lorcaserin
Ganesh S. Ghotekar, Devidas A. More, Viswanadh Nalla, M. Muthukrishnan
New Journal of Chemistry.2019; 43(43): 16876. CrossRef - Facing Overweight and Obesity: A Guide for Mental Health Professionals
Jonathan R. Stevens, Theodore A. Stern
Psychiatric Annals.2019; 49(2): 65. CrossRef - Adipose tissue as a possible therapeutic target for polyphenols: A case for Cyclopia extracts as anti-obesity nutraceuticals
Babalwa U. Jack, Christiaan J. Malherbe, Mokadi Mamushi, Christo J.F. Muller, Elizabeth Joubert, Johan Louw, Carmen Pheiffer
Biomedicine & Pharmacotherapy.2019; 120: 109439. CrossRef - Pathophysiology and Individualized Treatment of Hypothalamic Obesity Following Craniopharyngioma and Other Suprasellar Tumors: A Systematic Review
Laura van Iersel, Karen E Brokke, Roger A H Adan, Lauren C M Bulthuis, Erica L T van den Akker, Hanneke M van Santen
Endocrine Reviews.2019; 40(1): 193. CrossRef - Anti-obesity effects of dichloromethane leaf extract of Gnidia glauca in high fat diet-induced obese rats
Wycliffe Makori Arika, Cromwell Mwiti Kibiti, Joan Murugi Njagi, Mathew Piero Ngugi
Heliyon.2019; 5(11): e02800. CrossRef - Pharmacogenetics of amfepramone in healthy Mexican subjects reveals potential markers for tailoring pharmacotherapy of obesity: results of a randomised trial
Magdalena Gómez-Silva, Everardo Piñeyro-Garza, Rigoberto Vargas-Zapata, María Elena Gamino-Peña, Armando León-García, Mario Bermúdez de León, Adrián Llerena, Rafael B. R. León-Cachón
Scientific Reports.2019;[Epub] CrossRef - Biological Properties and Bioactive Components of Allium cepa L.: Focus on Potential Benefits in the Treatment of Obesity and Related Comorbidities
Mariangela Marrelli, Valentina Amodeo, Giancarlo Statti, Filomena Conforti
Molecules.2018; 24(1): 119. CrossRef - Neuromodulation for the treatment of eating disorders and obesity
Darrin J. Lee, Gavin J.B. Elias, Andres M. Lozano
Therapeutic Advances in Psychopharmacology.2018; 8(2): 73. CrossRef - Nanomedicine for obesity treatment
Yuqi Zhang, Jicheng Yu, Li Qiang, Zhen Gu
Science China Life Sciences.2018; 61(4): 373. CrossRef - Effects of long-term oral nitrate administration on adiposity in normal adult female rats
Fatemeh Bakhtiarzadeh, Fatemeh Siavoshi, Sevda Gheibi, Khosrow Kashfi, Roghaieh Samadi, Sajad Jeddi, Asghar Ghasemi
Life Sciences.2018; 210: 76. CrossRef - The correction of the metabolic parameters of msg-induced obesity in rats by 2-[4-(benzyloxy) phenoxy] acetic acid
Victoria Konopelniuk, Tetyana Falalyeyeva, Olena Tsyryuk, Yuliia Savchenko, Iryna Prybytko, Nazarii Kobyliak, Oleksandr Kovalchuk, Aleksandr Boyko, Viatcheslav V. Arkhipov, Yurii Moroz, Liudmyla Ostapchenko
Journal of Nutrition & Intermediary Metabolism.2018; 13: 1. CrossRef - Body weight perception, disordered weight control behaviors, and depressive symptoms among Korean adults: The Korea National Health and Nutrition Examination Survey 2014
Yongjoo Kim, S. Bryn Austin, S. V. Subramanian, Ichiro Kawachi, Chung-Ying Lin
PLOS ONE.2018; 13(6): e0198841. CrossRef - Effect of sprouted fenugreek seeds on various diseases: a review
Firoz khan, Kapil Negi, Tinku Kumar
Journal of Diabetes, Metabolic Disorders & Control.2018; 5(4): 119. CrossRef - The free, esterified, and insoluble-bound phenolic profiles of Rhus chinensis Mill. fruits and their pancreatic lipase inhibitory activities with molecular docking analysis
Chengting Zhang, Yanli Ma, Fanding Gao, Yingxin Zhao, Shengbao Cai, Mingjie Pang
Journal of Functional Foods.2018; 40: 729. CrossRef - Future Pharmacotherapy for Obesity: New Anti-obesity Drugs on the Horizon
Gitanjali Srivastava, Caroline Apovian
Current Obesity Reports.2018; 7(2): 147. CrossRef - Bariatric surgery for obese patients with kidney disease
Muffazal Lakdawala, Carlyne Remedios
Journal of Renal Nutrition and Metabolism.2018; 4(4): 107. CrossRef - Fibroblast Growth Factor 21: A Versatile Regulator of Metabolic Homeostasis
Lucas D. BonDurant, Matthew J. Potthoff
Annual Review of Nutrition.2018; 38(1): 173. CrossRef - Antiobesity Effect of Tricin, a Methylated Cereal Flavone, in High-Fat-Diet-Induced Obese Mice
Dabeen Lee, Jee-Young Imm
Journal of Agricultural and Food Chemistry.2018; 66(38): 9989. CrossRef - Antiadipogenic Effects of Loganic Acid in 3T3-L1 Preadipocytes and Ovariectomized Mice
Eunkuk Park, Jeonghyun Kim, Subin Yeo, Gijeong Kim, Eun-Hee Ko, Sang Lee, Wan Li, Chun Choi, Seon-Yong Jeong
Molecules.2018; 23(7): 1663. CrossRef - Secondary Metabolites, Dietary Fiber and Conjugated Fatty Acids as Functional Food Ingredients against Overweight and Obesity
Kamila Kasprzak, Karolina Wojtunik-Kulesza, Tomasz Oniszczuk, Maciej Kuboń, Anna Oniszczuk
Natural Product Communications.2018; 13(8): 1934578X1801300. CrossRef - Antiobesity and Antioxidant Potentials of Selected Palestinian Medicinal Plants
Rana M. Jamous, Salam Y. Abu-Zaitoun, Rola J. Akkawi, Mohammed S. Ali-Shtayeh
Evidence-Based Complementary and Alternative Medicine.2018; 2018: 1. CrossRef - Transient receptor potential (TRP) channels: a metabolic TR(i)P to obesity prevention and therapy
M. Bishnoi, P. Khare, L. Brown, S. K. Panchal
Obesity Reviews.2018; 19(9): 1269. CrossRef - Perilipin-2 deletion promotes carbohydrate-mediated browning of white adipose tissue at ambient temperature
Andrew E. Libby, Elise S. Bales, Jenifer Monks, David J. Orlicky, James L. McManaman
Journal of Lipid Research.2018; 59(8): 1482. CrossRef - Circulating molecules that control brown/beige adipocyte differentiation and thermogenic capacity
Raissa G. Ludwig, Andréa L. Rocha, Marcelo A. Mori
Cell Biology International.2018; 42(6): 701. CrossRef - Inhibitory effect of ethanolic extract ofRamulus morion adipogenic differentiation of 3T3-L1 cells and their antioxidant activity
Seonwook Hwang, Jeong-Keun Kim, In-Ho Kim, Young-Hee Lim
Journal of Food Biochemistry.2018; 42(2): e12469. CrossRef - Antihyperglycemic activities of twig extract of indigenous cinnamon (Cinnamomum osmophloeum) on high‐fat diet and streptozotocin‐induced hyperglycemic rats
Gong‐Min Lin, Chia‐Yun Hsu, Shang‐Tzen Chang
Journal of the Science of Food and Agriculture.2018; 98(15): 5908. CrossRef - Cross-sectional associations between gender-linked personality traits and use of weight-loss and muscle-building products among U.S. young adults
Vivienne M Hazzard, Kelley A Borton, Katherine W Bauer, Kendrin R Sonneville
Eating Disorders.2018; 26(5): 418. CrossRef - 2020 vision – An overview of prospects for diabetes management and prevention in the next decade
Chih-Yuan Wang, David L. Neil, Philip Home
Diabetes Research and Clinical Practice.2018; 143: 101. CrossRef - In vitro α-amylase, α-glucosidase, lipase inhibitory and cytotoxic activities of tuber extracts of Kedrostis africana (L.) Cogn
Jeremiah Oshiomame Unuofin, Gloria Aderonke Otunola, Anthony Jide Afolayan
Heliyon.2018; 4(9): e00810. CrossRef - Inhibitory effect of astaxanthin on pancreatic lipase with inhibition kinetics integrating molecular docking simulation
Xiping Du, Manli Bai, Ying Huang, Zedong Jiang, Feng Chen, Hui Ni, Qingbiao Li
Journal of Functional Foods.2018; 48: 551. CrossRef - The Effects of Bariatric Surgery on Renal Outcomes: a Systematic Review and Meta-analysis
Stefana Catalina Bilha, Ionut Nistor, Alina Nedelcu, Mehmet Kanbay, Viorel Scripcariu, Daniel Timofte, Dimitrie Siriopol, Adrian Covic
Obesity Surgery.2018; 28(12): 3815. CrossRef - Camptocormia and myalgia as the revealing symptoms of a drug-induced myopathy related to chronic orlistat intake: a case report
Frédéric London, André Thévenon, Camille Levisse, Fançois Cassim, Céline Tard
Acta Neurologica Belgica.2018; 118(1): 115. CrossRef - Effects of Dietary Intake of Japanese Mushrooms on Visceral Fat Accumulation and Gut Microbiota in Mice
Takamitsu Shimizu, Koichiro Mori, Kenji Ouchi, Mamoru Kushida, Tsuyoshi Tsuduki
Nutrients.2018; 10(5): 610. CrossRef - Alpha-lipoic acid supplement in obesity treatment: A systematic review and meta-analysis of clinical trials
Nazli Namazi, Bagher Larijani, Leila Azadbakht
Clinical Nutrition.2018; 37(2): 419. CrossRef - Ilex paraguariensis modulates fat metabolism in Caenorhabditis elegans through purinergic system (ADOR-1) and nuclear hormone receptor (NHR-49) pathways
Marina Lopes Machado, Leticia Priscilla Arantes, Priscila Gubert, Daniele Coradini Zamberlan, Thayanara Cruz da Silva, Tássia Limana da Silveira, Aline Boligon, Félix Alexandre Antunes Soares, Myon-Hee Lee
PLOS ONE.2018; 13(9): e0204023. CrossRef - Heshouwu (Polygonum multiflorum Thunb.) ethanol extract suppresses pre-adipocytes differentiation in 3T3-L1 cells and adiposity in obese mice
Ra-Yeong Choi, Hae-In Lee, Ju Ri Ham, Sung-Tae Yee, Kyung-Yun Kang, Mi-Kyung Lee
Biomedicine & Pharmacotherapy.2018; 106: 355. CrossRef - The Gut Microbiota as a Mediator of Metabolic Benefits after Bariatric Surgery
Fernando F. Anhê, Thibault V. Varin, Jonathan D. Schertzer, André Marette
Canadian Journal of Diabetes.2017; 41(4): 439. CrossRef - Antiobesity, antioxidant and hepatoprotective effects of Diallyl trisulphide (DATS) alone or in combination with Orlistat on HFD induced obese rats
Sivakumar Annamalai, Lavanya Mohanam, Veena Raja, Alwin Dev, Venkataraman Prabhu
Biomedicine & Pharmacotherapy.2017; 93: 81. CrossRef - A 8-Week, Randomized, Double-Blind, Placebo-Controlled Human Trial to Evaluate the Efficacy and Safety of Punica granatum L.ㆍ Actinidia chinensis Planch. Mixed Extract on Body Fat
Jin-Bong Choi, Ji-Eun Lee, Yun-Kyoung Do
Journal of Korean Medicine for Obesity Research.2017; 17(2): 87. CrossRef - Pharmabiotics as an Emerging Medication for Metabolic Syndrome and Its Related Diseases
Thi Thanh Binh Nguyen, Yan Yan Jin, Hea-Jong Chung, Seong-Tschool Hong
Molecules.2017; 22(10): 1795. CrossRef - Genotoxicity of antiobesity drug orlistat and effect of caffeine intervention: an in vitro study
Manoswini Chakrabarti, Ilika Ghosh, Aditi Jana, Manosij Ghosh, Anita Mukherjee
Drug and Chemical Toxicology.2017; 40(3): 339. CrossRef - Antidiabetic, antidyslipidemic and toxicity profile of ENV-2: A potent pyrazole derivative against diabetes and related diseases
Eduardo Hernández-Vázquez, Hugo Ocampo-Montalban, Litzia Cerón-Romero, Miguel Cruz, Jaime Gómez-Zamudio, Guadalupe Hiriart-Valencia, Rafael Villalobos-Molina, Angelica Flores-Flores, Samuel Estrada-Soto
European Journal of Pharmacology.2017; 803: 159. CrossRef - Caffeic acid methyl and ethyl esters exert potential antidiabetic effects on glucose and lipid metabolism in cultured murine insulin-sensitive cells through mechanisms implicating activation of AMPK
Hoda M. Eid, Farah Thong, Abir Nachar, Pierre S. Haddad
Pharmaceutical Biology.2017; 55(1): 2026. CrossRef - Antihyperlidemic Effects of Mangosteen (Garcinia mangostana L.) Pericarp Ethanolic Extract In High-Carbohydrate Wistar Rats
Alkilany Salem Abuzaid, Elin Yulinah Sukandar, Neng Fisheri Kurniati, I. Ketut Adnyana
JOURNAL OF NATURAL REMEDIES.2017; 17(4): 1. CrossRef - PRIORITIES FOR HEALTH ECONOMIC METHODOLOGICAL RESEARCH: RESULTS OF AN EXPERT CONSULTATION
David Tordrup, Christos Chouaid, Pim Cuijpers, William Dab, Johanna Maria van Dongen, Jaime Espin, Bengt Jönsson, Christian Léonard, David McDaid, Martin McKee, José Pereira Miguel, Anita Patel, Jean-Yves Reginster, Walter Ricciardi, Maureen Rutten-van Mo
International Journal of Technology Assessment in Health Care.2017; 33(6): 609. CrossRef - Recent Advances in Effect‐directed Enzyme Assays based on Thin‐layer Chromatography
Sarah Bräm, Evelyn Wolfram
Phytochemical Analysis.2017; 28(2): 74. CrossRef - Phenolics and neolignans isolated from the fruits of Juglans mandshurica Maxim. and their effects on lipolysis in adipocytes
SeonJu Park, Nanyoung Kim, Guijae Yoo, Sang-Nam Kim, Hyun-Jung Kwon, Kiwon Jung, Dong-Chan Oh, Yun-Hee Lee, Seung Hyun Kim
Phytochemistry.2017; 137: 87. CrossRef - Assessment of essential oil as a potential anti-obesity agent: a narrative review
Aswir Abd Rashed, Mohd Naeem Mohd Nawi, Kasmawati Sulaiman
Journal of Essential Oil Research.2017; 29(1): 1. CrossRef - Transdermal Delivery of Anti‐Obesity Compounds to Subcutaneous Adipose Tissue with Polymeric Microneedle Patches
Aung Than, Ke Liang, Shaohai Xu, Lei Sun, Hongwei Duan, Fengna Xi, Chenjie Xu, Peng Chen
Small Methods.2017;[Epub] CrossRef - Lipase inhibitory activity of Lagenaria siceraria fruit as a strategy to treat obesity
Maria Maqsood, Dildar Ahmed, Iqra Atique, Wajeeha Malik
Asian Pacific Journal of Tropical Medicine.2017; 10(3): 305. CrossRef - Saffron: a promising natural medicine in the treatment of metabolic syndrome
Bibi Marjan Razavi, Hossein Hosseinzadeh
Journal of the Science of Food and Agriculture.2017; 97(6): 1679. CrossRef - Neuropsychiatric safety with liraglutide 3.0 mg for weight management: Results from randomized controlled phase 2 and 3a trials
Patrick M. O'Neil, Vanita R. Aroda, Arne Astrup, Robert Kushner, David C. W. Lau, Thomas A. Wadden, Jason Brett, Ana‐Paula Cancino, John P. H. Wilding
Diabetes, Obesity and Metabolism.2017; 19(11): 1529. CrossRef - Prostaglandin D2 enhances lipid accumulation through suppression of lipolysis via DP2 (CRTH2) receptors in adipocytes
Eri Wakai, Kosuke Aritake, Yoshihiro Urade, Ko Fujimori
Biochemical and Biophysical Research Communications.2017; 490(2): 393. CrossRef - Ayurvedic anti-diabetic formulation Lodhrasavam inhibits alpha-amylase, alpha-glucosidase and suppresses adipogenic activity in vitro
Megha Abhijit Butala, Subrahmanya Kumar Kukkupuni, Chethala N. Vishnuprasad
Journal of Ayurveda and Integrative Medicine.2017; 8(3): 145. CrossRef - StandardizedCirsium setidensNakai Ethanolic Extract Suppresses Adipogenesis and Regulates Lipid Metabolisms in 3T3-L1 Adipocytes and C57BL/6J Mice Fed High-Fat Diets
Bong-Yeon Cho, Mi-Ryeong Park, Jin-Ha Lee, Moon-Jin Ra, Kyoung Chan Han, Il-Jun Kang, Ok-Hwan Lee
Journal of Medicinal Food.2017; 20(8): 763. CrossRef - The Obesity–Impulsivity Axis: Potential Metabolic Interventions in Chronic Psychiatric Patients
Adonis Sfera, Carolina Osorio, Luzmin Acosta Inderias, Victoria Parker, Amy I. Price, Michael Cummings
Frontiers in Psychiatry.2017;[Epub] CrossRef - Hot-Water Extracts from Roots of Vitis thunbergii var. taiwaniana and Identified ε-Viniferin Improve Obesity in High-Fat Diet-Induced Mice
Yeh-Lin Lu, Shyr-Yi Lin, Sheng-Uei Fang, Ying-Ying Hsieh, Chiy-Rong Chen, Chi-Luan Wen, Chi-I Chang, Wen-Chi Hou
Journal of Agricultural and Food Chemistry.2017; 65(12): 2521. CrossRef - Peptide Fraction pOh2 Exerts Antiadipogenic Activity through Inhibition of C/EBP-αand PPAR-γExpression in 3T3-L1 Adipocytes
Thi Tuyet Nhung Nguyen, Thi Thu Ha, Thi Hoa Nguyen, Thi Hien Vu, Nam Hai Truong, Hoang Ha Chu, Dong Van Quyen
BioMed Research International.2017; 2017: 1. CrossRef - IN VITRO STUDIES OF SOME EDIBLE SPICES ON PANCREATIC LIPASE INHIBITORY ACTIVITY
S Mhatre, A. Bhagit, R. P Yadav
INDIAN DRUGS.2017; 54(02): 62. CrossRef - Combined Supplementation with Grape Pomace and Omija Fruit Ethanol Extracts Dose-Dependently Improves Body Composition, Plasma Lipid Profiles, Inflammatory Status, and Antioxidant Capacity in Overweight and Obese Subjects
Hye Jin Han, Un Ju Jung, Hye-Jin Kim, Su-Jung Cho, Ae Hyang Kim, Youngji Han, Myung-Sook Choi
Journal of Medicinal Food.2016; 19(2): 170. CrossRef - Arctigenin Inhibits Adipogenesis by Inducing AMPK Activation and Reduces Weight Gain in High‐Fat Diet‐Induced Obese Mice
Yo‐Han Han, Ji‐Ye Kee, Jinbong Park, Hye‐Lin Kim, Mi‐Young Jeong, Dae‐Seung Kim, Yong‐Deok Jeon, Yunu Jung, Dong‐Hyun Youn, JongWook Kang, Hong‐Seob So, Raekil Park, Jong‐Hyun Lee, Soyoung Shin, Su‐Jin Kim, Jae‐Young Um, Seung‐Heon Hong
Journal of Cellular Biochemistry.2016; 117(9): 2067. CrossRef - Evolution of pharmacological obesity treatments: focus on adverse side‐effect profiles
A. J. Krentz, K. Fujioka, M. Hompesch
Diabetes, Obesity and Metabolism.2016; 18(6): 558. CrossRef - Protective and curative effects of Bacillus subtilis SPB1 biosurfactant on high-fat-high-fructose diet induced hyperlipidemia, hypertriglyceridemia and deterioration of liver function in rats
Raida Zouari, Khaled Hamden, Abdelfattah El Feki, Khansa Chaabouni, Fatma Makni-Ayadi, Choumous Kallel, Fahima Sallemi, Semia Ellouze-Chaabouni, Dhouha Ghribi-Aydi
Biomedicine & Pharmacotherapy.2016; 84: 323. CrossRef - Pharmacotherapy in Treatment of Obesity
Jeanette N. Keith
Gastroenterology Clinics of North America.2016; 45(4): 663. CrossRef - Intestinal removal of free fatty acids from hosts by Lactobacilli for the treatment of obesity
Hea‐Jong Chung, Jae G. Yu, In‐Ah Lee, Ming‐Jie Liu, Yan‐Fei Shen, Satya P. Sharma, Mohammad A. H. M. Jamal, Jun‐Hyun Yoo, Hyeon‐Jin Kim, Seong‐Tshool Hong
FEBS Open Bio.2016; 6(1): 64. CrossRef - Role of glucagon-like peptide 1 receptor agonists in management of obesity
Diana Isaacs, Lalita Prasad-Reddy, Sneha Baxi Srivastava
American Journal of Health-System Pharmacy.2016; 73(19): 1493. CrossRef - Cardiovascular effects of bariatric surgery
Andrew J. Beamish, Torsten Olbers, Aaron S. Kelly, Thomas H. Inge
Nature Reviews Cardiology.2016; 13(12): 730. CrossRef - A new TLC bioautographic assay for qualitative and quantitative estimation of lipase inhibitors
Jihe Tang, Jinge Zhou, Qingjiu Tang, Tao Wu, Zhihong Cheng
Phytochemical Analysis.2016; 27(1): 5. CrossRef - Anti-obesity effects of artificial planting blueberry (Vaccinium ashei) anthocyanin in high-fat diet-treated mice
Tao Wu, Zenghong Jiang, Jinjin Yin, Hairong Long, Xiaodong Zheng
International Journal of Food Sciences and Nutrition.2016; 67(3): 257. CrossRef - Serious adverse events reported for antiobesity medicines: postmarketing experiences from the EU adverse event reporting system EudraVigilance
L Aagaard, C E Hallgreen, E H Hansen
International Journal of Obesity.2016; 40(11): 1742. CrossRef - The effect of high-fat diet on the structure of the colon and the possible protective role of L-arginine
Asmaa I. Ahmed
The Egyptian Journal of Histology.2016; 39(1): 25. CrossRef - Roles of calcium and Mitochondria-Associated Membranes in the development of obesity and diabetes
R.E. Cárdenas-Pérez, A. Camacho
Medicina Universitaria.2016; 18(70): 23. CrossRef - The efficacy of the appetite suppressant, diethylpropion, is dependent on both when it is given (day vs. night) and under conditions of high fat dietary restriction
B. Kalyanasundar, Jessica Solorio, Claudia I. Perez, Carlos Hoyo-Vadillo, Sidney A. Simon, Ranier Gutierrez
Appetite.2016; 100: 152. CrossRef - A review on possible therapeutic targets to contain obesity: The role of phytochemicals
Meriga Balaji, Muni Swamy Ganjayi, Gali E.N. Hanuma Kumar, Brahma Naidu Parim, Ramgopal Mopuri, Sreenivasulu Dasari
Obesity Research & Clinical Practice.2016; 10(4): 363. CrossRef - The effectiveness of interventions to treat obesity in survivors of childhood brain tumors: a systematic review protocol
Kuan-Wen Wang, Marlie Valencia, Laura Banfield, Ruth Chau, Adam Fleming, Sheila K. Singh, Sarah Burrow, Russell J. de Souza, Lehana Thabane, M. Constantine Samaan
Systematic Reviews.2016;[Epub] CrossRef - Role of the vagus nerve in the development and treatment of diet‐induced obesity
Guillaume de Lartigue
The Journal of Physiology.2016; 594(20): 5791. CrossRef - Hunger and Satiety Mechanisms and Their Potential Exploitation in the Regulation of Food Intake
Tehmina Amin, Julian G. Mercer
Current Obesity Reports.2016; 5(1): 106. CrossRef - Anti-adipogenic activity of Carduus crispus and its constituent apigenin in 3T3-L1 adipocytes by downregulating PPARγ and C/EBPα
Yoo Na Hong, Jaemoo Chun, Yeong Shik Kim
European Food Research and Technology.2016; 242(9): 1555. CrossRef - Cinnamaldehyde supplementation prevents fasting‐induced hyperphagia, lipid accumulation, and inflammation in high‐fat diet‐fed mice
Pragyanshu Khare, Sneha Jagtap, Yachna Jain, Ritesh K. Baboota, Priyanka Mangal, Ravneet K. Boparai, Kamlesh K. Bhutani, Shyam S. Sharma, Louis S. Premkumar, Kanthi K. Kondepudi, Kanwaljit Chopra, Mahendra Bishnoi
BioFactors.2016; 42(2): 201. CrossRef - Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: a systematic review
Igho J. Onakpoya, Carl J. Heneghan, Jeffrey K. Aronson
BMC Medicine.2016;[Epub] CrossRef - Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity
Mariangela Marrelli, Filomena Conforti, Fabrizio Araniti, Giancarlo Statti
Molecules.2016; 21(10): 1404. CrossRef - Cannabinoid receptor 1 ligands revisited: Pharmacological assessment in the ACTOne system
Chaela S. Presley, Ammaar H. Abidi, Bob M. Moore
Analytical Biochemistry.2016; 498: 8. CrossRef - A Review of Combination Effects and Adverse Effects of Yerba Mate () on the Treatment of Obesity
Jae Hyun Ahn, Min Ho Lee, Seung Hoon Lee, Do Young Choi, Jae Dong Lee
The Acupuncture.2016; 33(2): 135. CrossRef - Glucagon increases energy expenditure independently of brown adipose tissue activation in humans
V. Salem, C. Izzi‐Engbeaya, C. Coello, D. B. Thomas, E. S. Chambers, A. N. Comninos, A. Buckley, Z. Win, A. Al‐Nahhas, E. A. Rabiner, R. N. Gunn, H. Budge, M. E. Symonds, S. R. Bloom, T. M. Tan, W. S. Dhillo
Diabetes, Obesity and Metabolism.2016; 18(1): 72. CrossRef - Marine Algae as a Potential Source for Anti-Obesity Agents
Chu Wan-Loy, Phang Siew-Moi
Marine Drugs.2016; 14(12): 222. CrossRef - Weight loss intervention through lifestyle modification or pharmacotherapy for obstructive sleep apnoea in adults
Marzieh Hosseini Araghi, Yen-Fu Chen, Alison Jagielski, Sopna Mannan Choudhury, Dev Banerjee, G Neil Thomas, Shahrad Taheri
Cochrane Database of Systematic Reviews.2016;[Epub] CrossRef - Preclinical models for obesity research
Perry Barrett, Julian G. Mercer, Peter J. Morgan
Disease Models & Mechanisms.2016; 9(11): 1245. CrossRef - Pharmacotherapy for the management of obesity
Dhiren Patel
Metabolism.2015; 64(11): 1376. CrossRef - The Expected Net Present Value of Developing Weight Management Drugs in the Context of Drug Safety Litigation
Anita Chawla, Ginger Carls, Edmund Deng, Edward Tuttle
PharmacoEconomics.2015; 33(7): 749. CrossRef - Modeling energy intake by adding homeostatic feedback and drug intervention
Peter Gennemark, Stephan Hjorth, Johan Gabrielsson
Journal of Pharmacokinetics and Pharmacodynamics.2015; 42(1): 79. CrossRef - A natural solution for obesity: Bioactives for the prevention and treatment of weight gain. A review
Cristina Torres-Fuentes, Harriët Schellekens, Timothy G. Dinan, John F. Cryan
Nutritional Neuroscience.2015; 18(2): 49. CrossRef - Target identification and validation in brain reward dysfunction
Luis F. Alguacil, Carmen González-Martín
Drug Discovery Today.2015; 20(3): 347. CrossRef - Weights and measures: are bariatric surgery guidelines realistic?
Youssof Oskrochi, Azeem Majeed, Graham Easton
Journal of the Royal Society of Medicine.2015; 108(7): 252. CrossRef - Biting off more than we can chew: is BMI the correct standard for bariatric surgery eligibility?
Youssof Oskrochi, Azeem Majeed, Graham Easton
British Journal of General Practice.2015; 65(638): 482. CrossRef - Fisetin Suppresses Lipid Accumulation in Mouse Adipocytic 3T3-L1 Cells by Repressing GLUT4-Mediated Glucose Uptake through Inhibition of mTOR-C/EBPα Signaling
Marina Watanabe, Mitsuhiro Hisatake, Ko Fujimori
Journal of Agricultural and Food Chemistry.2015; 63(20): 4979. CrossRef - Modulation of food consumption and sleep–wake cycle in mice by the neutral CB1 antagonist ABD459
Anushka V. Goonawardena, Andrea Plano, Lianne Robinson, Ruth Ross, Iain Greig, Roger G. Pertwee, Robert E. Hampson, Bettina Platt, Gernot Riedel
Behavioural Pharmacology.2015; 26(3): 289. CrossRef - Suppressive Effect of Acorn (Quercus acutissima Carr.) Extracts in 3T3-L1 Preadipocytes
Ji-Yeon Kim, Jin Lee, Chang-Won Lee, Ae-Jung Kim
The Korean Journal of Food And Nutrition.2015; 28(4): 650. CrossRef - Discovery of Rimonabant and its potential analogues as anti-TB drug candidates
J. M. Gajbhiye, N. A. More, Manoj D. Patil, R. Ummanni, S. S. Kotapalli, P. Yogeeswari, D. Sriram, V. H. Masand
Medicinal Chemistry Research.2015; 24(7): 2960. CrossRef - Seapolynol Extracted from Ecklonia cava Inhibits Adipocyte Differentiation in Vitro and Decreases Fat Accumulation in Vivo
Hui-Jeon Jeon, Hyeon-Son Choi, Yeon-Joo Lee, Ji-Hyun Hwang, Ok-Hwan Lee, Min-Jung Seo, Kui-Jin Kim, Boo-Yong Lee
Molecules.2015; 20(12): 21715. CrossRef - Inhibition of opioid systems in the hypothalamus as well as the mesolimbic area suppresses feeding behavior of mice
H. Ikeda, C. Ardianto, N. Yonemochi, L. Yang, T. Ohashi, M. Ikegami, H. Nagase, J. Kamei
Neuroscience.2015; 311: 9. CrossRef - Medicinal Plants and Their Inhibitory Activities against Pancreatic Lipase: A Review
Atefehalsadat Seyedan, Mohammed Abdullah Alshawsh, Mustafa Ahmed Alshagga, Sanaz Koosha, Zahurin Mohamed
Evidence-Based Complementary and Alternative Medicine.2015; 2015: 1. CrossRef - Access to weight reduction interventions for overweight and obese patients in UK primary care: population-based cohort study
H. P. Booth, A. T. Prevost, M. C. Gulliford
BMJ Open.2015; 5(1): e006642. CrossRef - Myocardin-Related Transcription Factor A Regulates Conversion of Progenitors to Beige Adipocytes
Meghan E. McDonald, Chendi Li, Hejiao Bian, Barbara D. Smith, Matthew D. Layne, Stephen R. Farmer
Cell.2015; 160(1-2): 105. CrossRef - Phentermine Plus Topiramate for Weight Reduction
Ömer Kartal, Ayşe Tuğba Kartal
Clinical Pediatrics.2015; 54(3): 302. CrossRef - New perspectives on the development of antiobesity drugs
Luca Costantino, Daniela Barlocco
Future Medicinal Chemistry.2015; 7(3): 315. CrossRef - Operon for Biosynthesis of Lipstatin, the Beta-Lactone Inhibitor of Human Pancreatic Lipase
Tingli Bai, Daozhong Zhang, Shuangjun Lin, Qingshan Long, Yemin Wang, Hongyu Ou, Qianjin Kang, Zixin Deng, Wen Liu, Meifeng Tao, H. Nojiri
Applied and Environmental Microbiology.2014; 80(24): 7473. CrossRef - Orlistat‐induced fulminant hepatic failure
D. Sall, J. Wang, M. Rashkin, M. Welch, C. Droege, D. Schauer
Clinical Obesity.2014; 4(6): 342. CrossRef - Pycnogenol Supplementation Promotes Lipolysis via Activation of cAMP-Dependent PKA in ob/ob Mice and Primary-Cultured Adipocytes
Jin-Nyoung HO, Ok-Kyung KIM, Da-Eun NAM, Woojin JUN, Jeongmin LEE
Journal of Nutritional Science and Vitaminology.2014; 60(6): 429. CrossRef - Treatment of obesity with the resveratrol-enriched rice DJ-526
So-Hyeon Baek, Hea-Jong Chung, Heui-Kwan Lee, Roshan D'Souza, Youngju Jeon, Hyeon-Jin Kim, Soon-Jong Kweon, Seong-Tshool Hong
Scientific Reports.2014;[Epub] CrossRef - Pharmacotherapy for weight loss: the cardiovascular effects of the old and new agents
C. P. Walter, B. E. Bleske, M. P. Dorsch
Journal of Clinical Pharmacy and Therapeutics.2014; 39(5): 475. CrossRef - Inhibitory effects of sweet cherry anthocyanins on the obesity development in C57BL/6 mice
Tao Wu, Qiong Tang, Zhuoping Yu, Zichun Gao, Hao Hu, Wei Chen, Xiaodong Zheng, Ting Yu
International Journal of Food Sciences and Nutrition.2014; 65(3): 351. CrossRef - Cardiovascular effects of phentermine and topiramate
Jens Jordan, Arne Astrup, Stefan Engeli, Krzysztof Narkiewicz, Wesley W. Day, Nick Finer
Journal of Hypertension.2014; 32(6): 1178. CrossRef - Modulation of inflammatory gene transcription after long-term coffee consumption
Swantje Winkler, Natalie Dieminger, Volker Blust, Annett Riedel, Tamara Bakuradze, Gina Montoya, Ute Hassmann, Roman Lang, Thomas Hofmann, Veronika Somoza, Elke Richling, Gerhard Bytof, Herbert Stiebitz, Ingo Lantz, Dorothea Schipp, Jochen Raedle, Doris M
Food Research International.2014; 63: 428. CrossRef - Monoterpene phenolic compound thymol prevents high fat diet induced obesity in murine model
Mohammad Rafiul Haque, S. H. Ansari, A. K. Najmi, Mohammed Afroz Ahmad
Toxicology Mechanisms and Methods.2014; 24(2): 116. CrossRef - Fenugreek Seed Extract Inhibit Fat Accumulation and Ameliorates Dyslipidemia in High Fat Diet-Induced Obese Rats
Parveen Kumar, Uma Bhandari, Shrirang Jamadagni
BioMed Research International.2014; 2014: 1. CrossRef - Há irracionalidades no consumo de inibidores de apetite no Brasil? Uma análise farmacoeconométrica de dados em painel
Daniel Marques Mota, Márcia Gonçalves de Oliveira, Rafael Filiacci Bovi, Sidarta Figueredo Silva, Jeane Araújo Fernandes Cunha, José Angelo Divino
Ciência & Saúde Coletiva.2014; 19(5): 1389. CrossRef - Effects of anti-obesity drugs, phentermine and mahuang, on the behavioral patterns in Sprague-Dawley rat model
Ryeo-Eun Go, Kyung-A Hwang, Seung-Hee Kim, Min-Young Lee, Cho-Won Kim, So-Ye Jeon, Yun-Bae Kim, Kyung-Chul Choi
Laboratory Animal Research.2014; 30(2): 73. CrossRef - Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight‐loss outcomes
Steven R. Smith, Patrick M. O'Neil, Arne Astrup, Nicholas Finer, Matilde Sanchez‐Kam, Kyle Fraher, Randi Fain, William R. Shanahan
Obesity.2014; 22(10): 2137. CrossRef - American Association of Clinical Endocrinologists and American College of Endocrinology Consensus Conference on Obesity: Building an Evidence Base for Comprehensive Action
W. Timothy Garvey, Alan J. Garber, Jeffrey I. Mechanick, George A. Bray, Samuel Dagogo-Jack, Daniel Einhorn, George Grunberger, Yehuda Handelsman, Charles H. Hennekens, Daniel L. Hurley, Janet McGill, Pasquale Palumbo, Guillermo Umpierrez
Endocrine Practice.2014; 20(9): 956. CrossRef - New Antiobesity Agents
Grace Shyh, Angela Cheng-Lai
Cardiology in Review.2014; 22(1): 43. CrossRef - Effect of broccoli phytochemical extract on release of fatty acids from salmon muscle and salmon oil during in vitro digestion
K. E. Aarak, B. Kirkhus, S. Johansen, G. E. Vegarud, G. I. A. Borge
Food Funct..2014; 5(9): 2331. CrossRef - Association between obesity and prescribed medication use in England
Jonas Minet Kinge, Stephen Morris
Economics & Human Biology.2014; 15: 47. CrossRef - Therapeutic Phytogenic Compounds for Obesity and Diabetes
Hee Jung, Yun Lim, Eun-Kyoung Kim
International Journal of Molecular Sciences.2014; 15(11): 21505. CrossRef - Milk protein-derived peptides induce 5-HT2C-mediated satiety in vivo
Harriët Schellekens, Alice B. Nongonierma, Gerard Clarke, Wesley E.P.A. van Oeffelen, Richard J. FitzGerald, Timothy G. Dinan, John F. Cryan
International Dairy Journal.2014; 38(1): 55. CrossRef - Devil's Claw to Suppress Appetite—Ghrelin Receptor Modulation Potential of a Harpagophytum procumbens Root Extract
Cristina Torres-Fuentes, Wessel F. Theeuwes, Michael K. McMullen, Anna K. McMullen, Timothy G. Dinan, John F. Cryan, Harriët Schellekens, Zane Andrews
PLoS ONE.2014; 9(7): e103118. CrossRef - Peripheral targets in obesity treatment: a comprehensive update
A. Chatzigeorgiou, E. Kandaraki, A. G. Papavassiliou, M. Koutsilieris
Obesity Reviews.2014; 15(6): 487. CrossRef - Carboxylesterase-2 is a highly sensitive target of the antiobesity agent orlistat with profound implications in the activation of anticancer prodrugs
Da Xiao, Deshi Shi, Dongfang Yang, Benjamin Barthel, Tad H. Koch, Bingfang Yan
Biochemical Pharmacology.2013; 85(3): 439. CrossRef - The unrelenting fall of the pharmacological treatment of obesity
Guido Di Dalmazi, Valentina Vicennati, Renato Pasquali, Uberto Pagotto
Endocrine.2013; 44(3): 598. CrossRef - Illicit online marketing of lorcaserin before DEA scheduling
Bryan A. Liang, Tim K. Mackey, Ashley N. Archer‐Hayes, Linda M. Shinn
Obesity.2013; 21(5): 861. CrossRef - Macrocytic Anemia and Thrombocytopenia Induced by Orlistat
David Palacios-Martínez, Juan Carlos García-Álvarez, Nieves Montero-Santamaría, Olga Patricia Villar-Ruiz, Antonio Ruiz-García, Raquel Asunción Díaz-Alonso
International Journal of Endocrinology and Metabolism.2013;[Epub] CrossRef - Anti- and Pro-Lipase Activity of Selected Medicinal, Herbal and Aquatic Plants, and Structure Elucidation of an Anti-Lipase Compound
Muhammad Ado, Faridah Abas, Abdulkarim Mohammed, Hasanah Ghazali
Molecules.2013; 18(12): 14651. CrossRef - Postprandial metabolism of docosapentaenoic acid (DPA, 22:5n−3) and eicosapentaenoic acid (EPA, 20:5n−3) in humans
Kaisa M. Linderborg, Gunveen Kaur, Eliza Miller, Peter J. Meikle, Amy E. Larsen, Jacquelyn M. Weir, Anu Nuora, Christopher K. Barlow, Heikki P. Kallio, David Cameron-Smith, Andrew J. Sinclair
Prostaglandins, Leukotrienes and Essential Fatty Acids.2013; 88(4): 313. CrossRef - Functional food ingredients for the management of obesity and associated co-morbidities – A review
Ritesh K. Baboota, Mahendra Bishnoi, Padma Ambalam, Kanthi Kiran Kondepudi, Siddhartha M. Sarma, Ravneet K. Boparai, Koteswaraiah Podili
Journal of Functional Foods.2013; 5(3): 997. CrossRef - tert-Butylhydroquinone reduces lipid accumulation in C57BL/6 mice with lower body weight gain
Kung-Woo Nam, Yong Hyun Kim, Hyun Jung Kwon, Sang-Ki Rhee, Wan-Jong Kim, Man-Deuk Han
Archives of Pharmacal Research.2013; 36(7): 897. CrossRef - Sympathomimetic Activity of aHoodia gordoniiProduct: A Possible Mechanism of Cardiovascular Side Effects
Orsolya Roza, Norbert Lovász, István Zupkó, Judit Hohmann, Dezső Csupor
BioMed Research International.2013; 2013: 1. CrossRef - Gender and age differences in obesity among Korean adults
Jun Goo Kang, Cheol-Young Park
The Korean Journal of Internal Medicine.2013; 28(1): 19. CrossRef - Treating the obese diabetic
Julia Kenkre, Tricia Tan, Stephen Bloom
Expert Review of Clinical Pharmacology.2013; 6(2): 171. CrossRef - The toxicological assessment of two anti-obesity drugs in C. elegans
Layla Aitlhadj, Stephen R. Stürzenbaum
Toxicology Research.2013; 2(2): 145. CrossRef - Milk protein hydrolysates activate 5-HT2C serotonin receptors: influence of the starting substrate and isolation of bioactive fractions
Alice B. Nongonierma, Harriët Schellekens, Timothy G. Dinan, John F. Cryan, Richard J. FitzGerald
Food & Function.2013; 4(5): 728. CrossRef - A modeling approach for compounds affecting body composition
Peter Gennemark, Rasmus Jansson-Löfmark, Gina Hyberg, Maria Wigstrand, Dorota Kakol-Palm, Pernilla Håkansson, Daniel Hovdal, Peter Brodin, Maria Fritsch-Fredin, Madeleine Antonsson, Karolina Ploj, Johan Gabrielsson
Journal of Pharmacokinetics and Pharmacodynamics.2013; 40(6): 651. CrossRef - Efficacy and Tolerability of an Herbal Formulation for Weight Management
Judith S. Stern, Jan Peerson, Artatrana T. Mishra, Venkata Sadasiva Rao Mathukumalli, Poorna Rajeswari Konda
Journal of Medicinal Food.2013; 16(6): 529. CrossRef - A Case of Phendimetrazine Induced-Psychotic Disorder and Dependence
Ji-Ae Yun, Wu-Ri Park, Je-Chun Yu, Kyeong-Sook Choi
Journal of Korean Neuropsychiatric Association.2013; 52(5): 402. CrossRef - Honeysuckle anthocyanin supplementation prevents diet-induced obesity in C57BL/6 mice
Tao Wu, Zhuoping Yu, Qiong Tang, Haizhao Song, Zichun Gao, Wei Chen, Xiaodong Zheng
Food & Function.2013; 4(11): 1654. CrossRef - Novel Acylethanolamide Derivatives That Modulate Body Weight through Enhancement of Hypothalamic Pro-Opiomelanocortin (POMC) and/or Decreased Neuropeptide Y (NPY)
Yosefa Avraham, Jehoshua Katzhendler, Lia Vorobeiv, Shira Merchavia, Chana Listman, Eithan Kunkes, Fida’ Harfoush, Sawsan Salameh, Aviva F. Ezra, Nikolaos C. Grigoriadis, Elliot M. Berry, Yousef Najajreh
Journal of Medicinal Chemistry.2013; 56(5): 1811. CrossRef - Synthesis, characterization, and biological assessment of the four stereoisomers of the H3 receptor antagonist 5-fluoro-2-methyl-N-[2-methyl-4-(2-methyl[1,3′]bipyrrolidinyl-1′-yl)phenyl]benzamide
Zhongli Gao, William J. Hurst, Etienne Guillot, Raisa Nagorny, Marie-Pierre Pruniaux, James A. Hendrix, Pascal G. George
Bioorganic & Medicinal Chemistry Letters.2013; 23(14): 4044. CrossRef - Efficacy and Safety of Taeeumjowi-tang in Obese Korean Adults: A Double-Blind, Randomized, and Placebo-Controlled Pilot Trial
Sunju Park, Won Nahmkoong, ChunHoo Cheon, Jeong-Su Park, Bo-Hyoung Jang, Yongcheol Shin, Kyung-Soo Kim, Hoyeon Go, Yun-Kyung Song, Seong-Gyu Ko
Evidence-Based Complementary and Alternative Medicine.2013; 2013: 1. CrossRef - New and Unconventional Treatments for Obstructive Sleep Apnea
Jose Angelo A. De Dios, Steven D. Brass
Neurotherapeutics.2012; 9(4): 702. CrossRef - Assessing Psychiatric Adverse Effects during Clinical Drug Development
Matthew V. Rudorfer, Mi Hillefors
Pharmaceutical Medicine.2012; 26(6): 363. CrossRef - Anti-obesity Effect of HT048, a Herbal Combination, in High Fat Diet-Induced Obese Rats
Dong Lim, MiKyung Song, Juyeon Park, Sang Park, Nak Kim, Bhakta Gaire, Ho-Young Choi, Hocheol Kim
Molecules.2012; 17(12): 14765. CrossRef - One-Year Effectiveness of a 3-Week Balneotherapy Program for the Treatment of Overweight or Obesity
Thierry Hanh, Patrick Serog, Jérôme Fauconnier, Pierre Batailler, Florence Mercier, Christian F. Roques, Patrick Blin
Evidence-Based Complementary and Alternative Medicine.2012; 2012: 1. CrossRef - Was it worth the weight? - Drug review on two new weight loss agents: lorcaserin (Belviq®) and phentermine/topiramate ER (QsymiaTM)
Aaron Crawford, Tiffany-Jade Kreys
Mental Health Clinician.2012; 2(6): 144. CrossRef - Diabetes: Have We Got It All Wrong?
Barbara E. Corkey
Diabetes Care.2012; 35(12): 2432. CrossRef - Inhibitors of fatty acid synthesis in prokaryotes and eukaryotes as anti-infective, anticancer and anti-obesity drugs
Jingxin Wang, Ryan Hudson, Herman O Sintim
Future Medicinal Chemistry.2012; 4(9): 1113. CrossRef - Outcomes of Laparoscopic Roux-en-Y Gastric Bypass Versus Laparoscopic Adjustable Gastric Banding in Adolescents
David Y. Lee, Hamza Guend, Koji Park, Jun Levine, Ronald E. Ross, James J. McGinty, Julio A. Teixeira
Obesity Surgery.2012; 22(12): 1859. CrossRef

 KDA
KDA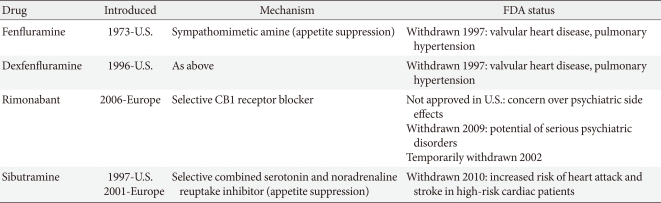
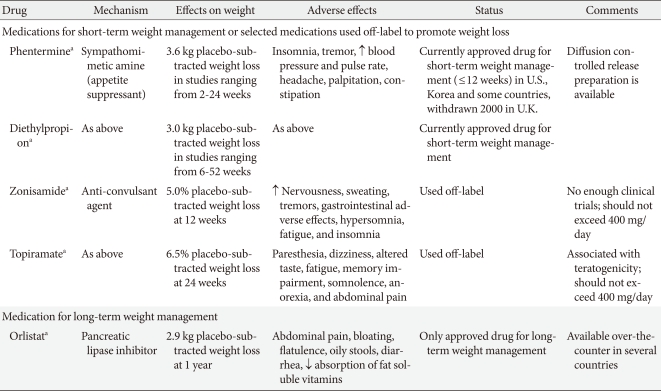
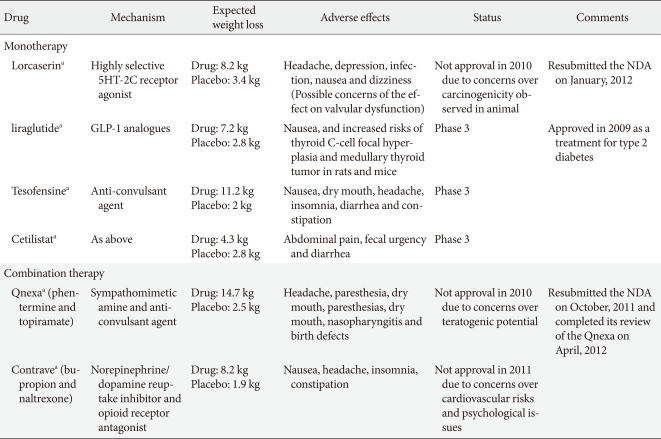
 PubReader
PubReader Cite
Cite



