
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
- Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
- Ji-Hae Park, Soyeon Kwon, Young Mi Park
- Diabetes Metab J. 2024;48(2):215-230. Published online September 26, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0332
- 2,308 View
- 193 Download
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub - Background
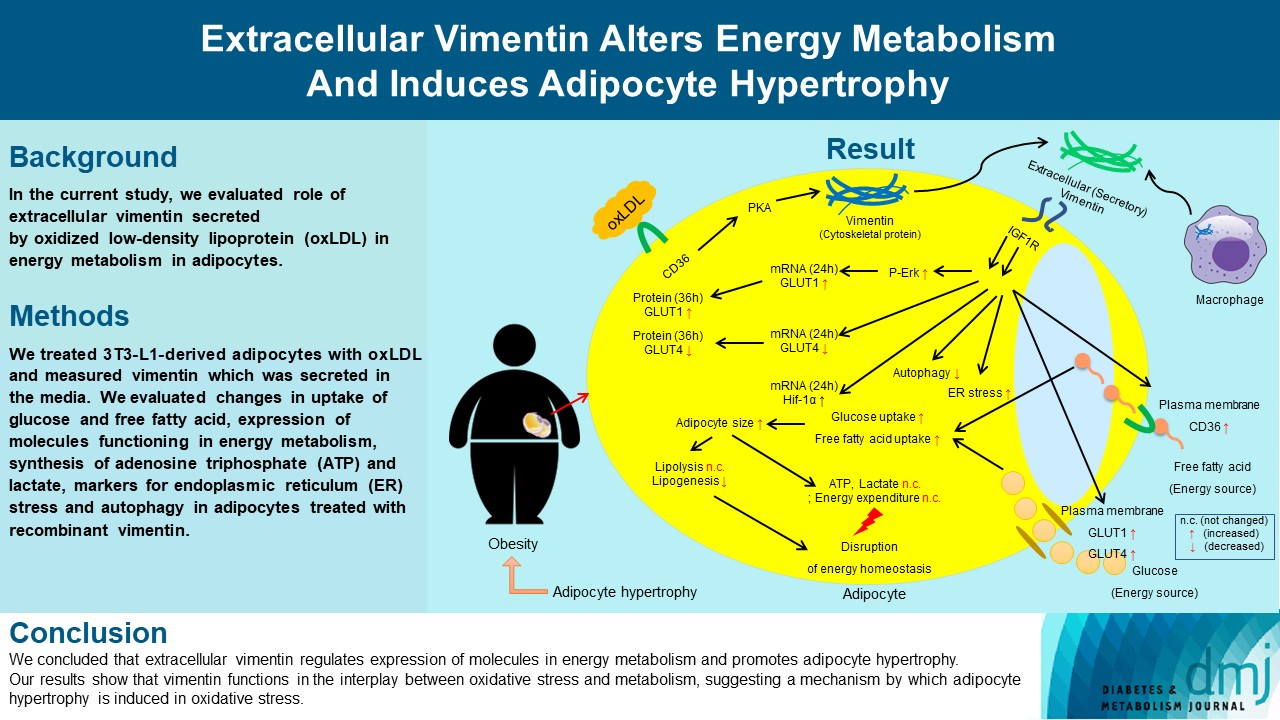
Previous studies have reported that oxidative stress contributes to obesity characterized by adipocyte hypertrophy. However, mechanism has not been studied extensively. In the current study, we evaluated role of extracellular vimentin secreted by oxidized low-density lipoprotein (oxLDL) in energy metabolism in adipocytes.
Methods
We treated 3T3-L1-derived adipocytes with oxLDL and measured vimentin which was secreted in the media. We evaluated changes in uptake of glucose and free fatty acid, expression of molecules functioning in energy metabolism, synthesis of adenosine triphosphate (ATP) and lactate, markers for endoplasmic reticulum (ER) stress and autophagy in adipocytes treated with recombinant vimentin.
Results
Adipocytes secreted vimentin in response to oxLDL. Microscopic evaluation revealed that vimentin treatment induced increase in adipocyte size and increase in sizes of intracellular lipid droplets with increased intracellular triglyceride. Adipocytes treated with vimentin showed increased uptake of glucose and free fatty acid with increased expression of plasma membrane glucose transporter type 1 (GLUT1), GLUT4, and CD36. Vimentin treatment increased transcription of GLUT1 and hypoxia-inducible factor 1α (Hif-1α) but decreased GLUT4 transcription. Adipose triglyceride lipase (ATGL), peroxisome proliferator-activated receptor γ (PPARγ), sterol regulatory element-binding protein 1 (SREBP1), diacylglycerol O-acyltransferase 1 (DGAT1) and 2 were decreased by vimentin treatment. Markers for ER stress were increased and autophagy was impaired in vimentin-treated adipocytes. No change was observed in synthesis of ATP and lactate in the adipocytes treated with vimentin.
Conclusion
We concluded that extracellular vimentin regulates expression of molecules in energy metabolism and promotes adipocyte hypertrophy. Our results show that vimentin functions in the interplay between oxidative stress and metabolism, suggesting a mechanism by which adipocyte hypertrophy is induced in oxidative stress.
- Basic Research
- Vimentin Deficiency Prevents High-Fat Diet-Induced Obesity and Insulin Resistance in Mice
- SeoYeon Kim, Inyeong Kim, Wonkyoung Cho, Goo Taeg Oh, Young Mi Park
- Diabetes Metab J. 2021;45(1):97-108. Published online June 15, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0198

- 7,482 View
- 232 Download
- 15 Web of Science
- 17 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
Background Obesity and type 2 diabetes mellitus are world-wide health problems, and lack of understanding of their linking mechanism is one reason for limited treatment options. We determined if genetic deletion of vimentin, a type 3 intermediate filament, affects obesity and type 2 diabetes mellitus.
Methods We fed vimentin-null (
Vim −/−) mice and wild-type mice a high-fat diet (HFD) for 10 weeks and measured weight change, adiposity, blood lipids, and glucose. We performed intraperitoneal glucose tolerance tests and measured CD36, a major fatty acid translocase, and glucose transporter type 4 (GLUT4) in adipocytes from both groups of mice.Results Vim −/− mice fed an HFD showed less weight gain, less adiposity, improved glucose tolerance, and lower serum level of fasting glucose. However, serum triglyceride and non-esterified fatty acid levels were higher inVim −/− mice than in wild-type mice. Vimentin-null adipocytes showed 41.1% less CD36 on plasma membranes, 27% less uptake of fatty acids, and 50.3% less GLUT4, suggesting defects in intracellular trafficking of these molecules.Conclusion We concluded that vimentin deficiency prevents obesity and insulin resistance in mice fed an HFD and suggest vimentin as a central mediator linking obesity and type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
Ji-Hae Park, Soyeon Kwon, Young Mi Park
Diabetes & Metabolism Journal.2024; 48(2): 215. CrossRef - Neutrophils display distinct post-translational modifications in response to varied pathological stimuli
Pooja Yedehalli Thimmappa, Aswathy S Nair, Sian D'silva, Anjana Aravind, Sandeep Mallya, Sreelakshmi Pathappillil Soman, Kanive Parashiva Guruprasad, Shamee Shastry, Rajesh Raju, Thottethodi Subrahmanya Keshava Prasad, Manjunath B Joshi
International Immunopharmacology.2024; 132: 111950. CrossRef - Extracellular Vesicles as Carriers of Adipokines and Their Role in Obesity
Tamara Camino, Nerea Lago-Baameiro, María Pardo
Biomedicines.2023; 11(2): 422. CrossRef - Bioinformatics and Next-Generation Data Analysis for Identification of Genes and Molecular Pathways Involved in Subjects with Diabetes and Obesity
Prashanth Ganekal, Basavaraj Vastrad, Satish Kavatagimath, Chanabasayya Vastrad, Shivakumar Kotrashetti
Medicina.2023; 59(2): 309. CrossRef - Modified Signaling of Membrane Formyl Peptide Receptors in NADPH-Oxidase Regulation in Obesity-Resistant Mice
Irina Tikhonova, Alsu Dyukina, Elvira Shaykhutdinova, Valentina Safronova
Membranes.2023; 13(3): 306. CrossRef - Plasma Cytokeratin-18 Fragment Level Reflects the Metabolic Phenotype in Obesity
Joanna Goralska, Urszula Razny, Anna Gruca, Anna Zdzienicka, Agnieszka Micek, Aldona Dembinska-Kiec, Bogdan Solnica, Malgorzata Malczewska-Malec
Biomolecules.2023; 13(4): 675. CrossRef - Blueberry and Blackberry Anthocyanins Ameliorate Metabolic Syndrome by Modulating Gut Microbiota and Short-Chain Fatty Acids Metabolism in High-Fat Diet-Fed C57BL/6J Mice
Lanlan Du, Han Lü, Yan Chen, Xiaohua Yu, Tunyu Jian, Huifang Zhao, Wenlong Wu, Xiaoqin Ding, Jian Chen, Weilin Li
Journal of Agricultural and Food Chemistry.2023; 71(40): 14649. CrossRef - An analogue of the Prolactin Releasing Peptide reduces obesity and promotes adult neurogenesis
Sara KM Jörgensen, Alena Karnošová, Simone Mazzaferro, Oliver Rowley, Hsiao-Jou Cortina Chen, Sarah J Robbins, Sarah Christofides, Florian T Merkle, Lenka Maletínská, David Petrik
EMBO Reports.2023; 25(1): 351. CrossRef - Cytoskeleton alterations in non-alcoholic fatty liver disease
João Pessoa, José Teixeira
Metabolism.2022; 128: 155115. CrossRef - Recent Advances in the Treatment of Insulin Resistance Targeting Molecular and Metabolic Pathways: Fighting a Losing Battle?
Marta Wolosowicz, Slawomir Prokopiuk, Tomasz W. Kaminski
Medicina.2022; 58(4): 472. CrossRef - Roles of vimentin in health and disease
Karen M. Ridge, John E. Eriksson, Milos Pekny, Robert D. Goldman
Genes & Development.2022; 36(7-8): 391. CrossRef - Plasma Membrane Localization of CD36 Requires Vimentin Phosphorylation; A Mechanism by Which Macrophage Vimentin Promotes Atherosclerosis
Seo Yeon Kim, Se-Jin Jeong, Ji-Hae Park, Wonkyoung Cho, Young-Ho Ahn, Youn-Hee Choi, Goo Taeg Oh, Roy L. Silverstein, Young Mi Park
Frontiers in Cardiovascular Medicine.2022;[Epub] CrossRef - Camel Proteins and Enzymes: A Growing Resource for Functional Evolution and Environmental Adaptation
Mahmoud Kandeel, Abdulla Al-Taher, Katharigatta N. Venugopala, Mohamed Marzok, Mohamed Morsy, Sreeharsha Nagaraja
Frontiers in Veterinary Science.2022;[Epub] CrossRef - Brown Adipose Tissue Sheds Extracellular Vesicles That Carry Potential Biomarkers of Metabolic and Thermogenesis Activity Which Are Affected by High Fat Diet Intervention
Tamara Camino, Nerea Lago-Baameiro, Aurelio Sueiro, Susana Belén Bravo, Iván Couto, Francisco Fernando Santos, Javier Baltar, Felipe F. Casanueva, María Pardo
International Journal of Molecular Sciences.2022; 23(18): 10826. CrossRef - Dietary tea seed saponin combined with aerobic exercise attenuated lipid metabolism and oxidative stress in mice fed a high‐fat diet (HFD)
Wenjing Cao, Keying Wang, Chanhua Liang, Yanming Su, Shuang Liu, Jiali Li, Huishan Qing, Zhen Zeng, Ling Dai, Jia‐Le Song
Journal of Food Biochemistry.2022;[Epub] CrossRef - Influence of Protein Carbonylation on Human Adipose Tissue Dysfunction in Obesity and Insulin Resistance
M. Carmen Navarro-Ruiz, M. Carmen Soler-Vázquez, Alberto Díaz-Ruiz, Juan R. Peinado, Andrea Nieto Calonge, Julia Sánchez-Ceinos, Carmen Tercero-Alcázar, Jaime López-Alcalá, Oriol A. Rangel-Zuñiga, Antonio Membrives, José López-Miranda, María M. Malagón, R
Biomedicines.2022; 10(12): 3032. CrossRef - The Role of Adipose Tissue Lipolysis in Diet-Induced Obesity: Focus on Vimentin
Eun Roh, Hye Jin Yoo
Diabetes & Metabolism Journal.2021; 45(1): 43. CrossRef
- Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
- Characterization of Preadipocyte factor-1 (Pref-1) Expressing Pancreatic Cells.
- Marie Rhee, Sun Hee Suh, Youn Joo Yang, Ji Won Kim, Sung Yoon Jeon, Oak Kee Hong, Seung Hyun Ko, Yoon Hee Choi, Bong Yun Cha, Ho Yong Son, Kun Ho Yoon
- Korean Diabetes J. 2005;29(6):507-516. Published online November 1, 2005
- 1,169 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Preadipocyte factor-1/Delta-like 1(Pref-1/Dlk1) is a type I membrane protein that has six epidermal growth factor (EGF)-like repeats in its extracellular and a short cytoplasmic domain. It is widely expressed in embryonic tissues, whereas its expressions were limited in adult and postnatal stage. To characterize the Pref-1 expressing cells during pancreas development and regeneration after birth, we analyzed Pref-1 expression in embryonic and adult partial pancreatectomized rat pancreas, and primary cultured neonatal pig pancreatic cells. METHODS: Whole fetuses or pieces of rat pancreas were obtained at E20. 90% partial pancreatectomy (Px) and sham operation were done using 5 week-old Sprague-Dawley rats. Experimental animals were divided into 11 groups by time of killing after surgery; 0, 1, 3, 6 and 12 hours, 1, 2, 3, 5, 7, and 14 days. All tissues were immunostained with Pref-1 and analysed by reverse transcriptase (RT)-PCR. Porcine neonatal pancreas cell clusters (NPCCs) were prepared from neonatal pigs aged 1-2 days. Cells were harvested on day 0, 3, 4, 5, 6, and 7 after dispersion. All cells were immunostained with Pref-1 and other specific cell markers such as Pan-cytokeratin (Pan-CK), vimentin (VT) and islet hormones, and confirmed by Western blot, RT-PCR and fluorescence activated cell sorting (FACS) analysis. RESULTS: In the rat embryonic pancreas at E20, Pref-1 expression was restricted only in the small branching ductules. In adult rat pancreas, Pref-1 was not expressed at all. Whereas, Pref-1 transiently expressed in the small regenerating duct cells located in foci of regeneration in Px model, then completely disappeared at day 7. The Pref-1 mRNA measured by RT-PCR was peaked at day 3 after Px and then gradually disappeared. Pref-1 expression pattern was also reproduced in monolayer cultured NPCCs. In NPCCs, protein levels of Pref-1 were peaked at day 0 to day 4 then gradually disappeared until day 7 by western blot. Most of Pref-1 expressing cells were co-stained with cytokeratin. The proportion of Pref-1 expressing cells in dispersed NPCCs were counted and isolated by FACS at 3 days after culture were 25% and then decreased over time during 7 days culture period. CONCLUSIONS: Pref-1 expression was regained in adult pancreatic cells during regeneration in vivo and in vitro and Pref-1 might be a useful marker for the pancreatic protodifferentiated cells.
- In Vitro Expansion and Differentiation of Islet Precursor Cells from Cultured Neonatal Porcine Pancreatic Tissue.
- Yu Bae Ahn, Kun Ho Yoon, Sun Hee Seo, Seung Hyun Ko, Ki Ho Song, Je Ho Han, Soon Jip Yoo, Hyun Sik Son, Moo Il Kang, Bong Yun Cha, Kwang Woo Lee, Ho Young Son, Sung Koo Kang
- Korean Diabetes J. 2000;24(3):310-322. Published online January 1, 2001
- 929 View
- 17 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Neonatal porcine pancreas is an attractive alternative source for islet transplantation because of its growth potential and availability. Porcine neonatal pancreatic cell clusters (NPCCs) consist mainly of protodifferentiated cells expressing both the duct cell marker pancytokeratin and islet hormones. In this study, we investigated to expand and mature the pancreas duct cells contained in porcine NPCCs with extracellular matrix. METHODS: For NPCCs, pancreas obtained from neonatal pigs were minced, digested with collagenase and cultured overnight. Then NPCCs were further dispersed to small cell groups and cultured on HTB-9 extracellular matrix: the tissue attached and formed monolayer patches. At the 3rd and 8th days, tissue was fixed, immunostained for pancytokeratin (panCK), vimentin (VT) and islet hormones. RESULTS: During 5 days culture, the total cell numbers increased 3.2 fold on the matrix, and 1.6 fold on the sticky dish, respectively. Insulin positive cells (Ins+) were 6.0% of total cells at day 3 and increased 1.6 fold in numbers at day 8. There was significant increase in DNA content of NPCCs in monolayers on both sticky dishes and HTB-9 matrix. In contrast, insulin content of both groups decreased during culture periods. Until 8 days of culture after dispersion of porcine NPCC, most duct cells costained with panCK and VT. CONCLUSION: We observed NPCCs were composed of many of duct cells which were known to be endocrine precursor cells and monolayer culture of NPCC withextracellular matrix resulted in the proliferation and differentiation of pancreatic duct cells.
- The Changes of Expression of Intermediate Flament in Pancreatic Duct Cells During Proliferation and Differentiation after 90% Pancreatectomy in Rats.
- Seung Hyeon Ko, Kun Ho Yoon, Sun Hee Seo, Jung Min Lee, Ki Won Oh, Sang Ah Chang, Hye Soo Kim, Yoo Bae Ahn, Hyun Shik Son, Moo Il Kang, Bong Yun Cha, Kwang Woo Lee, Ho Young Son, Sung Koo Kang
- Korean Diabetes J. 2000;24(2):191-201. Published online January 1, 2001
- 1,038 View
- 16 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
Neogenesis of the beta calls from ductal cells is the main mechanism of the increased beta cell mass after partial pancreatectomy. For the transdifferentiation from the duct cells to the beta cells, de-differentiation of the duct cells is needed because duct cells are also terminally differentiated cells already. But there was no clear evidence of de-differentiation of the duct cells during duct call proliferation so far. Herein we report the changes of intermediate filament protein expression in rapidly proliferating duct cells after partial pancreatectomy for the evidence of de-differentiation of the duct cells. METHODS: 45 week-old Sprague-Dawley rats weighing 80~120 g were used. 90% partial pancreatectomy was done. Experimental animals were divided into 5 subgroups by date of killing after surgery: 1, 3, 7, 14, 30 days, Pancreas remnant was excised and immunohistochemical stain was done for pancytokeratin (Pan-CK) as a epithelial cell marker and vimentin (VT) as a mesenchymal cell marker. We observed the double stained slide with pan-CK and VT antibody using confocal microscope for costaining analysis over time. The sections were also immunostained with anti-insulin antibody for the quantification of the beta cell mass by point-counting methods. RESULTS: We observed impaired glucose tolerance and diabetes were developed affer 90% pancreatectomy. Significant increase of the weight of pancreatic remnant, beta cell and duct cell mass were observed about 14 days after pancreatectomy. We observed the co-expression of VT and pan-CK intermediate filament protein in rapidly proliferating duct cells in the area of common pancreatic duct and main duct at one day after partial pancreatectomy. 3 days affer partial pancreatectomy, VT and pan-CK costained duct cells were mainly observed in the rageneration focus of the duct cell proliferation. 30 days after partial pancreatectomy, we could not find any costaining duct calls in the remnant pancreas. CONCLUSION: The vimentin intermediate filament, a marker of mesenchymal cell was expressed in proliferating ductal cells after pancreatectomy. We could suspect that pancytokeratin and vimentin co-expression is a good marker for de-differentiation of proliferating duct cells.

 KDA
KDA
 First
First Prev
Prev





