- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Articles
- Page Path
- HOME > Diabetes Metab J > Volume 47(6); 2023 > Article
-
Original ArticleBasic Research Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition
-
Ho Gyun Lee1
 , Il Hyeon Jung1, Byong Seo Park1, Hye Rim Yang1, Kwang Kon Kim2, Thai Hien Tu1, Jung-Yong Yeh1, Sewon Lee3,4, Sunggu Yang4,5, Byung Ju Lee2, Jae Geun Kim1,4
, Il Hyeon Jung1, Byong Seo Park1, Hye Rim Yang1, Kwang Kon Kim2, Thai Hien Tu1, Jung-Yong Yeh1, Sewon Lee3,4, Sunggu Yang4,5, Byung Ju Lee2, Jae Geun Kim1,4 , Il Seong Nam-Goong6
, Il Seong Nam-Goong6
-
Diabetes & Metabolism Journal 2023;47(6):784-795.
DOI: https://doi.org/10.4093/dmj.2022.0261
Published online: August 23, 2023
1Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, Incheon, Korea
2Department of Biological Science, University of Ulsan, Ulsan, Korea
3Division of Sport Science, College of Arts & Physical Education, Incheon National University, Incheon, Korea
4Research Center of Brain-Machine Interface, Incheon National University, Incheon, Korea
5Department of Nano-Bioengineering, College of Life Science and Technology, Incheon National University, Incheon, Korea
6Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
-
Corresponding authors: Jae Geun Kim
 Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, 119 Academy-ro, Yeonsu-gu, Incheon 22012, Korea E-mail: jgkim@inu.ac.kr
Division of Life Sciences, College of Life Sciences and Bioengineering, Incheon National University, 119 Academy-ro, Yeonsu-gu, Incheon 22012, Korea E-mail: jgkim@inu.ac.kr -
Il Seong Nam-Goong
 Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, 877 Bangeojinsunhwan-doro, Dong-gu, Ulsan 44033, Korea E-mail: paul@uuh.ulsan.kr
Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, 877 Bangeojinsunhwan-doro, Dong-gu, Ulsan 44033, Korea E-mail: paul@uuh.ulsan.kr
Copyright © 2023 Korean Diabetes Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
-
Background
- Sodium-glucose cotransporter 2 (SGLT-2) inhibitors are currently used to treat patients with diabetes. Previous studies have demonstrated that treatment with SGLT-2 inhibitors is accompanied by altered metabolic phenotypes. However, it has not been investigated whether the hypothalamic circuit participates in the development of the compensatory metabolic phenotypes triggered by the treatment with SGLT-2 inhibitors.
-
Methods
- Mice were fed a standard diet or high-fat diet and treated with dapagliflozin, an SGLT-2 inhibitor. Food intake and energy expenditure were observed using indirect calorimetry system. The activity of hypothalamic neurons in response to dapagliflozin treatment was evaluated by immunohistochemistry with c-Fos antibody. Quantitative real-time polymerase chain reaction was performed to determine gene expression patterns in the hypothalamus of dapagliflozin-treated mice.
-
Results
- Dapagliflozin-treated mice displayed enhanced food intake and reduced energy expenditure. Altered neuronal activities were observed in multiple hypothalamic nuclei in association with appetite regulation. Additionally, we found elevated immunosignals of agouti-related peptide neurons in the paraventricular nucleus of the hypothalamus.
-
Conclusion
- This study suggests the functional involvement of the hypothalamus in the development of the compensatory metabolic phenotypes induced by SGLT-2 inhibitor treatment.
- As glucose is completely reabsorbed from proximal renal tubules, urinary glucose excretion does not occur under euglycemic conditions [1]. Sodium-glucose cotransporters (SGLTs) mainly participate in glucose reabsorption in the apical membrane of proximal tubular epithelial cells. In particular, SGLT-2 is responsible for 90% of renal glucose reabsorption under normal glucose homeostasis conditions [2]. In this context, patients retaining mutation of the SGLT-2 gene and a genetic mouse line with SGLT-2 deletion revealed elevated glycosuria [3,4]. Thus, the modulation of SGLT-2 functions can be considered as an effective strategy for improving hyperglycemia [5]. SGLT-2 inhibitors are currently used as glucose-lowering drugs in the treatment of type 2 diabetes [6]. As the treatment with SGLT-2 inhibitors is accompanied by multiple physiological alterations linked with whole-body energy homeostasis, much attention has been paid to identify the detailed mechanisms underlying the pathophysiological effects of SGLT-2 inhibitors beyond their therapeutic effects on impaired glucose metabolism [7]. Several lines of evidence have shown that long-term treatment with SGLT-2 inhibitors leads to a hyperphagic response to compensate for the limited glucose availability. This might explain why patients with diabetes treated with SGLT-2 inhibitors do not display effective weight loss [8]. The hypothalamus is the central unit that controls whole-body energy metabolism by integration of the neuronal and endocrine systems [9,10]. Particularly, energy intake and expenditure are tightly coupled with the activities of hypothalamic neurocircuitry and metabolic coupling between neurons and glial cells in the hypothalamic nuclei [11,12]. However, it is not yet clear whether hyperphagia triggered by SGLT-2 inhibitors is associated with altered activity of hypothalamic neurocircuitry. In this study, we confirmed the metabolic phenotypes of mice treated with a SGLT-2 inhibitor using indirect calorimetry and feeding monitor systems. Furthermore, we investigated the patterns of neuronal activity in multiple hypothalamic nuclei involved in the regulation of appetite and energy expenditure by assessing the number of c-Fos-positive cells. Finally, we evaluated the projection of agouti-related peptide (Agrp) neurons into the paraventricular nucleus (Pvn) of the hypothalamus. Overall, the current study determined the involvement of the hypothalamus in compensatory behavioral outcomes revealed during long-term treatment with SGLT-2 inhibitors.
INTRODUCTION
- Animals
- Six-week-old C57B/L6 male mice with an initial body weight of 20±2 g (Dae Han Bio Link, Eumseong, Korea) were maintained under specific pathogen-free conditions at 22°C and given access to food and water ad libitum. All animals were maintained in humidity-controlled room with a 12 hour-12 hour light-dark cycle, with lights on from 7:00 AM to 7:00 PM. The timetable of animal experiment was described in Fig. 1.
- All animal care and procedures used were in accordance with the guidelines and approval of the Institutional Animal Care and Use Committee (IACUC) at the University of Ulsan (Permission number: ISN-20-010).
- Measurement of O2 consumption, CO2 production, and energy expenditure
- Four weeks after vehicle or dapagliflozin treatment in mice during standard diet (SD) or high-fat diet (HFD) feeding conditions, metabolic parameters, including oxygen consumption (VO2), carbon dioxide emission (VCO2), and energy expenditure were measured using an indirect calorimetry system (Promethion, Sable Systems, Las Vegas, NV, USA). Mice were acclimated to the metabolic cages for 48 hours prior to data collection under 12 hours light/dark cycle condition. During the measurement, saline or dapagliflozin were injected into the mice by oral gavage. The respiratory exchange ratio (RER) was calculated as the ratio of VCO2 to VO2. Data acquisition and instrument control were coordinated by the MetaScreen software version 2.3.12 (Sable Systems), and the obtained raw data were processed using Expe-Data version 1.9.14 (Sable Systems).
- Immunohistochemistry
- Mice were anesthetized and perfused transcardially with 0.9% saline (w/v), followed by fixation with 4% paraformaldehyde in phosphate buffer (PB, 0.1 M, pH 7.4). Fixative brains were harvested and post-fixed overnight with 4% paraformaldehyde in PB. The coronal section (thickness, 50 µm) was prepared using a vibratome (5,100 mz Campden Instruments, Leicestershire, UK). After several washes with PB buffer, the sections were preincubated with 0.3% Triton X-100 (Sigma-Aldrich) for 30 minutes at room temperature (RT). The sections were then incubated with primary antibodies against rabbit c-Fos (1:1,000, Santa Cruz Biotechnology, Dallas, TX, USA), and Rabbit Agrp (1:1,000, Phoenix pharmaceuticals Inc., Burlingame, CA, USA), overnight at 4°C. The next day, sections were washed in PB for 30 minutes and incubated with the following secondary antibodies at RT: goat anti-rabbit Alexa Fluor 488 (1:1,000, Invitrogen, Carlsbad, CA, USA), goat anti-rabbit Alexa Fluor 594 (1:1,000, Invitrogen). The sections were then mounted onto glass slides and covered by coverslips with a drop of mounting medium (Dako North America Inc., Carpinteria, CA, USA).
- Image capture and analyses
- Stained brain slides (50 µm) were captured at 10 µm intervals using a fluorescence microscope (Axioplan2 Imaging, Carl Zeiss Microimaging Inc., Thornwood, NY, USA). The captured fluorescence images were Z-stacked and merged into one. For all immunohistochemical analyses, the slice sections were anatomically matched with the mouse brain atlas (Pvn: between −0.82 and −1.06 mm from bregma, arcuate nucleus [Arc], ventromedial hypothalamus [Vmh], dorsomedialhypothalamus [Dmh], lateral hypothalamus [Lh]: between −1.46 and −1.94 mm from bregma). The number of c-Fos-positive cells was evaluated manually using ImageJ software version 1.47 (National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/; accessed on 20 March 2021) by an unbiased observer. Fiber intensity and particle number of Agrp-positive immunosignals in the Pvn were evaluated using the ImageJ software.
- Quantitative real-time reverse transcription-polymerase chain reaction
- Total RNA was extracted from the hypothalamus according to the Tri-Reagent protocol (Invitrogen). cDNA was synthesized from total RNA using a high-capacity cDNA reverse transcription kit (Intron Biotechnology, Seoul, Korea). Real-time polymerase chain reaction (PCR) amplification of the cDNA was detected using the SYBR Green Real-time PCR Master Mix (Toyobo Co. Ltd., Osaka, Japan) in a Light Cycler 480 (Roche Diagnostics Ltd., Rotkreuz, Switzerland). The results were analyzed by the Light Cycler 480 SW 1.5.1 and normalized to the levels of β-actin, a housekeeping gene. The primer sequences are as follows; Agrp, F-TGC AGA CCG AGC AGA AGA AG and R-ACT CGT GCA GCC TTA CAC AG; pro-opiomelanocortin (Pomc), F-TCC TAC TCC ATG GAG CAC TTC and R-CT CGT TCT CAG CAA CGT TG; suppressor of cytokine signaling 3 (Socs3), F-GCC TCA AGA CCT TCA GCT CC and R-TGT CGC GGA TAA GAA AGG TG; forkhead box protein O1 (Foxo1), F-TTT CTA AGT GGC CTG CGA GT and R-GGT GGA TAC ACC AGG GAA TG; neuropeptide Y (Npy), F-ATG CTA GGT AAC AAG CGA ATG G and R-TGT CGA AGA GCG GAG TAG TAT; β-actin, F-TGG AAT CCT GTG GCA TCC ATG AAA C and R-TAA AAC GCA GCT CAG TAA CAG TAA CAG TCC G.
- Statistical analysis
- The number of mice in each experimental group is indicated in the figure legends. Statistical analyses were performed using GraphPad Prism version 6.0 software (GraphPad Software, San Diego, CA, USA). Unpaired two-tail Student’s t-test was performed to analyze the significance between the two experimental groups. Comparisons of multiple groups were evaluated by one-way analysis of variance (ANOVA). P value ≤0.05 was considered statistically significant. The values are represented as the mean±standard error of the mean.
METHODS
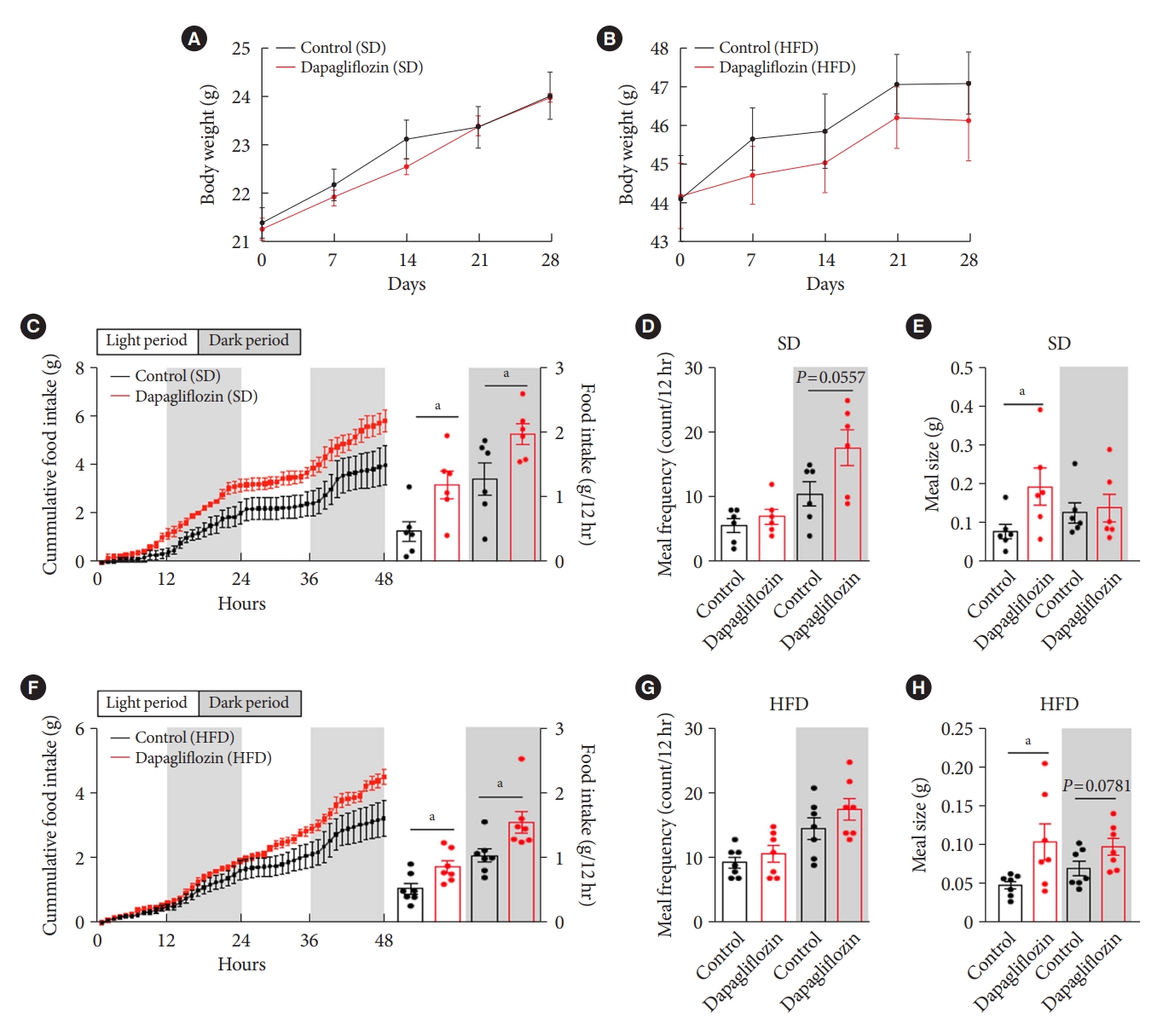
- Dapagliflozin treatment increased food intake without a significant change in body weight
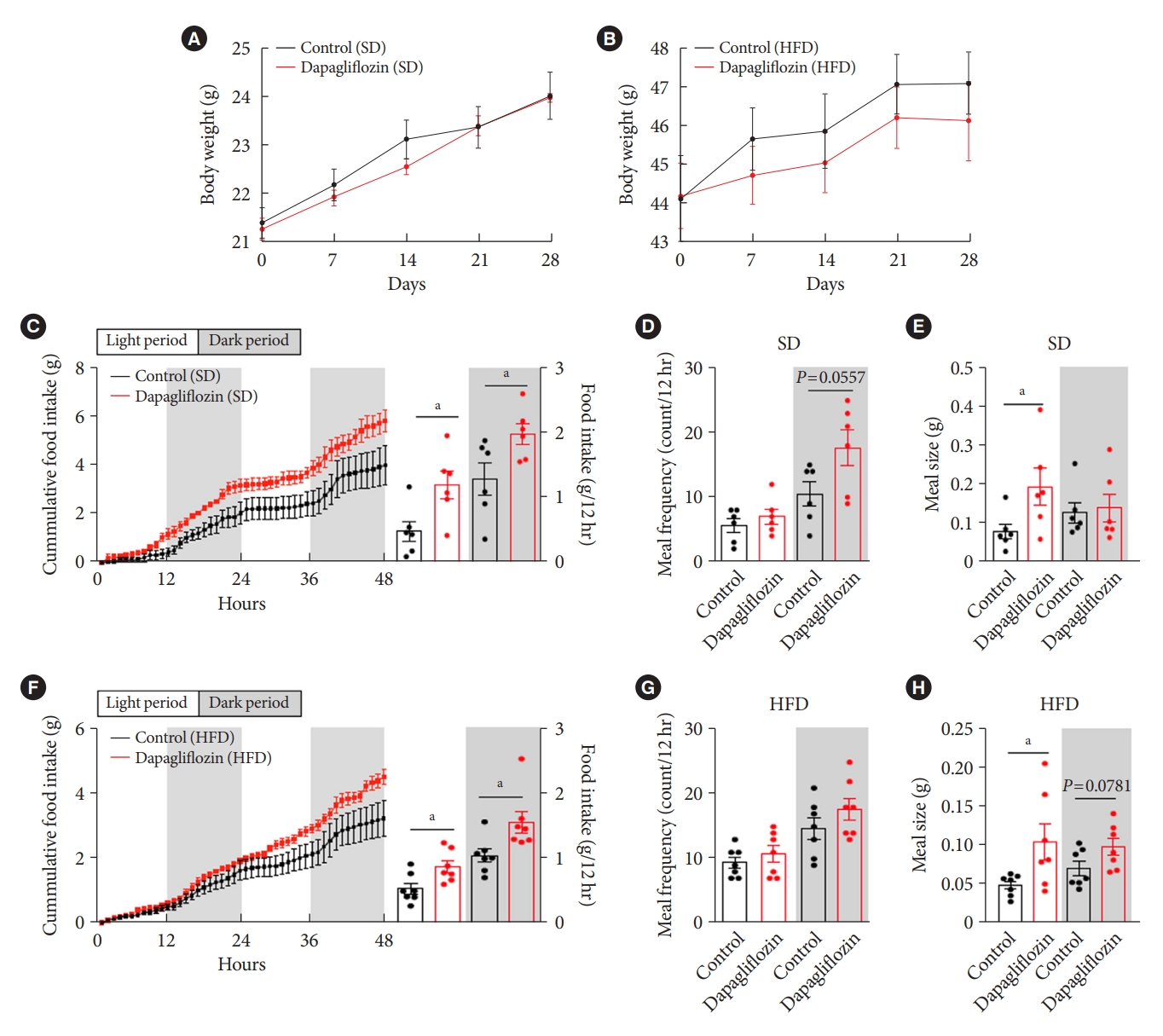
- To confirm the effects of the SGLT-2 inhibitor on metabolic phenotypes, mice were treated with dapagliflozin, and changes in body weight and food intake were investigated. Long-term exposure to dapagliflozin did not induce a significant change in body weight under SD or HFD feeding conditions (Fig. 2A and B). However, dapagliflozin-treated mice displayed an increase in food intake under SD or HFD feeding conditions (Fig. 2C and F). Additionally, dapagliflozin treatment led to a tendency of high meal frequency during the dark period and increased meal size during the light period under SD feeding conditions (Fig. 2D and E). In accordance with the feeding patterns observed in SD-treated mice, dapagliflozin-treated HFD-fed mice showed increased food intake and meal size compared with vehicle-treated control mice (Fig. 2F). Moreover, no alteration of meal frequency and a significant elevation of meal size were observed in dapagliflozin-treated mice under HFD feeding condition (Fig. 2G and H). These observations are consistent with previous findings showing increased appetite in human patients and rodent models treated with SGLT-2 inhibitors.
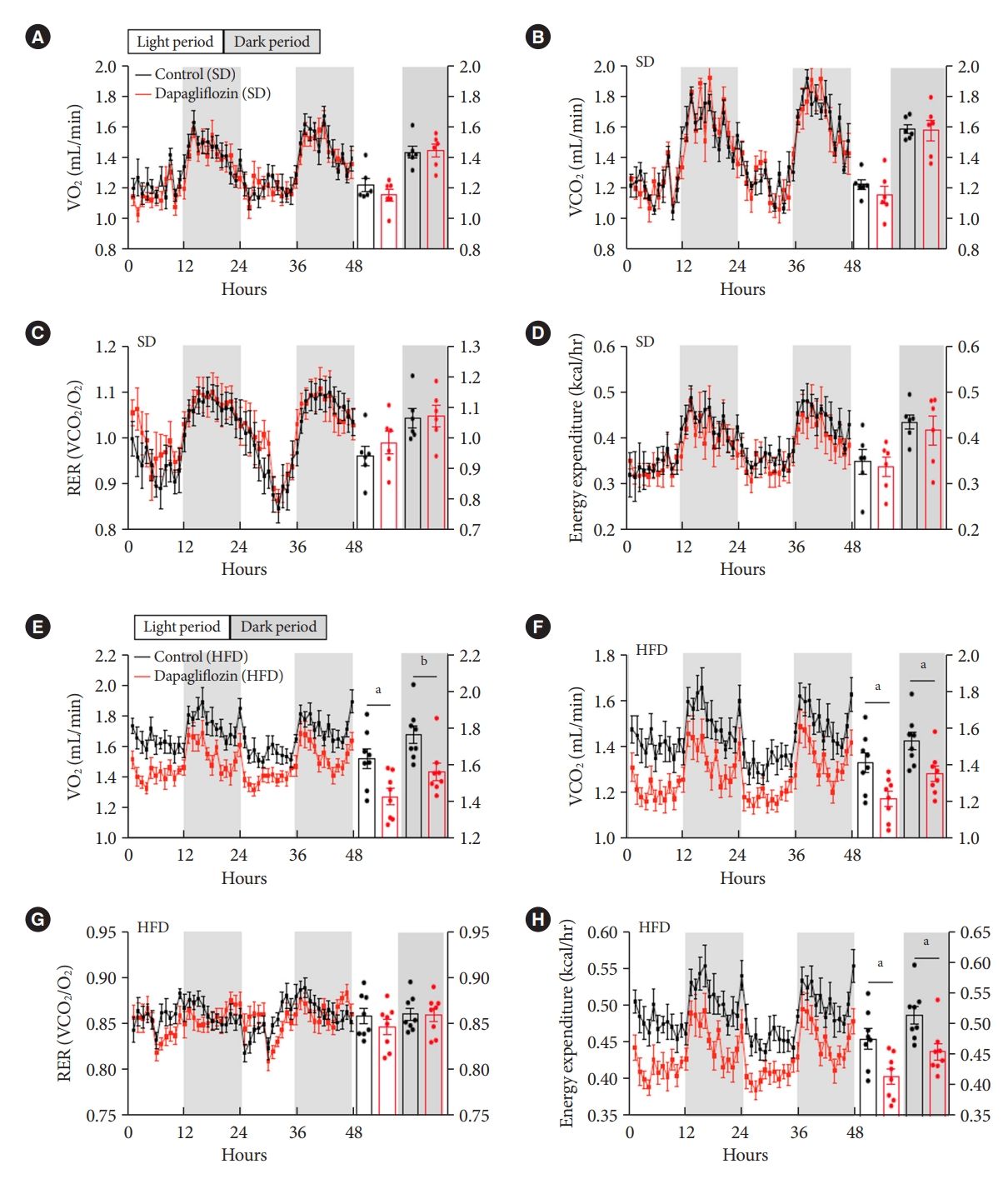
- Dapagliflozin treatment reduced energy expenditure under HFD feeding conditions
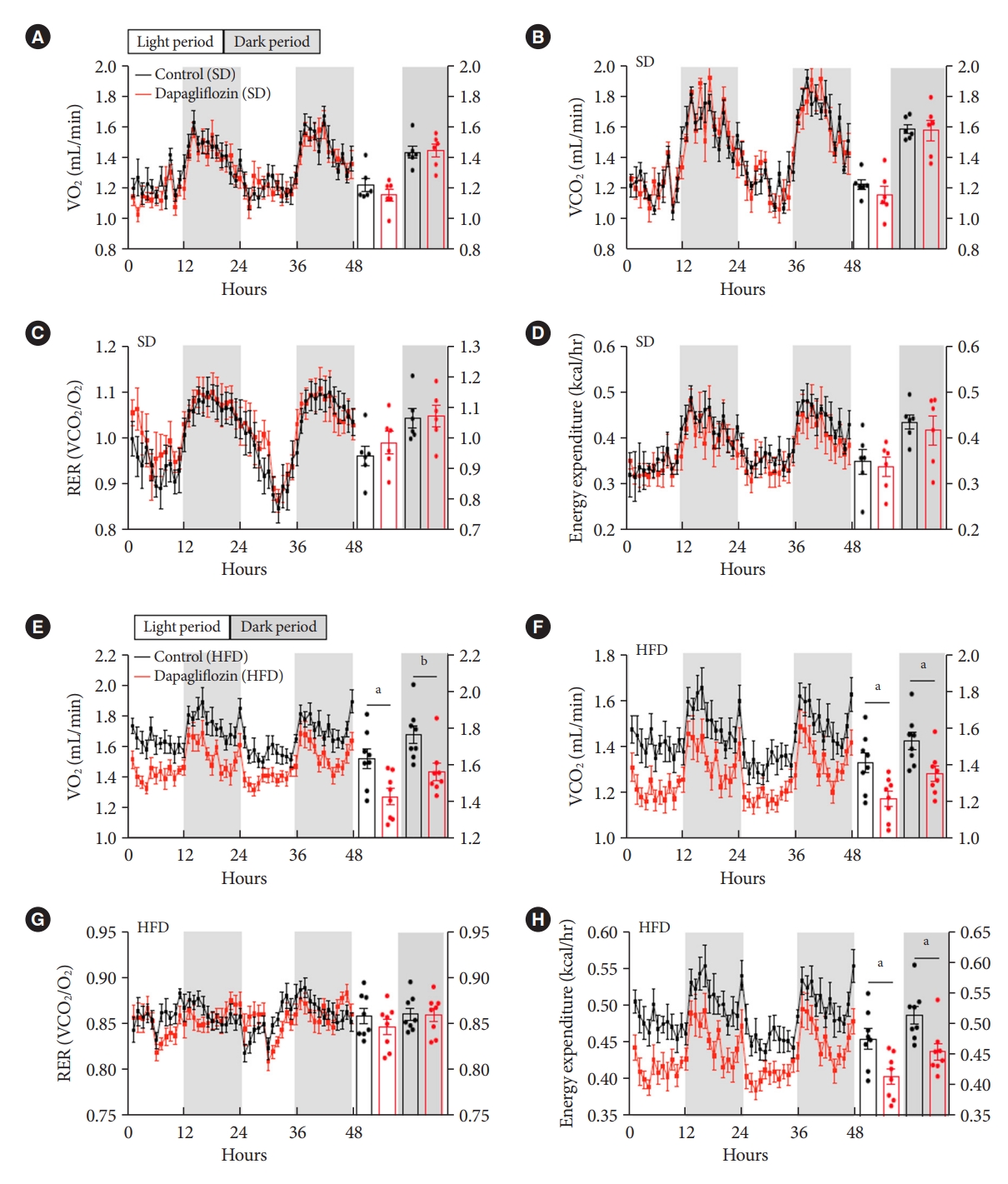
- To further investigate the impact of SGLT-2 inhibitors on energy expenditure, mice were subjected to measurement of VO2, VCO2, and RER after dapagliflozin treatment using an indirect calorimetry system. Under SD feeding conditions, dapagliflozin-treated mice showed no alteration in VO2, VCO2, RER, or energy expenditure compared with the control mice (Fig. 3A-D). However, dapagliflozin treatment led to a significant decrease in VO2, VCO2, and energy expenditure without a change in RER in HFD-fed mice (Fig. 3E-H). Collectively, these results indicate that the treatment with SGLT-2 inhibitor leads to reduced energy expenditure during the overnutrition.
- Altered neuronal activities in the hypothalamic nuclei of dapagliflozin-treated mice
- As the regulation of appetite and energy expenditure is mainly controlled by the hypothalamic neurocircuitry, we determined the activities of neurons in multiple hypothalamic nuclei involved in the energy homeostasis of the dapagliflozin-treated mice by detecting immunosignals of c-Fos protein, a molecular marker for neuronal activity under both SD and HFD feeding conditions. Consistent with the results, including feeding behaviors and energy expenditure, dapagliflozin treatment significantly increased the number of c-Fos-positive cells in the Arc and Lh of SD-fed mice (Fig. 4A and B). In contrast, dapagliflozin treatment decreased the number of c-Fos-positive cells in Vmh and Pvn of SD-fed mice (Fig. 4A and B). Under HFD feeding conditions, dapagliflozin treatment resulted in an increase in c-Fos immunosignals in the Arc and a decrease in c-Fos immunosignals in the Lh (Fig. 4C and D). This suggests that altered activities of hypothalamic neurons are coupled with metabolic phenotypes, including elevated food intake and decreased energy expenditure, as observed in dapagliflozin-treated mice.
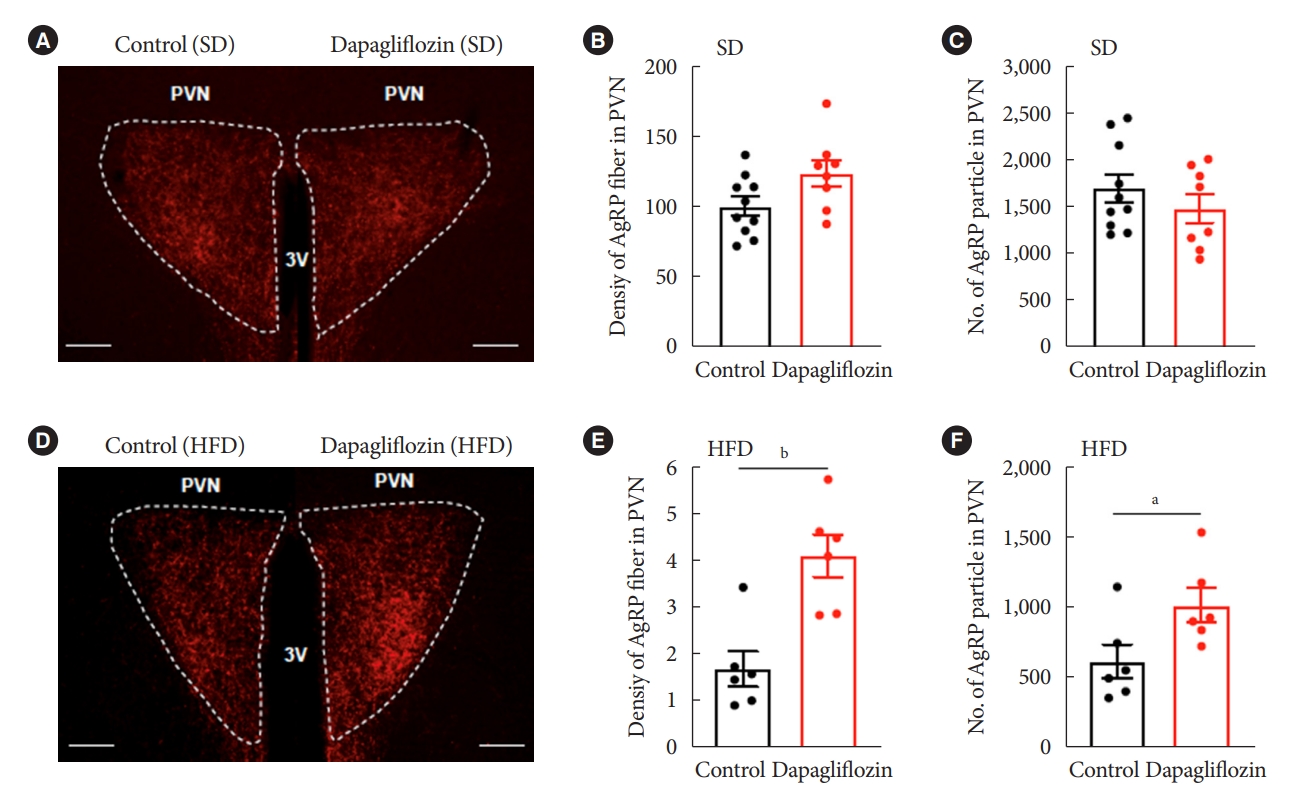
- Dapagliflozin treatment enhanced immunosignals of Agrp in the Pvn of HFD-fed mice
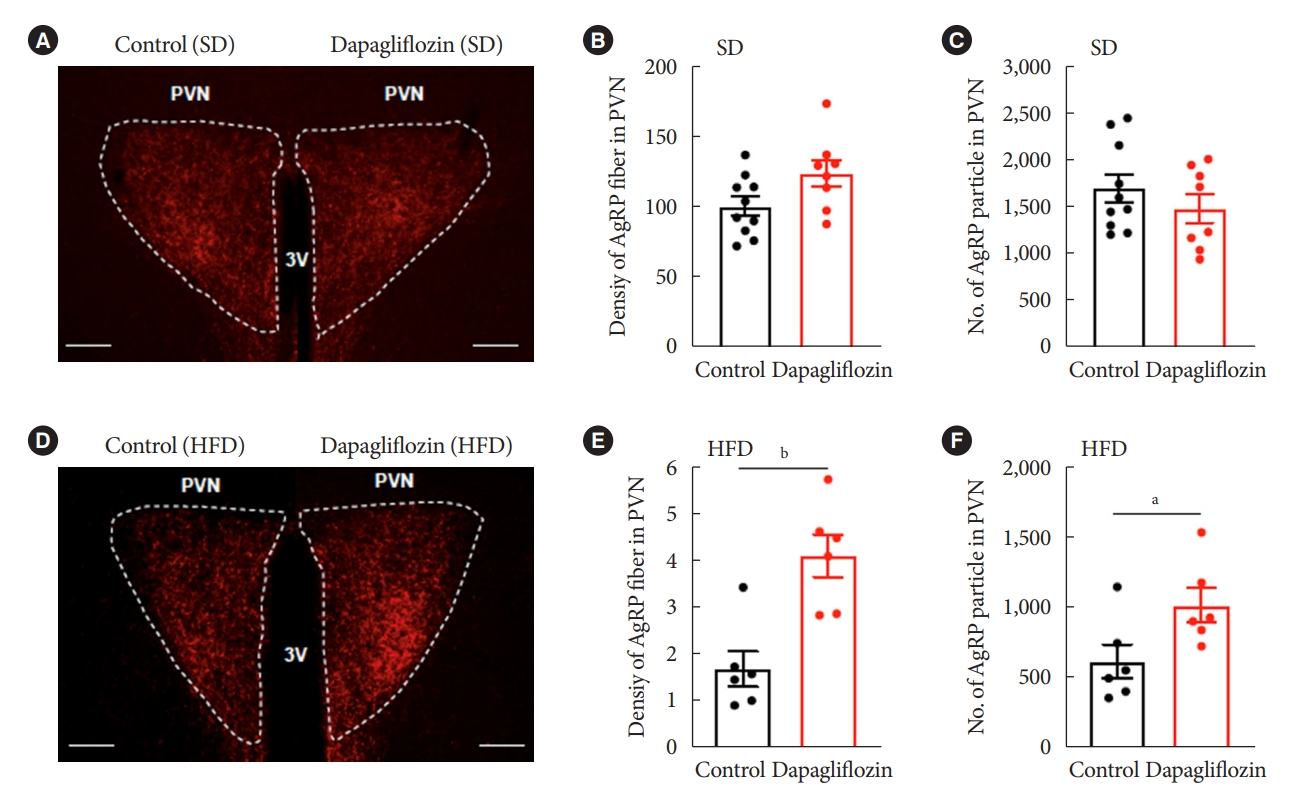
- Hunger-promoting Agrp neurons in the Arc control energy intake and expenditure by releasing Agrp neuropeptide and gamma-aminobutyric acid to the Pvn [13]. Based on this and our results demonstrating altered c-Fos-positive cells in the hypothalamic Arc and Pvn, we evaluated the Agrp-positive immunosignals in the Pvn, one of the projection sites that mediate the effect of Agrp neurons on feeding. Under SD feeding conditions, dapagliflozin treatment did not alter fiber density or particle numbers of Agrp-positive immunosignals in Pvn (Fig. 5A-C). However, HFD-fed mice showed a significant increase in fiber density and particle numbers of Agrp-positive immunosignals in Pvn in response to dapagliflozin treatment (Fig. 5D-F). These observations suggest that the hyperphagic response observed in dapagliflozin-treated mice was associated with the functions of hunger-promoting Agrp neurons in the hypothalamic Pvn.
- Hypothalamic mRNA expression patterns in response to dapagliflozin treatment
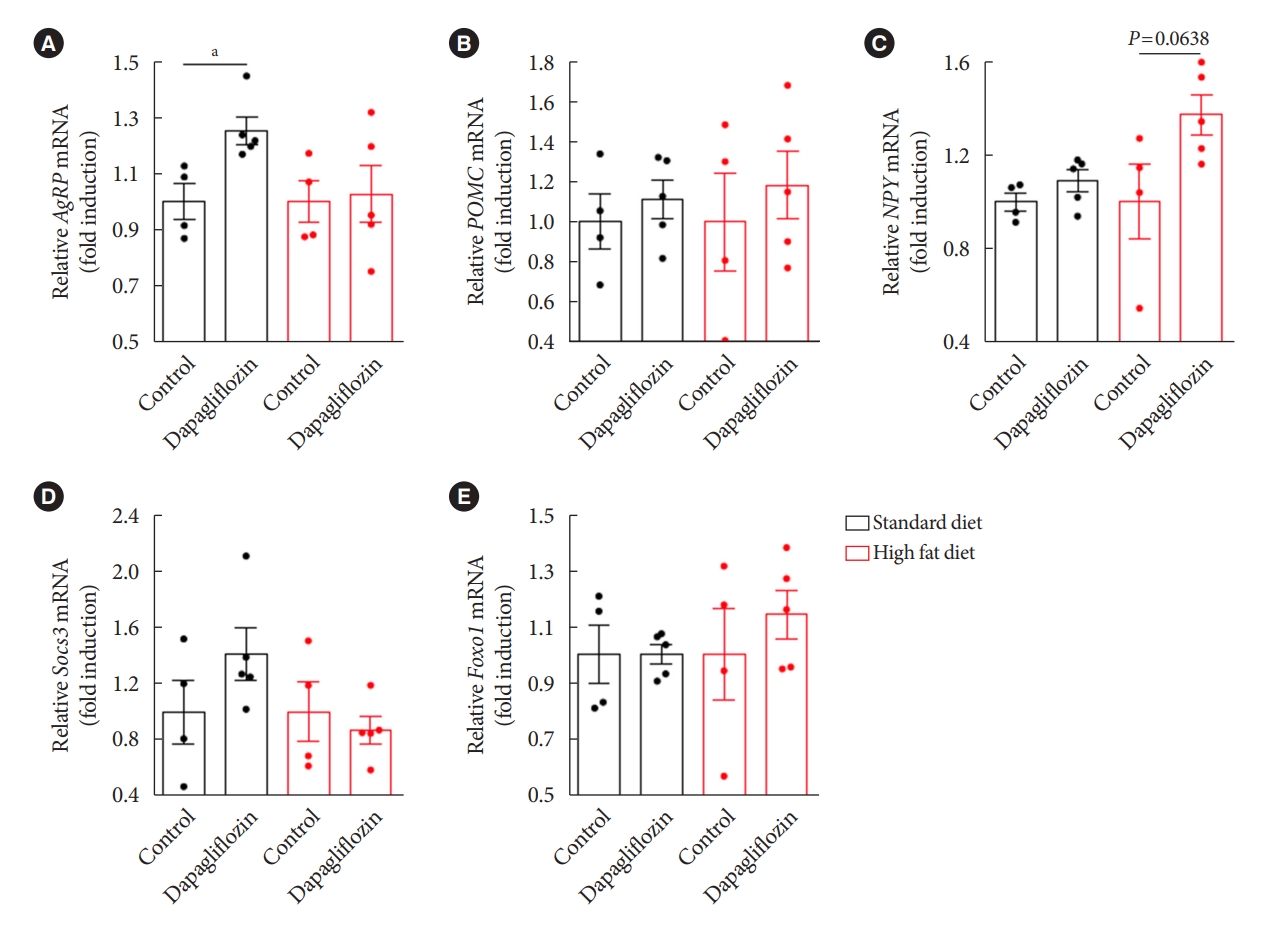
- To obtain molecular evidence supporting the effect of dapagliflozin on hypothalamic control of energy metabolism, we further investigated expression patterns of hypothalamic genes in association with appetite regulation by performing qPCR after treatment with dapagliflozin. Consistent with the findings showing increased food intake and elevated Agrp projection to the Pvn in response to dapagliflozin treatment, we observed that dapagliflozin-treated mice showed a significant increase in the level of Agrp mRNA in the hypothalamus under SD feeding conditions (Fig. 6A). However, no altered mRNA levels of Pomc, an anorexigenic peptide, or Npy, an orexigenic peptide, were observed in the hypothalamus of dapagliflozin-treated mice under SD feeding conditions (Fig. 6B and C). Additionally, dapagliflozin treatment did not affect the mRNA expression of genes involved in the signaling of appetite-regulating hormones, such as Foxo1 or Socs3 in the hypothalamus of SD-fed mice (Fig. 6D and E). No significant difference in the expression of hypothalamic genes involved in appetite regulation was observed in dapagliflozin-treated mice compared to that in vehicle-treated control mice under HFD feeding conditions. These observations further support that hypothalamic Agrp neurons mediate the hyperphagic response revealed during long-term treatment with SGLT-2 inhibitors.
RESULTS
- In this study, we verified the effects of SGLT-2 inhibitors on energy metabolism beyond their beneficial effects on hyperglycemia, suggesting the potential therapeutic applications of SGLT-2 inhibitors in the pathogenesis of obesity. Much attention has been paid to identify the physiological and pathological phenomena that occur during treatment with SGLT-2 inhibitors in patients with diabetes and in rodent models [14,15]. Particularly, it has recently been reported that long-term treatment with SGLT-2 inhibitors leads to compensatory metabolic processes to maintain whole-body energy homeostasis [16]. In this study, we successfully confirmed the effects of SGLT-2 inhibitors on increased appetite and reduced energy expenditure in both, SD- and HFD-fed mice. In accordance with clinical data, dapagliflozin-treated mice did not show critical body weight loss due to the compensatory metabolic processes to prevent the negative energy balance induced by increased urinary glucose output [17]. In this context, the modulation of hyperphagia observed in patients with diabetes treated with SGLT-2 inhibitors has been regarded as an effective strategy to expand its application in obesity pathogenesis [18].
- The hypothalamic neurocircuitry dynamically participates in the regulation of energy intake and expenditure, and perturbed circuit activity in the hypothalamus is directly associated with the development of obesity [19]. Based on this evidence, together with the current finding showing increased food intake in response to SGLT-2 inhibitor treatment, we further investigated the responsiveness of the hypothalamic neurocircuitry regulating whole-body energy metabolism to clarify the underlying mechanisms of the hyperphagic response revealed in the SGLT-2 inhibitor-treated models. Consistent with the feeding behavior patterns, dapagliflozin-treated mice displayed enhanced neuronal activity in the hypothalamic Arc and Lh, which are home to appetite-regulatory neurons [20]. Under SD feeding conditions, treatment with dapagliflozin reduces the activity of neurons in the hypothalamic Pvn, a nucleus retaining preganglionic neurons controlling the sympathetic nerve activity [21]. These cellular data are in good agreement with the findings of elevated food intake and reduced energy expenditure in dapagliflozin-treated mice.
- The hypothalamic melanocortin system regulates energy homeostasis by integrating various afferent metabolic signals, including hormonal, neuronal, and nutritional inputs [22]. Agrp neurons coexist with Pomc neurons in the hypothalamic Arc and promote hunger signals by interrupting the melanocortin pathway [23,24]. In this study, we observed that dapagliflozin-treated mice displayed enhanced Agrp fiber signals in Pvn and elevated Agrp mRNA in the hypothalamus, suggesting that dapagliflozin-induced hyperphagia might be coupled with the enhanced function of Agrp neurons. Interestingly, the metabolic phenotypes and the reactivity of neurons in the hypothalamus were observed in different patterns between the SD- and the HFD-fed groups. It has been well documented that the insensitivity of appetite-regulating hypothalamic neurons in response to the metabolic shifts were observed in the HFD-induced obesity models [25]. For instance, HFD-induced obesity models display sensitivity reduction of the hypothalamic neurocircuitry to the circulating leptin, thereby disrupting the homeostatic behaviors, including increased food intake and reduced energy expenditure [25,26]. Therefore, we assumed that the results showing different patterns between SD- and HFD-fed groups might be due to different responsiveness of the hypothalamic neurocircuitry to the reduced glucose availability triggered by dapagliflozin.
- Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), antidiabetic medicines, are widely used to lower body weight due to their anorexigenic effect [27]. It has recently been highlighted that the clinical applications of SGLT-2 inhibitors in combination with GLP-1 RAs can lead to the concomitant synergic effect on the modulation of weight loss and hyperglycemia [28]. Thus, the current findings provide insights into the pharmacological and physiological basis of SGLT-2 inhibitor treatment for establishing effective strategies that can be used for the treatment of obesity in patients with diabetes. In consideration of our findings showing decreased energy expenditure in response to dapagliflozin treatment, we suggest that exercise interventions in combination with SGLT-2 inhibitor treatment can be a useful guideline for better patient care.
- In view of SGLT-2 inhibitors altering systemic glucose availability, it is a compelling hypothesis that the glucose-sensing cells in the brain participate in the development of compensatory metabolic phenotypes triggered by SGLT-2 inhibitors. It has recently been established that cells in circumventricular organs (CVOs) play a critical role in sensing the circulating level of glucose [29]; distinct glucose-sensing neurons and glial cells are present in multiple hypothalamic nuclei, including the Vmh, Dmh, Lh, Pvn, and Arc [30,31]. Therefore, further studies in the core glucose-sensing sites, such as CVOs and hypothalamic nuclei, are required for better understanding of the underlying mechanism of the compensatory metabolic phenotypes revealed during the treatment with SGLT-2 inhibitors.
- Collectively, this study confirms that treatment with SGLT-2 inhibitors leads to increased food intake and reduced energy expenditure accompanied by altered activities of the hypothalamic neurons, including Agrp neurons. This study addresses a gap in our understanding of the metabolic effects of SGLT-2 inhibitors beyond their antidiabetic properties and provides insight into the expanded application of SGLT-2 inhibitors for patients with diabetes accompanied by obesity risks.
DISCUSSION
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHOR CONTRIBUTIONS
Conception or design: J.G.K., I.S.N.G.
Acquisition, analysis, or interpretation of data: H.G.L., I.H.J., B.S.P., H.R.Y., K.K.K., T.H.T.
Drafting the work or revising: H.G.L., B.S.P., J.G.K., I.S.N.G.
Final approval of the manuscript: J.Y.Y., S.L., S.Y., B.J.L., J.G.K., I.S.N.G.
-
FUNDING
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A3072427) for Il Seong Nam-Goong, and supported by an Incheon National University (Foreign post-doctor program-2018) Research Grant for Thai Hien Tu.
NOTES
-
Acknowledgements
- None

- 1. Abdul-Ghani MA, Norton L, DeFronzo RA. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am J Physiol Renal Physiol 2015;309:F889-900.ArticlePubMedPMC
- 2. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733-94.ArticlePubMed
- 3. Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, et al. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol 2014;306:F188-93.ArticlePubMedPMC
- 4. Neuwirt H, Burtscher A, Cherney D, Mayer G, Ebenbichler C. Tubuloglomerular feedback in renal glucosuria: mimicking long-term SGLT-2 inhibitor therapy. Kidney Med 2019;2:76-9.ArticlePubMedPMC
- 5. Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia 2017;60:215-25.ArticlePubMedPDF
- 6. Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab 2016;18:783-94.ArticlePubMed
- 7. Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia 2018;61:2098-107.ArticlePubMedPDF
- 8. Komiya C, Tsuchiya K, Shiba K, Miyachi Y, Furuke S, Shimazu N, et al. Ipragliflozin improves hepatic steatosis in obese mice and liver dysfunction in type 2 diabetic patients irrespective of body weight reduction. PLoS One 2016;11:e0151511.ArticlePubMedPMC
- 9. Saper CB, Lowell BB. The hypothalamus. Curr Biol 2014;24:R1111-6.ArticlePubMed
- 10. Dietrich MO, Horvath TL. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci 2013;36:65-73.ArticlePubMed
- 11. Lopez-Gambero AJ, Martinez F, Salazar K, Cifuentes M, Nualart F. Brain glucose-sensing mechanism and energy homeostasis. Mol Neurobiol 2019;56:769-96.ArticlePubMedPDF
- 12. Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A 2010;107:14875-80.ArticlePubMedPMC
- 13. Cansell C, Denis RG, Joly-Amado A, Castel J, Luquet S. Arcuate AgRP neurons and the regulation of energy balance. Front Endocrinol (Lausanne) 2012;3:169.ArticlePubMedPMC
- 14. Kusakabe T, Yokota S, Shimizu M, Inoue T, Tanaka M, Ohue-Kitano R, et al. Differential effects of sodium-glucose cotransporter 2 inhibitor and low-carbohydrate diet on body composition and metabolic profile in obese diabetic db/db mice. BMJ Open Diabetes Res Care 2020;8:e001303.ArticlePubMedPMC
- 15. Inoue H, Morino K, Ugi S, Tanaka-Mizuno S, Fuse K, Miyazawa I, et al. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: a randomized clinical trial. J Diabetes Investig 2019;10:1012-21.ArticlePubMedPMCPDF
- 16. Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1730-5.ArticlePubMedPMCPDF
- 17. Chiba Y, Yamada T, Tsukita S, Takahashi K, Munakata Y, Shirai Y, et al. Dapagliflozin, a sodium-glucose co-transporter 2 inhibitor, acutely reduces energy expenditure in BAT via neural signals in mice. PLoS One 2016;11:e0150756.ArticlePubMedPMC
- 18. van Ruiten CC, Veltman DJ, Schrantee A, van Bloemendaal L, Barkhof F, Kramer MH, et al. Effects of dapagliflozin and combination therapy with exenatide on food-cue induced brain activation in patients with type 2 diabetes. J Clin Endocrinol Metab 2022;107:e2590-9.ArticlePubMedPMCPDF
- 19. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661-71.ArticlePubMedPDF
- 20. Nguyen T, Wen S, Gong M, Yuan X, Xu D, Wang C, et al. Dapagliflozin activates neurons in the central nervous system and regulates cardiovascular activity by inhibiting SGLT-2 in mice. Diabetes Metab Syndr Obes 2020;13:2781-99.PubMedPMC
- 21. Takeda K, Ono H, Ishikawa K, Ohno T, Kumagai J, Ochiai H, et al. Central administration of sodium-glucose cotransporter-2 inhibitors increases food intake involving adenosine monophosphate-activated protein kinase phosphorylation in the lateral hypothalamus in healthy rats. BMJ Open Diabetes Res Care 2021;9:e002104.ArticlePubMedPMC
- 22. Kim JD, Leyva S, Diano S. Hormonal regulation of the hypothalamic melanocortin system. Front Physiol 2014;5:480.ArticlePubMedPMC
- 23. Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis 2008;18:158-68.ArticlePubMed
- 24. Sobrino Crespo C, Perianes Cachero A, Puebla Jimenez L, Barrios V, Arilla Ferreiro E. Peptides and food intake. Front Endocrinol (Lausanne) 2014;5:58.PubMedPMC
- 25. Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 2007;5:181-94.ArticlePubMed
- 26. Park BS, Kang D, Kim KK, Jeong B, Lee TH, Park JW, et al. Hypothalamic TTF-1 orchestrates the sensitivity of leptin. Mol Metab 2022;66:101636.ArticlePubMedPMC
- 27. Lu K, Chen X, Yan J, Li X, Huang C, Wan Q, et al. The effect of feeding behavior on hypothalamus in obese type 2 diabetic rats with glucagon-like peptide-1 receptor agonist intervention. Obes Facts 2018;11:181-94.ArticlePubMedPMCPDF
- 28. Brown E, Wilding JP, Barber TM, Alam U, Cuthbertson DJ. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: mechanistic possibilities. Obes Rev 2019;20:816-28.ArticlePubMedPDF
- 29. Bentsen MA, Mirzadeh Z, Schwartz MW. Revisiting how the brain senses glucose-and why. Cell Metab 2019;29:11-7.ArticlePubMedPMC
- 30. Yoon NA, Diano S. Hypothalamic glucose-sensing mechanisms. Diabetologia 2021;64:985-93.ArticlePubMedPMCPDF
- 31. Routh VH, Hao L, Santiago AM, Sheng Z, Zhou C. Hypothalamic glucose sensing: making ends meet. Front Syst Neurosci 2014;8:236.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Jae Hyun Bae
Diabetes & Metabolism Journal.2024; 48(1): 157. CrossRef - Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
Ho Gyun Lee, Il Hyeon Jung, Byong Seo Park, Hye Rim Yang, Kwang Kon Kim, Thai Hien Tu, Jung-Yong Yeh, Sewon Lee, Sunggu Yang, Byung Ju Lee, Jae Geun Kim, Il Seong Nam-Goong
Diabetes & Metabolism Journal.2024; 48(1): 159. CrossRef
- Figure
- Related articles
-
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Altered Metabolic Phenotypes and Hypothalamic Neuronal Activity Triggered by Sodium-Glucose Cotransporter 2 Inhibition (Diabetes Metab J 2023;47:784-95)
- Renoprotective Mechanism of Sodium-Glucose Cotransporter 2 Inhibitors: Focusing on Renal Hemodynamics

 KDA
KDA PubReader
PubReader ePub Link
ePub Link Cite
Cite