
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Complications
- Peripheral Neuropathy Phenotyping in Rat Models of Type 2 Diabetes Mellitus: Evaluating Uptake of the Neurodiab Guidelines and Identifying Future Directions
- Md Jakir Hossain, Michael D. Kendig, Meg E. Letton, Margaret J. Morris, Ria Arnold
- Diabetes Metab J. 2022;46(2):198-221. Published online March 24, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0347
- 5,220 View
- 225 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Diabetic peripheral neuropathy (DPN) affects over half of type 2 diabetes mellitus (T2DM) patients, with an urgent need for effective pharmacotherapies. While many rat and mouse models of T2DM exist, the phenotyping of DPN has been challenging with inconsistencies across laboratories. To better characterize DPN in rodents, a consensus guideline was published in 2014 to accelerate the translation of preclinical findings. Here we review DPN phenotyping in rat models of T2DM against the ‘Neurodiab’ criteria to identify uptake of the guidelines and discuss how DPN phenotypes differ between models and according to diabetes duration and sex. A search of PubMed, Scopus and Web of Science databases identified 125 studies, categorised as either diet and/or chemically induced models or transgenic/spontaneous models of T2DM. The use of diet and chemically induced T2DM models has exceeded that of transgenic models in recent years, and the introduction of the Neurodiab guidelines has not appreciably increased the number of studies assessing all key DPN endpoints. Combined high-fat diet and low dose streptozotocin rat models are the most frequently used and well characterised. Overall, we recommend adherence to Neurodiab guidelines for creating better animal models of DPN to accelerate translation and drug development.
-
Citations
Citations to this article as recorded by- SIRT3 alleviates painful diabetic neuropathy by mediating the FoxO3a‐PINK1‐Parkin signaling pathway to activate mitophagy
Jing Yang, Zhuoying Yu, Ye Jiang, Zixian Zhang, Yue Tian, Jie Cai, Min Wei, Yanhan Lyu, Dongsheng Yang, Shixiong Shen, Guo‐Gang Xing, Min Li
CNS Neuroscience & Therapeutics.2024;[Epub] CrossRef - Compound Qiying Granules alleviates diabetic peripheral neuropathy by inhibiting endoplasmic reticulum stress and apoptosis
Yan Hu, Chen Chen, Zhengting Liang, Tao Liu, Xiaoling Hu, Guanying Wang, Jinxia Hu, Xiaolin Xie, Zhiyan Liu
Molecular Medicine.2023;[Epub] CrossRef - HCV affects KATP channels through GnT-IVa-mediated N-glycosylation of GLUT2 on the surface of pancreatic β-cells leading to impaired insulin secretion
Ben Niu, Lijing Ma, Lixuan Yao, Yating Zhang, Heng Su
Endocrine.2023;[Epub] CrossRef - Multimodal Comparison of Diabetic Neuropathy in Aged Streptozotocin-Treated Sprague–Dawley and Zucker Diabetic Fatty Rats
Annalisa Canta, Valentina A. Carozzi, Alessia Chiorazzi, Cristina Meregalli, Norberto Oggioni, Virginia Rodriguez-Menendez, Barbara Sala, Roberto Cosimo Melcangi, Silvia Giatti, Raffaella Lombardi, Roberto Bianchi, Paola Marmiroli, Guido Cavaletti
Biomedicines.2022; 11(1): 20. CrossRef
- SIRT3 alleviates painful diabetic neuropathy by mediating the FoxO3a‐PINK1‐Parkin signaling pathway to activate mitophagy
- Basic Research
- Application of Animal Models in Diabetic Cardiomyopathy
- Wang-Soo Lee, Jaetaek Kim
- Diabetes Metab J. 2021;45(2):129-145. Published online March 25, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0285

- 9,168 View
- 332 Download
- 9 Web of Science
- 14 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
- Diabetic heart disease is a growing and important public health risk. Apart from the risk of coronary artery disease or hypertension, diabetes mellitus (DM) is a well-known risk factor for heart failure in the form of diabetic cardiomyopathy (DiaCM). Currently, DiaCM is defined as myocardial dysfunction in patients with DM in the absence of coronary artery disease and hypertension. The underlying pathomechanism of DiaCM is partially understood, but accumulating evidence suggests that metabolic derangements, oxidative stress, increased myocardial fibrosis and hypertrophy, inflammation, enhanced apoptosis, impaired intracellular calcium handling, activation of the renin-angiotensin-aldosterone system, mitochondrial dysfunction, and dysregulation of microRNAs, among other factors, are involved. Numerous animal models have been used to investigate the pathomechanisms of DiaCM. Despite some limitations, animal models for DiaCM have greatly advanced our understanding of pathomechanisms and have helped in the development of successful disease management strategies. In this review, we summarize the current pathomechanisms of DiaCM and provide animal models for DiaCM according to its pathomechanisms, which may contribute to broadening our understanding of the underlying mechanisms and facilitating the identification of possible new therapeutic targets.
-
Citations
Citations to this article as recorded by- Chitosan Versus Dapagliflozin in a Diabetic Cardiomyopathy Mouse Model
Georgică Târtea, Aurel Popa-Wagner, Veronica Sfredel, Smaranda Ioana Mitran, Alexandra Oltea Dan, Anca-Maria Țucă, Alexandra Nicoleta Preda, Victor Raicea, Eugen Țieranu, Dragoș Cozma, Radu Vătășescu
International Journal of Molecular Sciences.2024; 25(4): 2118. CrossRef - Mitochondrial energy metabolism in diabetic cardiomyopathy: Physiological adaption, pathogenesis, and therapeutic targets
Wanlin Ye, Kun Han, Maodi Xie, Sheyu Li, Guo Chen, Yanyan Wang, Tao Li
Chinese Medical Journal.2024; 137(8): 936. CrossRef - Liraglutide Attenuates Diabetic Cardiomyopathy via the ILK/PI3K/AKT/PTEN Signaling Pathway in Rats with Streptozotocin-Induced Type 2 Diabetes Mellitus
Shatha M. Alobaid, Rahaf M. Alshahrani, Asma S. Alonazi, Nawal M. Alrasheed, Maha A. Alamin, Tahani K. Alshammari, Anfal F. Bin Dayel, Doaa M. Elnagar, Rana R. Alotaibi, Lama A. Almuthnabi, Dalia H. Almasud, Shahad E. Al-Ammar, Shahad O. Almadhi, Reema A.
Pharmaceuticals.2024; 17(3): 374. CrossRef - An Overview of Diabetic Cardiomyopathy
Abdul Quaiyoom, Ranjeet Kumar
Current Diabetes Reviews.2024;[Epub] CrossRef - Evaluation and Management of Patients With Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon
International Journal of Heart Failure.2023; 5(1): 1. CrossRef - Evaluation and Management of Patients with Diabetes and Heart Failure: A Korean Diabetes Association and Korean Society of Heart Failure Consensus Statement
Kyu-Sun Lee, Junghyun Noh, Seong-Mi Park, Kyung Mook Choi, Seok-Min Kang, Kyu-Chang Won, Hyun-Jai Cho, Min Kyong Moon
Diabetes & Metabolism Journal.2023; 47(1): 10. CrossRef - Machine learning for spatial stratification of progressive cardiovascular dysfunction in a murine model of type 2 diabetes mellitus
Andrya J. Durr, Anna S. Korol, Quincy A. Hathaway, Amina Kunovac, Andrew D. Taylor, Saira Rizwan, Mark V. Pinti, John M. Hollander, Yoshihiro Fukumoto
PLOS ONE.2023; 18(5): e0285512. CrossRef - Hyperglycemic memory in diabetic cardiomyopathy
Jiabing Zhan, Chen Chen, Dao Wen Wang, Huaping Li
Frontiers of Medicine.2022; 16(1): 25. CrossRef - Murine Models of Obesity
Tânia Martins, Catarina Castro-Ribeiro, Sílvia Lemos, Tiago Ferreira, Elisabete Nascimento-Gonçalves, Eduardo Rosa, Paula Alexandra Oliveira, Luís Miguel Antunes
Obesities.2022; 2(2): 127. CrossRef - The Role of Mitochondria in Metabolic Syndrome–Associated Cardiomyopathy
Jiayu Li, Jingye Li, Yijun Chen, Wenyu Hu, Xuhe Gong, Hui Qiu, Hui Chen, Yanguo Xin, Hongwei Li, Tao Li
Oxidative Medicine and Cellular Longevity.2022; 2022: 1. CrossRef - Guidelines on models of diabetic heart disease
Lisa C. Heather, Anne D. Hafstad, Ganesh V. Halade, Romain Harmancey, Kimberley M. Mellor, Paras K. Mishra, Erin E. Mulvihill, Miranda Nabben, Michinari Nakamura, Oliver J. Rider, Matthieu Ruiz, Adam R. Wende, John R. Ussher
American Journal of Physiology-Heart and Circulatory Physiology.2022; 323(1): H176. CrossRef - Extracellular vesicle therapy for non-ischemic heart failure: A systematic review of preclinical studies
Ramana Vaka, Sophie Van Remortel, Valentina Ly, Darryl R. Davis
Extracellular Vesicle.2022; 1: 100009. CrossRef - Effect of a Six-week Endurance Exercise Program and Empagliflozin Consumption on Some Structural and Functional Indices of the Heart in Male Diabetic Rats
Eftekhar Mohammadi, Mohammad Fathi, Farzaneh Chehel Cheraghi, Afshin Nazari
journal of ilam university of medical sciences.2022; 30(3): 1. CrossRef - Cardiac Phosphodiesterases Are Differentially Increased in Diabetic Cardiomyopathy
Rita Hanna, Wared Nour-Eldine, Youakim Saliba, Carole Dagher-Hamalian, Pia Hachem, Pamela Abou-Khalil, Delphine Mika, Audrey Varin, Magali Samia El Hayek, Laëtitia Pereira, Nassim Farès, Grégoire Vandecasteele, Aniella Abi-Gerges
Life Sciences.2021; 283: 119857. CrossRef
- Chitosan Versus Dapagliflozin in a Diabetic Cardiomyopathy Mouse Model
- Complications
- Lost in Translation? Measuring Diabetic Neuropathy in Humans and Animals
- Heung Yong Jin, Seong-Su Moon, Nigel A. Calcutt
- Diabetes Metab J. 2021;45(1):27-42. Published online December 15, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0216
- 8,399 View
- 224 Download
- 13 Web of Science
- 12 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
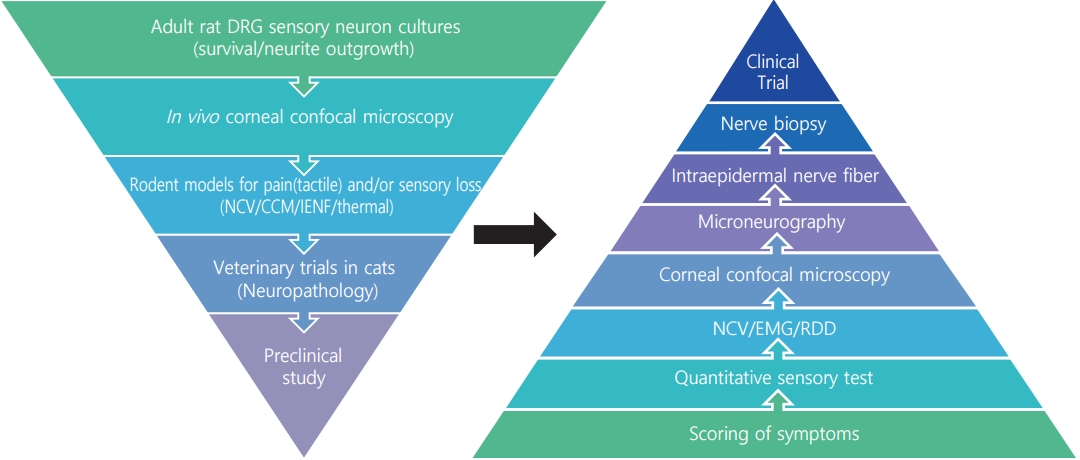
- The worldwide diabetes epidemic is estimated to currently afflict almost 500 million persons. Long-term diabetes damages multiple organ systems with the blood vessels, eyes, kidneys and nervous systems being particularly vulnerable. These complications of diabetes reduce lifespan, impede quality of life and impose a huge social and economic burden on both the individual and society. Peripheral neuropathy is a debilitating complication that will impact over half of all persons with diabetes. There is no treatment for diabetic neuropathy and a disturbingly long history of therapeutic approaches showing promise in preclinical studies but failing to translate to the clinic. These failures have prompted re-examination of both the animal models and clinical trial design. This review focuses on the functional and structural parameters used as indices of peripheral neuropathy in preclinical and clinical studies and the extent to which they share a common pathogenesis and presentation. Nerve conduction studies in large myelinated fibers have long been the mainstay of preclinical efficacy screening programs and clinical trials, supplemented by quantitative sensory tests. However, a more refined approach is emerging that incorporates measures of small fiber density in the skin and cornea alongside these traditional assays at both preclinical and clinical phases.
-
Citations
Citations to this article as recorded by- Peripheral Neuropathy in Diabetes Mellitus: Pathogenetic Mechanisms and Diagnostic Options
Raffaele Galiero, Alfredo Caturano, Erica Vetrano, Domenico Beccia, Chiara Brin, Maria Alfano, Jessica Di Salvo, Raffaella Epifani, Alessia Piacevole, Giuseppina Tagliaferri, Maria Rocco, Ilaria Iadicicco, Giovanni Docimo, Luca Rinaldi, Celestino Sardu, T
International Journal of Molecular Sciences.2023; 24(4): 3554. CrossRef - Bidirectional association between diabetic peripheral neuropathy and vitamin B12 deficiency: Two longitudinal 9-year follow-up studies using a national sample cohort
Heung Yong Jin, Kyung Ae Lee, Yu Ji Kim, In Sun Gwak, Tae Sun Park, Sang Woo Yeom, Jong Seung Kim
Primary Care Diabetes.2023; 17(5): 436. CrossRef - Advanced Drug Delivery System for Management of Chronic Diabetes
Wound Healing
Harish Bhardwaj, Sulekha Khute, Ram Sahu, Rajendra Kumar Jangde
Current Drug Targets.2023; 24(16): 1239. CrossRef - A Real-World Analysis of High-Frequency 10 kHz Spinal Cord Stimulation for the Treatment of Painful Diabetic Peripheral Neuropathy
Jeffrey L. Chen, Andrew W. Hesseltine, Sara E. Nashi, Shawn M. Sills, Tory L. McJunkin, Sandeep Patil, Manish Bharara, David L. Caraway, Elizabeth S. Brooks
Journal of Diabetes Science and Technology.2022; 16(2): 282. CrossRef - Using Corneal Confocal Microscopy to Identify Therapeutic Agents for Diabetic Neuropathy
Corinne G. Jolivalt, May Madi Han, Annee Nguyen, Fiona Desmond, Carlos Henrique Alves Jesus, Daniela C. Vasconselos, Andrea Pedneault, Natalie Sandlin, Sage Dunne-Cerami, Katie E. Frizzi, Nigel A. Calcutt
Journal of Clinical Medicine.2022; 11(9): 2307. CrossRef - Glycyrrhizic acid promotes sciatic nerves recovery in type 1 diabetic rats and protects Schwann cells from high glucose-induced cytotoxicity
Min Shi, Xiangcheng Zhang, Ridong Zhang, Hong Zhang, Dalong Zhu, Xiao Han
The Journal of Biomedical Research.2022; 36(3): 181. CrossRef - Novel mechanisms of pain in painful diabetic neuropathy
Rayaz A. Malik
Nature Reviews Endocrinology.2022; 18(8): 459. CrossRef - An induced pluripotent stem cell-based model identifies molecular targets of vincristine neurotoxicity
Neng-Wei Tsai, Cheng-Chen Lin, Ti-Yen Yeh, Yu-An Chiu, Hsin-Hui Chiu, Hsiang-Po Huang, Sung-Tsang Hsieh
Disease Models & Mechanisms.2022;[Epub] CrossRef - Corneal Confocal Microscopy: A Biomarker for Diabetic Peripheral Neuropathy
Ioannis N. Petropoulos, Georgios Ponirakis, Maryam Ferdousi, Shazli Azmi, Alise Kalteniece, Adnan Khan, Hoda Gad, Bilal Bashir, Andrew Marshall, Andrew J.M. Boulton, Handrean Soran, Rayaz A. Malik
Clinical Therapeutics.2021; 43(9): 1457. CrossRef - Lost in Translation? Measuring Diabetic Neuropathy in Humans and Animals (Diabetes Metab J 2021;45:27-42)
Otto Jesus Hernandez Fustes
Diabetes & Metabolism Journal.2021; 45(3): 452. CrossRef - Lost in Translation? Measuring Diabetic Neuropathy in Humans and Animals (Diabetes Metab J 2021;45:27-42)
Heung Yong Jin, Seong-Su Moon, Nigel A. Calcutt
Diabetes & Metabolism Journal.2021; 45(3): 457. CrossRef - Sterculia tragacantha Lindl Leaf Extract Ameliorates STZ-Induced Diabetes, Oxidative Stress, Inflammation and Neuronal Impairment
Amos Sunday Onikanni, Bashir Lawal, Augustine O Olusola, Janet O Olugbodi, Saidu Sani, Basiru Olaitan Ajiboye, Omotayo B Ilesanmi, Mohammed Alqarni, Gomaa Mostafa-Hedeab, Ahmad J Obaidullah, Gaber El-Saber Batiha, Alexander TH Wu
Journal of Inflammation Research.2021; Volume 14: 6749. CrossRef
- Peripheral Neuropathy in Diabetes Mellitus: Pathogenetic Mechanisms and Diagnostic Options
- Pattern of Stress-Induced Hyperglycemia according to Type of Diabetes: A Predator Stress Model
- Jin-Sun Chang, Young-Hye You, Shin-Young Park, Ji-Won Kim, Hun-Sung Kim, Kun-Ho Yoon, Jae-Hyoung Cho
- Diabetes Metab J. 2013;37(6):475-483. Published online December 12, 2013
- DOI: https://doi.org/10.4093/dmj.2013.37.6.475
- 4,051 View
- 50 Download
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background We aimed to quantify stress-induced hyperglycemia and differentiate the glucose response between normal animals and those with diabetes. We also examined the pattern in glucose fluctuation induced by stress according to type of diabetes.
Methods To load psychological stress on animal models, we used a predator stress model by exposing rats to a cat for 60 minutes and measured glucose level from the beginning to the end of the test to monitor glucose fluctuation. We induced type 1 diabetes model (T1D) for ten Sprague-Dawley rats using streptozotocin and used five Otsuka Long-Evans Tokushima Fatty rats as obese type 2 diabetes model (OT2D) and 10 Goto-Kakizaki rats as nonobese type 2 diabetes model (NOT2D). We performed the stress loading test in both the normal and diabetic states and compared patterns of glucose fluctuation among the three models. We classified the pattern of glucose fluctuation into A, B, and C types according to speed of change in glucose level.
Results Increase in glucose, total amount of hyperglycemic exposure, time of stress-induced hyperglycemia, and speed of glucose increase were significantly increased in all models compared to the normal state. While the early increase in glucose after exposure to stress was higher in T1D and NOT2D, it was slower in OT2D. The rate of speed of the decrease in glucose level was highest in NOT2D and lowest in OT2D.
Conclusion The diabetic state was more vulnerable to stress compared to the normal state in all models, and the pattern of glucose fluctuation differed among the three types of diabetes. The study provides basic evidence for stress-induced hyperglycemia patterns and characteristics used for the management of diabetes patients.
-
Citations
Citations to this article as recorded by- Stress hyperglycemia as first sign of asymptomatic type 1 diabetes: an instructive case
Wei-De Wang, Chun-Hao Chu, Chiung-Hsi Tien, Shuo-Yu Wang, Shih-Yao Liu, Chien-Ming Lin
BMC Pediatrics.2021;[Epub] CrossRef - Genetic determinants of obesity heterogeneity in type II diabetes
Somayeh Alsadat Hosseini Khorami, Mohd Sokhini Abd Mutalib, Mohammad Feili Shiraz, Joseph Anthony Abdullah, Zulida Rejali, Razana Mohd Ali, Huzwah Khaza’ai
Nutrition & Metabolism.2020;[Epub] CrossRef - Sex Dimorphic Responses of the Hypothalamus–Pituitary–Thyroid Axis to Maternal Separation and Palatable Diet
Lorraine Jaimes-Hoy, Fidelia Romero, Jean-Louis Charli, Patricia Joseph-Bravo
Frontiers in Endocrinology.2019;[Epub] CrossRef - Hesperidin protects against stress induced gastric ulcer through regulation of peroxisome proliferator activator receptor gamma in diabetic rats
Shimaa M. Elshazly, Dalia M. Abd El Motteleb, Islam A.A.E-H. Ibrahim
Chemico-Biological Interactions.2018; 291: 153. CrossRef - Physiology and Neurobiology of Stress and the Implications for Physical Health
B Sivaprakash
Annals of SBV.2014; 3(1): 25. CrossRef
- Stress hyperglycemia as first sign of asymptomatic type 1 diabetes: an instructive case

 KDA
KDA
 First
First Prev
Prev





