
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
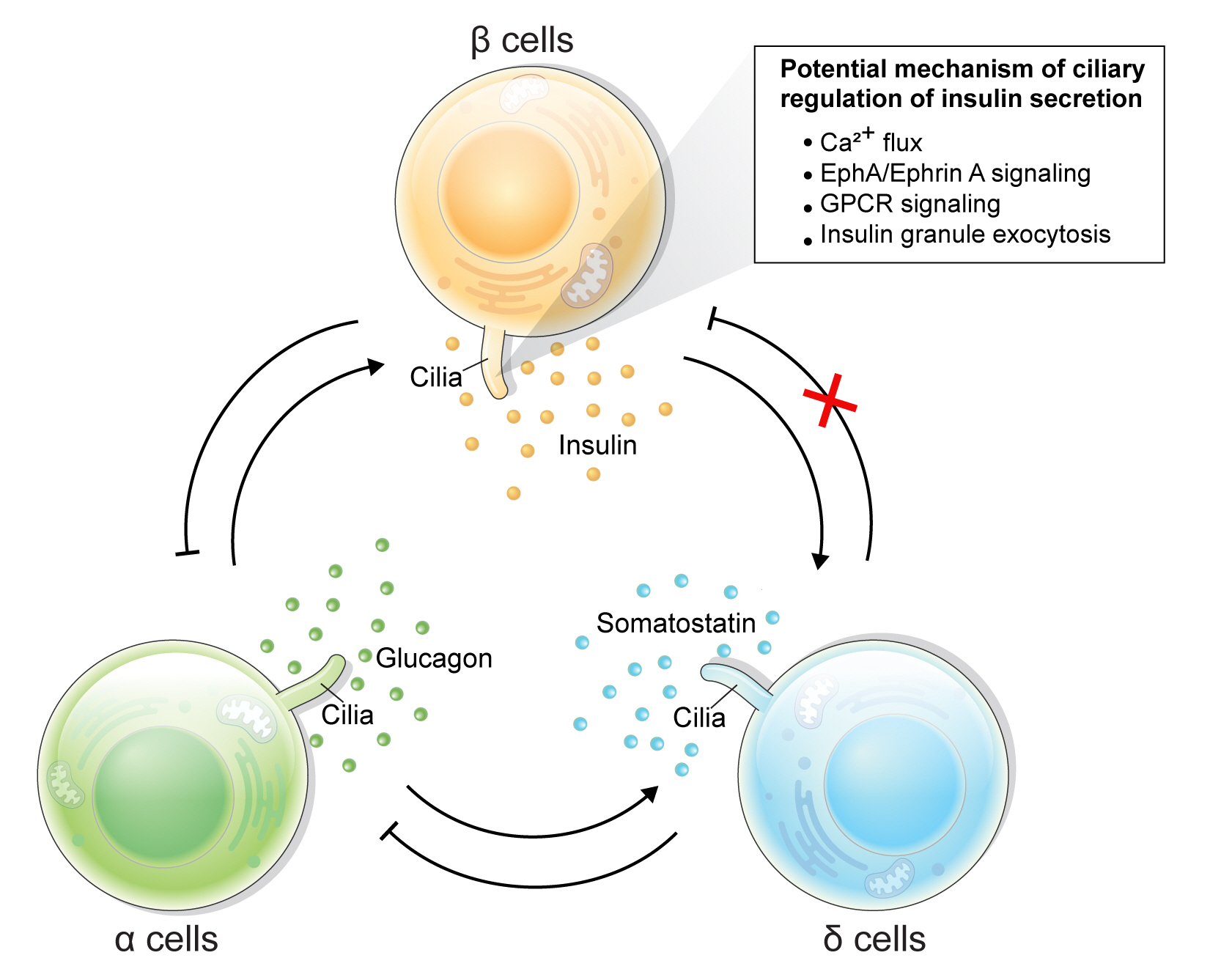
- Rediscovering Primary Cilia in Pancreatic Islets
- Eun Young Lee, Jing W. Hughes
- Diabetes Metab J. 2023;47(4):454-469. Published online April 28, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0442
- 2,593 View
- 240 Download
- 1 Web of Science
- 1 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Primary cilia are microtubule-based sensory and signaling organelles on the surfaces of most eukaryotic cells. Despite their early description by microscopy studies, islet cilia had not been examined in the functional context until recent decades. In pancreatic islets as in other tissues, primary cilia facilitate crucial developmental and signaling pathways in response to extracellular stimuli. Many human developmental and genetic disorders are associated with ciliary dysfunction, some manifesting as obesity and diabetes. Understanding the basis for metabolic diseases in human ciliopathies has been aided by close examination of cilia action in pancreatic islets at cellular and molecular levels. In this article, we review the evidence for ciliary expression on islet cells, known roles of cilia in pancreas development and islet hormone secretion, and summarize metabolic manifestations of human ciliopathy syndromes. We discuss emerging data on primary cilia regulation of islet cell signaling and the structural basis of cilia-mediated cell crosstalk, and offer our interpretation on the role of cilia in glucose homeostasis and human diseases.
-
Citations
Citations to this article as recorded by- Beta cell primary cilia mediate somatostatin responsiveness via SSTR3
Samantha E. Adamson, Zipeng A. Li, Jing W. Hughes
Islets.2023;[Epub] CrossRef
- Beta cell primary cilia mediate somatostatin responsiveness via SSTR3
- Basic Research
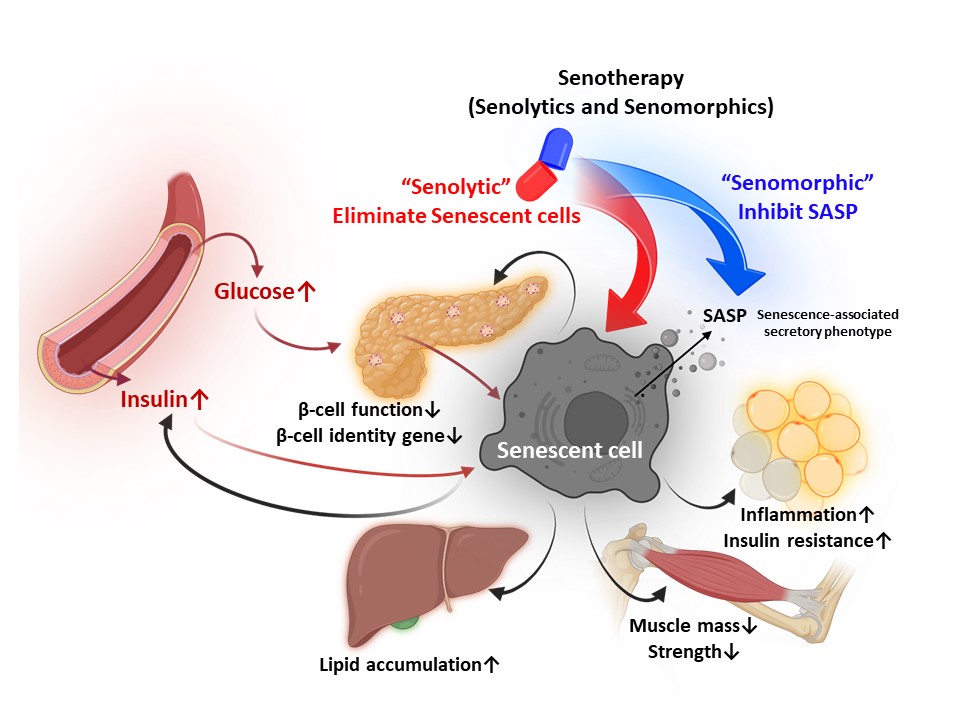
- Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications
- Kanako Iwasaki, Cristian Abarca, Cristina Aguayo-Mazzucato
- Diabetes Metab J. 2023;47(4):441-453. Published online March 6, 2023
- DOI: https://doi.org/10.4093/dmj.2022.0416
- 4,693 View
- 417 Download
- 3 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Cellular senescence is accelerated by hyperglycemia through multiple pathways. Therefore, senescence is an important cellular mechanism to consider in the pathophysiology of type 2 diabetes mellitus (T2DM) and an additional therapeutic target. The use of drugs that remove senescent cells has led to improvements in blood glucose levels and diabetic complications in animal studies. Although the removal of senescent cells is a promising approach for the treatment of T2DM, two main challenges limit its clinical application: the molecular basis of cellular senescence in each organ is yet to be understood, and the specific effect of removing senescent cells in each organ has to be determined. This review aims to discuss future applications of targeting senescence as a therapeutic option in T2DM and elucidate the characteristics of cellular senescence and senescence-associated secretory phenotype in the tissues important for regulating glucose levels: pancreas, liver, adipocytes, and skeletal muscle.
-
Citations
Citations to this article as recorded by- Amide Alkaloids as Privileged Sources of Senomodulators for Therapeutic Purposes in Age-Related Diseases
Mazzarine Dotou, Aurore L’honoré, Roba Moumné, Chahrazade El Amri
Journal of Natural Products.2024; 87(3): 617. CrossRef - Study on the Pathogenesis of Cell Senescence in Non-Alcoholic Fatty Liver
丽媛 黄
Medical Diagnosis.2024; 14(01): 76. CrossRef - Senescent adipocytes and type 2 diabetes – current knowledge and perspective concepts
Weronika Kruczkowska, Julia Gałęziewska, Mateusz Kciuk, Adrianna Gielecińska, Elżbieta Płuciennik, Zbigniew Pasieka, Lin-Yong Zhao, Yi-Jin Yu, Damian Kołat, Żaneta Kałuzińska-Kołat
Biomolecular Concepts.2024;[Epub] CrossRef - The Effect of Long-Term Passage on Porcine SMCs’ Function and the Improvement of TGF-β1 on Porcine SMCs’ Secretory Function in Late Passage
Yan-Yan Zheng, Ze-Nan Hu, Zheng Liu, Yi-Chen Jiang, Ren-Peng Guo, Shi-Jie Ding, Guang-Hong Zhou
Foods.2023; 12(14): 2682. CrossRef - Exploring the Relationship between Cellular Senescence Markers and Aging-Related Diseases
怡 罗
Advances in Clinical Medicine.2023; 13(08): 12298. CrossRef
- Amide Alkaloids as Privileged Sources of Senomodulators for Therapeutic Purposes in Age-Related Diseases
- Pathophysiology
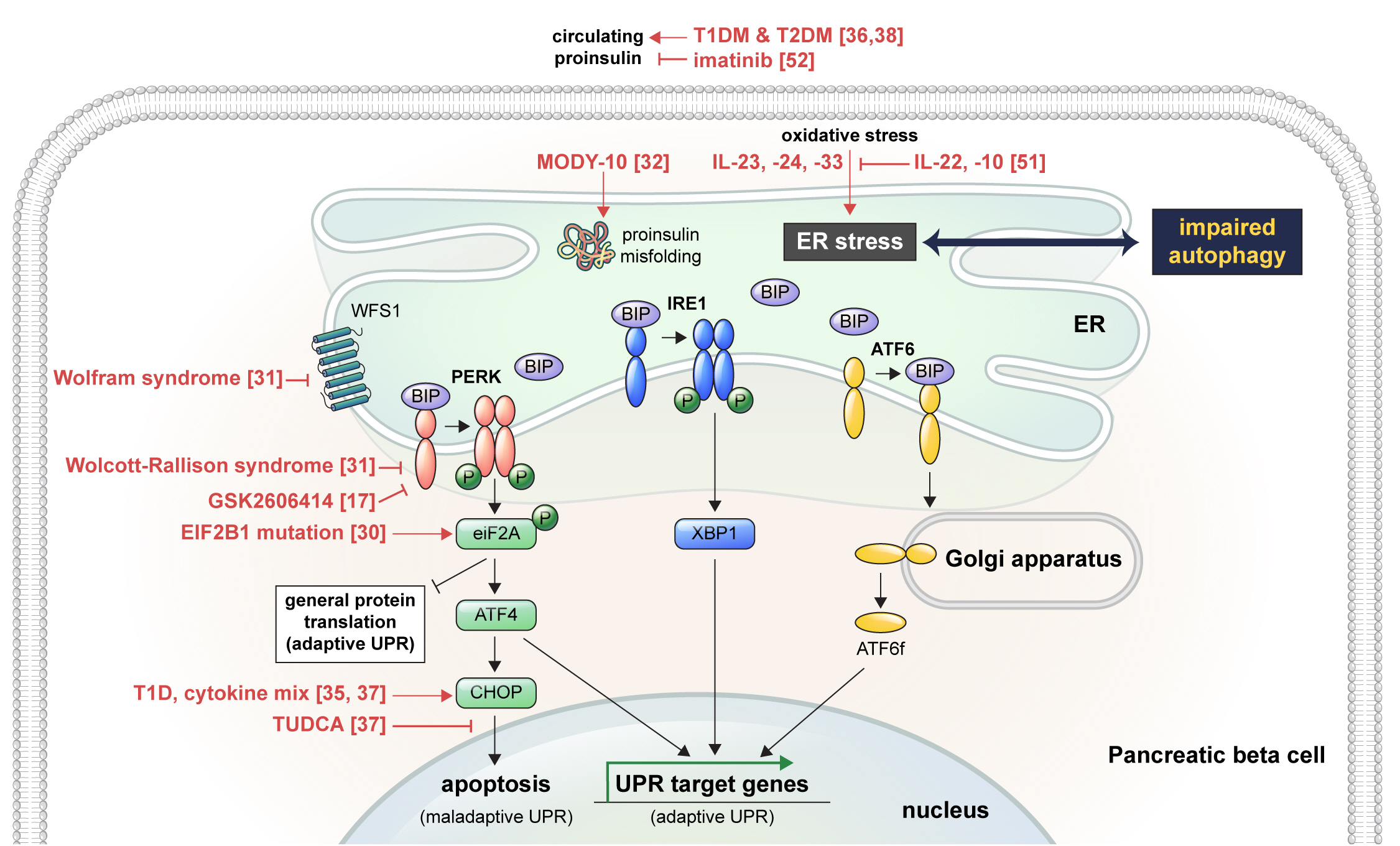
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells
- Seoil Moon, Hye Seung Jung
- Diabetes Metab J. 2022;46(4):533-542. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0070
- 4,536 View
- 250 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Pancreatic beta cell homeostasis is crucial for the synthesis and secretion of insulin; disruption of homeostasis causes diabetes, and is a treatment target. Adaptation to endoplasmic reticulum (ER) stress through the unfolded protein response (UPR) and adequate regulation of autophagy, which are closely linked, play essential roles in this homeostasis. In diabetes, the UPR and autophagy are dysregulated, which leads to beta cell failure and death. Various studies have explored methods to preserve pancreatic beta cell function and mass by relieving ER stress and regulating autophagic activity. To promote clinical translation of these research results to potential therapeutics for diabetes, we summarize the current knowledge on ER stress and autophagy in human insulin-secreting cells.
-
Citations
Citations to this article as recorded by- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
Diabetes & Metabolism Journal.2024; 48(2): 231. CrossRef - Endoplasmic reticulum stress: A possible connection between intestinal inflammation and neurodegenerative disorders
Giorgio Vivacqua, Romina Mancinelli, Stefano Leone, Rosa Vaccaro, Ludovica Garro, Simone Carotti, Ludovica Ceci, Paolo Onori, Luigi Pannarale, Antonio Franchitto, Eugenio Gaudio, Arianna Casini
Neurogastroenterology & Motility.2024;[Epub] CrossRef - Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells
Seok-Woo Hong, Jinmi Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2024; 39(2): 353. CrossRef - Pancreatic islet remodeling in cotadutide-treated obese mice
Renata Spezani, Thatiany Souza Marinho, Luiz E. Macedo Cardoso, Marcia Barbosa Aguila, Carlos Alberto Mandarim-de-Lacerda
Life Sciences.2023; 327: 121858. CrossRef - Modulation of Unfolded Protein Response Restores Survival and Function of β-Cells Exposed to the Endocrine Disruptor Bisphenol A
Laura Maria Daian, Gabriela Tanko, Andrei Mircea Vacaru, Luiza Ghila, Simona Chera, Ana-Maria Vacaru
International Journal of Molecular Sciences.2023; 24(3): 2023. CrossRef - Interplay of skeletal muscle and adipose tissue: sarcopenic obesity
Min Jeong Park, Kyung Mook Choi
Metabolism.2023; 144: 155577. CrossRef - Identification and analysis of type 2 diabetes-mellitus-associated autophagy-related genes
Kun Cui, Zhizheng Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Sestrin2 in diabetes and diabetic complications
Xiaodan Zhang, Zirui Luo, Jiahong Li, Yaxuan Lin, Yu Li, Wangen Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Crosstalk between autophagy and insulin resistance: evidence from different tissues
Asie Sadeghi, Maryam Niknam, Mohammad Amin Momeni-Moghaddam, Maryam Shabani, Hamid Aria, Alireza Bastin, Maryam Teimouri, Reza Meshkani, Hamed Akbari
European Journal of Medical Research.2023;[Epub] CrossRef - Beta cell lipotoxicity in the development of type 2 diabetes: the need for species-specific understanding
Patricia Thomas, Meurig T. Gallagher, Gabriela Da Silva Xavier
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Islet Studies and Transplantation
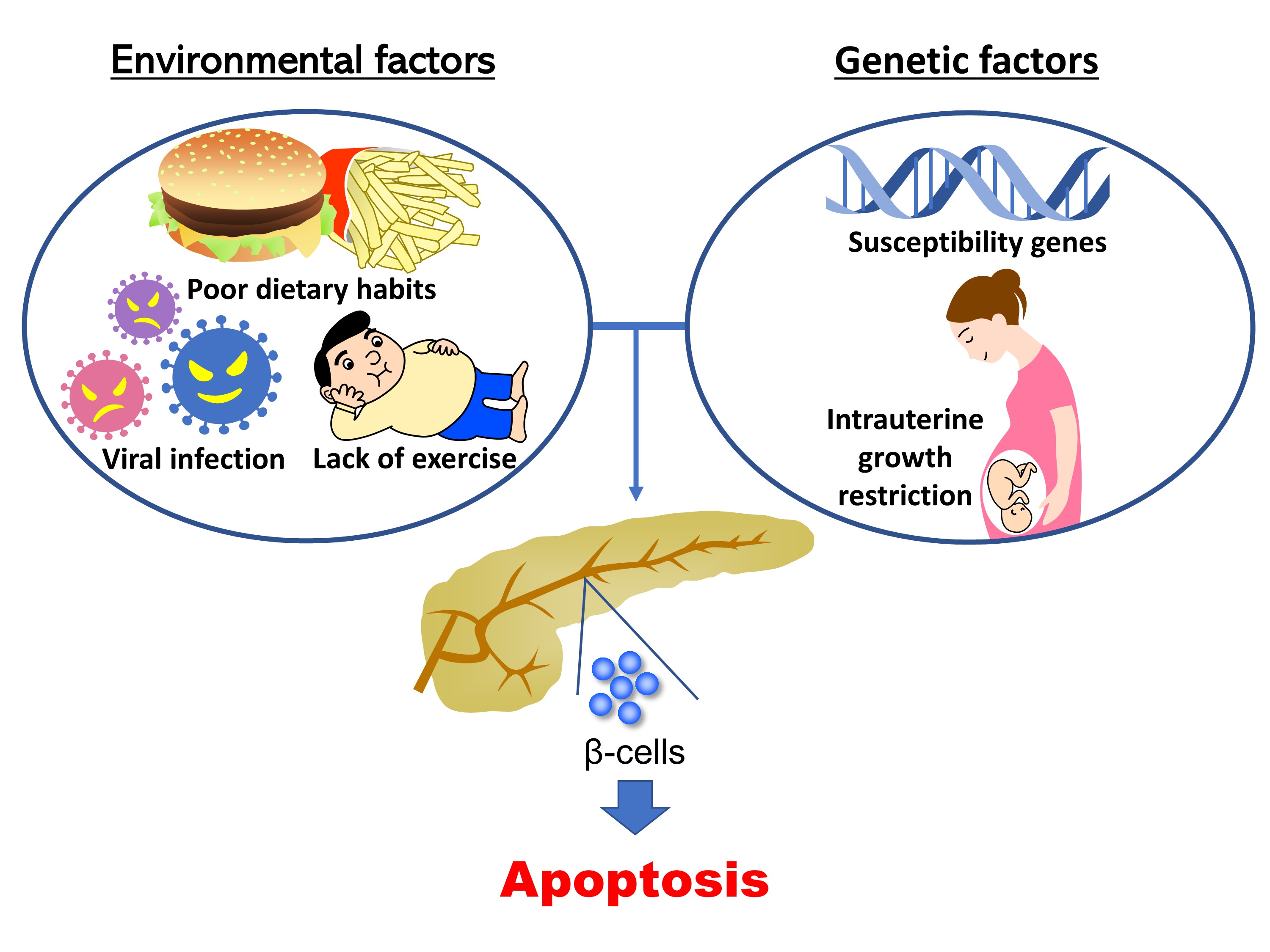
- Regulation of Pancreatic β-Cell Mass by Gene-Environment Interaction
- Shun-ichiro Asahara, Hiroyuki Inoue, Yoshiaki Kido
- Diabetes Metab J. 2022;46(1):38-48. Published online January 27, 2022
- DOI: https://doi.org/10.4093/dmj.2021.0045
- 4,520 View
- 197 Download
- 5 Web of Science
- 5 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
- The main pathogenic mechanism of diabetes consists of an increase in insulin resistance and a decrease in insulin secretion from pancreatic β-cells. The number of diabetic patients has been increasing dramatically worldwide, especially in Asian people whose capacity for insulin secretion is inherently lower than that of other ethnic populations. Causally, changes of environmental factors in addition to intrinsic genetic factors have been considered to have an influence on the increased prevalence of diabetes. Particular focus has been placed on “gene-environment interactions” in the development of a reduced pancreatic β-cell mass, as well as type 1 and type 2 diabetes mellitus. Changes in the intrauterine environment, such as intrauterine growth restriction, contribute to alterations of gene expression in pancreatic β-cells, ultimately resulting in the development of pancreatic β-cell failure and diabetes. As a molecular mechanism underlying the effect of the intrauterine environment, epigenetic modifications have been widely investigated. The association of diabetes susceptibility genes or dietary habits with gene-environment interactions has been reported. In this review, we provide an overview of the role of gene-environment interactions in pancreatic β-cell failure as revealed by previous reports and data from experiments.
-
Citations
Citations to this article as recorded by- Increased risk of incident diabetes after therapy with immune checkpoint inhibitor compared with conventional chemotherapy: A longitudinal trajectory analysis using a tertiary care hospital database
Minyoung Lee, Kyeongseob Jeong, Yu Rang Park, Yumie Rhee
Metabolism.2023; 138: 155311. CrossRef - The ameliorating effects of mesenchymal stem cells compared to α‐tocopherol on apoptosis and autophagy in streptozotocin‐induced diabetic rats: Implication of PI3K/Akt signaling pathway and entero‐insular axis
Heba A. Mubarak, Manal M. Kamal, Yossra Mahmoud, Fatma S. Abd‐Elsamea, Eman Abdelbary, Marwa G. Gamea, Reham I. El‐Mahdy
Journal of Cellular Biochemistry.2023; 124(11): 1705. CrossRef - Leptin Rs7799039 polymorphism is associated with type 2 diabetes mellitus Egyptian patients
Amal Ahmed Mohamed, Dina M. Abo-Elmatty, Alaa S. Wahba, Omnia Ezzat Esmail, Hadeer Saied Mahmoud Salim, Wafaa Salah Mohammed Hegab, Mona Mostafa Farid Ghanem, Nadia Youssef Riad, Doaa Ghaith, Lamiaa I Daker, Shorouk Issa, Noha Hassan Radwan, Eman Sultan,
Archives of Physiology and Biochemistry.2023; : 1. CrossRef - Association of Polygenic Variants with Type 2 Diabetes Risk and Their Interaction with Lifestyles in Asians
Haeng Jeon Hur, Hye Jeong Yang, Min Jung Kim, Kyun-Hee Lee, Myung-Sunny Kim, Sunmin Park
Nutrients.2022; 14(15): 3222. CrossRef - Chemical Compounds and Ambient Factors Affecting Pancreatic Alpha-Cells Mass and Function: What Evidence?
Gaia Chiara Mannino, Elettra Mancuso, Stefano Sbrignadello, Micaela Morettini, Francesco Andreozzi, Andrea Tura
International Journal of Environmental Research and Public Health.2022; 19(24): 16489. CrossRef
- Increased risk of incident diabetes after therapy with immune checkpoint inhibitor compared with conventional chemotherapy: A longitudinal trajectory analysis using a tertiary care hospital database
- Pathophysiology
- Relationships between Islet-Specific Autoantibody Titers and the Clinical Characteristics of Patients with Diabetes Mellitus
- Yiqian Zhang, Tong Yin, Xinlei Wang, Rongping Zhang, Jie Yuan, Yi Sun, Jing Zong, Shiwei Cui, Yunjuan Gu
- Diabetes Metab J. 2021;45(3):404-416. Published online July 21, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0239
- 6,361 View
- 161 Download
- 4 Web of Science
- 4 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
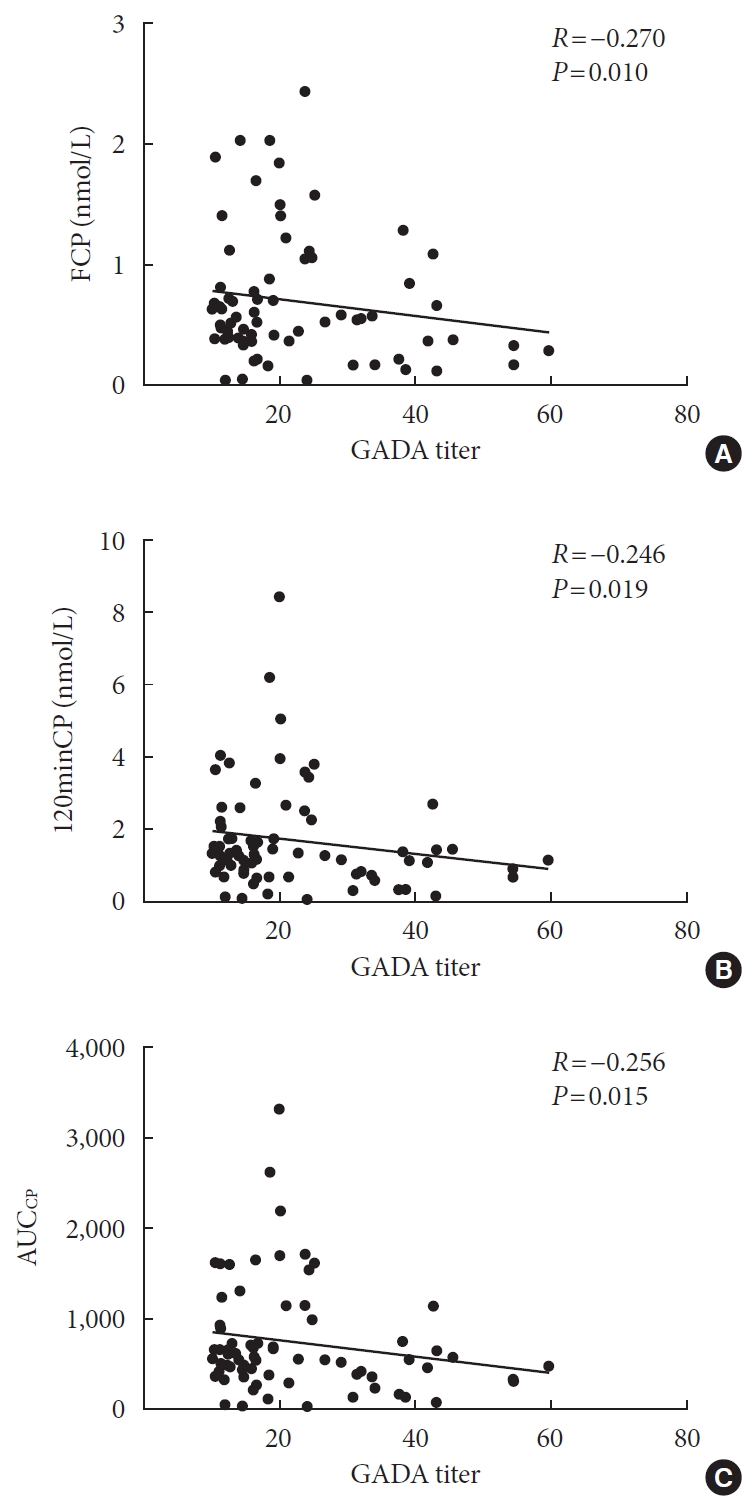
ePub Background Dysimmunity plays a key role in diabetes, especially type 1 diabetes mellitus. Islet-specific autoantibodies (ISAs) have been used as diagnostic markers for different phenotypic classifications of diabetes. This study was aimed to explore the relationships between ISA titers and the clinical characteristics of diabetic patients.
Methods A total of 509 diabetic patients admitted to Department of Endocrinology and Metabolism at the Affiliated Hospital of Nantong University were recruited. Anthropometric parameters, serum biochemical index, glycosylated hemoglobin, urinary microalbumin/creatinine ratio, ISAs, fat mass, and islet β-cell function were measured. Multiple linear regression analysis was performed to identify relationships between ISA titers and clinical characteristics.
Results Compared with autoantibody negative group, blood pressure, weight, total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), visceral fat mass, fasting C-peptide (FCP), 120 minutes C-peptide (120minCP) and area under C-peptide curve (AUCCP) of patients in either autoantibody positive or glutamate decarboxylase antibody (GADA) positive group were lower. Body mass index (BMI), waist circumference, triglycerides (TGs), body fat mass of patients in either autoantibody positive group were lower than autoantibody negative group. GADA titer negatively correlated with TC, LDL-C, FCP, 120minCP, and AUCCP. The islet cell antibody and insulin autoantibody titers both negatively correlated with body weight, BMI, TC, TG, and LDL-C. After adjusting confounders, multiple linear regression analysis showed that LDL-C and FCP negatively correlated with GADA titer.
Conclusion Diabetic patients with a high ISA titer, especially GADA titer, have worse islet β-cell function, but less abdominal obesity and fewer features of the metabolic syndrome.
-
Citations
Citations to this article as recorded by- Relationship between β-Cell Autoantibodies and Their Combination with Anthropometric and Metabolic Components and Microvascular Complications in Latent Autoimmune Diabetes in Adults
Tomislav Bulum, Marijana Vučić Lovrenčić, Jadranka Knežević Ćuća, Martina Tomić, Sandra Vučković-Rebrina, Lea Duvnjak
Biomedicines.2023; 11(9): 2561. CrossRef - RETRACTED ARTICLE: MiRNA-27a mediates insulin resistance in 3T3-L1 cells through the PPARγ
Yangming Zhuang, Ming Li
Molecular and Cellular Biochemistry.2022; 477(4): 1107. CrossRef - Correlation between Insulin Resistance and Microalbuminuria Creatinine Ratio in Postmenopausal Women
Han Na, Rong Wang, Hai-Long Zheng, Xiao-Pan Chen, Lin-Yang Zheng, Faustino R. Perez-Lopez
International Journal of Endocrinology.2022; 2022: 1. CrossRef - The longitudinal loss of islet autoantibody responses from diagnosis of type 1 diabetes occurs progressively over follow-up and is determined by low autoantibody titres, early-onset, and genetic variants
C L Williams, R Fareed, G L M Mortimer, R J Aitken, I V Wilson, G George, K M Gillespie, A J K Williams, Chitrabhanu Ballav, Atanu Dutta, Michelle Russell-Taylor, Rachel Besser, James Bursell, Shanthi Chandran, Sejal Patel, Anne Smith, Manohara Kenchaiah,
Clinical and Experimental Immunology.2022; 210(2): 151. CrossRef
- Relationship between β-Cell Autoantibodies and Their Combination with Anthropometric and Metabolic Components and Microvascular Complications in Latent Autoimmune Diabetes in Adults
- Basic Research
- Hypoxia Increases β-Cell Death by Activating Pancreatic Stellate Cells within the Islet
- Jong Jin Kim, Esder Lee, Gyeong Ryul Ryu, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
- Diabetes Metab J. 2020;44(6):919-927. Published online May 11, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0181
- 5,932 View
- 146 Download
- 15 Web of Science
- 16 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
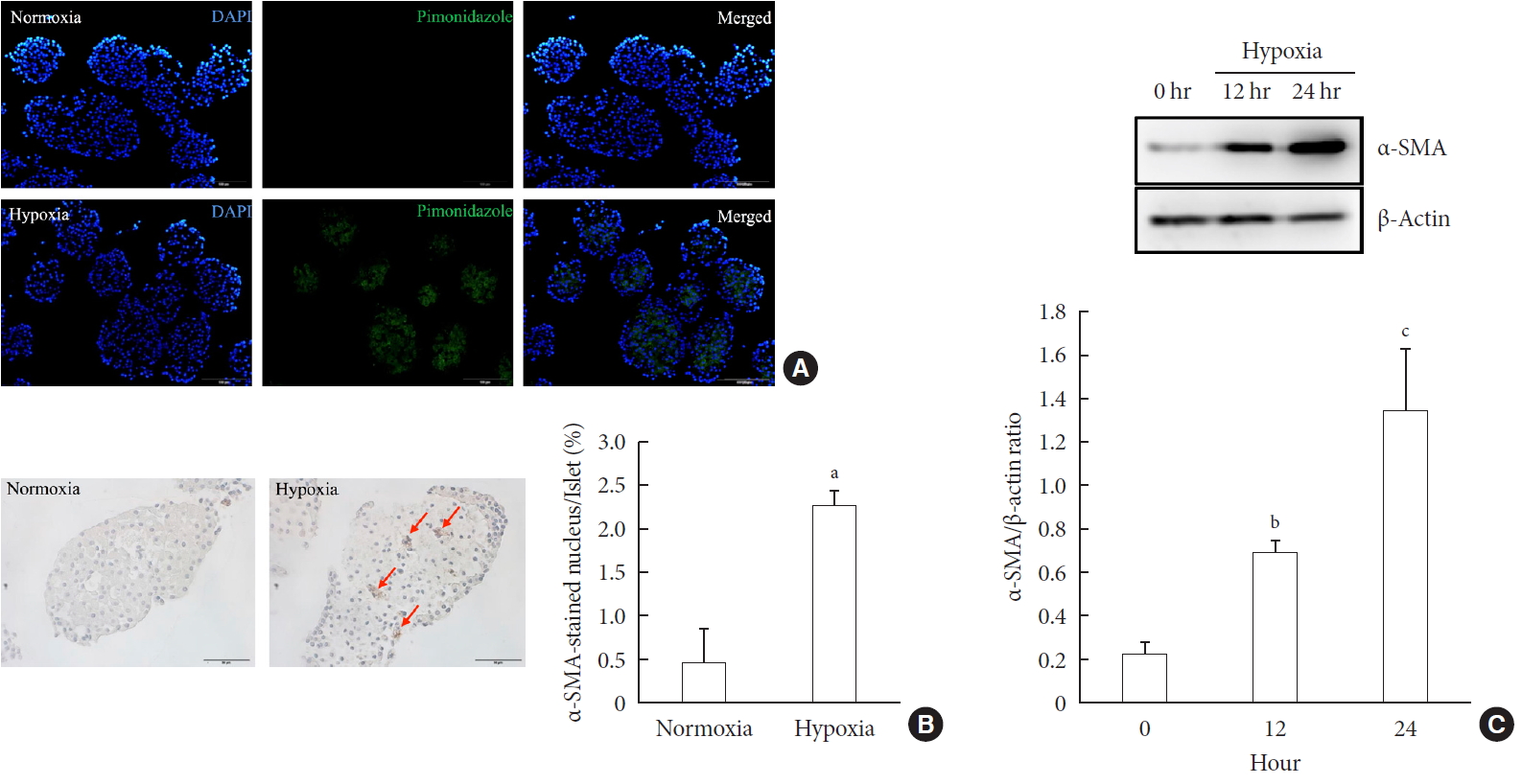
ePub Background Hypoxia can occur in pancreatic islets in type 2 diabetes mellitus. Pancreatic stellate cells (PSCs) are activated during hypoxia. Here we aimed to investigate whether PSCs within the islet are also activated in hypoxia, causing β-cell injury.
Methods Islet and primary PSCs were isolated from Sprague Dawley rats, and cultured in normoxia (21% O2) or hypoxia (1% O2). The expression of α-smooth muscle actin (α-SMA), as measured by immunostaining and Western blotting, was used as a marker of PSC activation. Conditioned media (hypoxia-CM) were obtained from PSCs cultured in hypoxia.
Results Islets and PSCs cultured in hypoxia exhibited higher expressions of α-SMA than did those cultured in normoxia. Hypoxia increased the production of reactive oxygen species. The addition of N-acetyl-L-cysteine, an antioxidant, attenuated the hypoxia-induced PSC activation in islets and PSCs. Islets cultured in hypoxia-CM showed a decrease in cell viability and an increase in apoptosis.
Conclusion PSCs within the islet are activated in hypoxia through oxidative stress and promote islet cell death, suggesting that hypoxia-induced PSC activation may contribute to β-cell loss in type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- Effects of hypoxia in the diabetic corneal stroma microenvironment
Purnima Sharma, Jian-Xing Ma, Dimitrios Karamichos
Experimental Eye Research.2024; 240: 109790. CrossRef - Visualizing hypoxic modulation of beta cell secretions via a sensor augmented oxygen gradient
Kai Duan, Mengyang Zhou, Yong Wang, Jose Oberholzer, Joe F. Lo
Microsystems & Nanoengineering.2023;[Epub] CrossRef - Pancreatic stellate cells promote pancreatic β-cell death through exosomal microRNA transfer in hypoxia
Esder Lee, Gyeong Ryul Ryu, Seung-Hyun Ko, Yu-Bae Ahn, Ki-Ho Song
Molecular and Cellular Endocrinology.2023; 572: 111947. CrossRef - Pancreatic stellate cells in pancreatic cancer: as potential targets for future therapy
Zhengfeng Wang, Ru He, Shi Dong, Wence Zhou
Frontiers in Oncology.2023;[Epub] CrossRef - Recent advances in the development of bioartificial pancreas using 3D bioprinting for the treatment of type 1 diabetes: a review
Anushikha Ghosh, Arka Sanyal, Abhik Mallick
Exploration of Medicine.2023; : 886. CrossRef - Pancreas and islet morphology in cystic fibrosis: clues to the etiology of cystic fibrosis-related diabetes
Sarah S. Malik, Diksha Padmanabhan, Rebecca L. Hull-Meichle
Frontiers in Endocrinology.2023;[Epub] CrossRef - Diabetic mellitus, vascular calcification and hypoxia: A complex and neglected tripartite relationship
Xue-Jiao Sun, Nai-Feng Liu
Cellular Signalling.2022; 91: 110219. CrossRef - HIF-1 and NRF2; Key Molecules for Malignant Phenotypes of Pancreatic Cancer
Shin Hamada, Ryotaro Matsumoto, Atsushi Masamune
Cancers.2022; 14(2): 411. CrossRef - Pancreatic Stellate Cells and Metabolic Alteration: Physiology and Pathophysiology
Shin Hamada, Ryotaro Matsumoto, Atsushi Masamune
Frontiers in Physiology.2022;[Epub] CrossRef - Exosomal miR-140–3p and miR-143–3p from TGF-β1-treated pancreatic stellate cells target BCL2 mRNA to increase β-cell apoptosis
Xiangyun Zhu, Dechen Liu, Guoqing Li, Mengmeng Zhi, Ji Sun, Liang Qi, Jingbo Li, Stephen J. Pandol, Ling Li
Molecular and Cellular Endocrinology.2022; 551: 111653. CrossRef - Mitochondria oxidative stress mediated nicotine-promoted activation of pancreatic stellate cells by regulating mitochondrial dynamics
Yue Yuan, Zhiren Li, Miaomiao Li, Tong Jin, Xiaoyun Zhang, Xinjuan Liu, Jianyu Hao
Toxicology in Vitro.2022; 84: 105436. CrossRef - Antioxidant Mitoquinone Alleviates Chronic Pancreatitis via Anti-Fibrotic and Antioxidant Effects
Miaomiao Li, Yue Yuan, Xue Han, Xinjuan Liu, Weizhen Zhang, Jianyu Hao
Journal of Inflammation Research.2022; Volume 15: 4409. CrossRef - Diabetic Ferroptosis and Pancreatic Cancer: Foe or Friend?
Le Li, Xing-jia Yu, Lei Gao, Long Cheng, Bei Sun, Gang Wang
Antioxidants & Redox Signaling.2022; 37(16-18): 1206. CrossRef - Melatonin Induces Apoptosis and Modulates Cyclin Expression and MAPK Phosphorylation in Pancreatic Stellate Cells Subjected to Hypoxia
Matias Estaras, Manuel R. Gonzalez-Portillo, Miguel Fernandez-Bermejo, Jose M. Mateos, Daniel Vara, Gerardo Blanco-Fernandez, Diego Lopez-Guerra, Vicente Roncero, Gines M. Salido, Antonio González
International Journal of Molecular Sciences.2021; 22(11): 5555. CrossRef - Integrated pancreatic microcirculatory profiles of streptozotocin‐induced and insulin‐administrated type 1 diabetes mellitus
Yuan Li, Bingwei Li, Bing Wang, Mingming Liu, Xiaoyan Zhang, Ailing Li, Jian Zhang, Honggang Zhang, Ruijuan Xiu
Microcirculation.2021;[Epub] CrossRef - Pancreatic stellate cells - rising stars in pancreatic pathologies
P Hrabák, M Kalousová, T Krechler, T Zima
Physiological Research.2021; (S4): S597. CrossRef
- Effects of hypoxia in the diabetic corneal stroma microenvironment
- Basic Research
- The Role of CD36 in Type 2 Diabetes Mellitus: β-Cell Dysfunction and Beyond
- Jun Sung Moon, Udayakumar Karunakaran, Elumalai Suma, Seung Min Chung, Kyu Chang Won
- Diabetes Metab J. 2020;44(2):222-233. Published online April 23, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0053
- 7,509 View
- 168 Download
- 17 Web of Science
- 15 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Impaired β-cell function is the key pathophysiology of type 2 diabetes mellitus, and chronic exposure of nutrient excess could lead to this tragedy. For preserving β-cell function, it is essential to understand the cause and mechanisms about the progression of β-cells failure. Glucotoxicity, lipotoxicity, and glucolipotoxicity have been suggested to be a major cause of β-cell dysfunction for decades, but not yet fully understood. Fatty acid translocase cluster determinant 36 (CD36), which is part of the free fatty acid (FFA) transporter system, has been identified in several tissues such as muscle, liver, and insulin-producing cells. Several studies have reported that induction of CD36 increases uptake of FFA in several cells, suggesting the functional interplay between glucose and FFA in terms of insulin secretion and oxidative metabolism. However, we do not currently know the regulating mechanism and physiological role of CD36 on glucolipotoxicity in pancreatic β-cells. Also, the downstream and upstream targets of CD36 related signaling have not been defined. In the present review, we will focus on the expression and function of CD36 related signaling in the pancreatic β-cells in response to hyperglycemia and hyperlipidemia (ceramide) along with the clinical studies on the association between CD36 and metabolic disorders.
-
Citations
Citations to this article as recorded by- Nrf2 inhibition regulates intracellular lipid accumulation in mouse insulinoma cells and improves insulin secretory function
Alpana Mukhuty, Samanwita Mandal, Chandrani Fouzder, Snehasis Das, Dipanjan Chattopadhyay, Tanmay Majumdar, Rakesh Kundu
Molecular and Cellular Endocrinology.2024; 581: 112112. CrossRef - CD36 gene variant rs1761667(G/A) as a biomarker in obese type 2 diabetes mellitus cases
Ashwin Kumar Shukla, Amreen Shamsad, Atar Singh Kushwah, Shalini Singh, Kauser Usman, Monisha Banerjee
Egyptian Journal of Medical Human Genetics.2024;[Epub] CrossRef - CD36 regulates macrophage and endothelial cell activation and multinucleate giant cell formation in anti neutrophil cytoplasm antibody vasculitis
Xiang Zhang, Catherine King, Alexander Dowell, Paul Moss, Lorraine Harper, Dimitrios Chanouzas, Xiong-zhong Ruan, Alan David Salama
Clinical Immunology.2024; 260: 109914. CrossRef - The association of soluble cluster of differentiation 36 with metabolic diseases: A potential biomarker and therapeutic target
Yun Li, Yaxi Chen, Xiong Z. Ruan
Pediatric Discovery.2023;[Epub] CrossRef - The role of candidate transport proteins in β‐cell long‐chain fatty acid uptake: Where are we now?
Christina Clavelo‐Farrow, Patricia Thomas
Diabetic Medicine.2023;[Epub] CrossRef - SARS-CoV-2 in the pancreas and the impaired islet function in COVID-19 patients
Ningfei Ji, Mingshun Zhang, Liang Ren, Yunyun Wang, Bicheng Hu, Jie Xiang, Yingyun Gong, Chaojie Wu, Guoqiang Qu, Wenqiu Ding, Zhiqiang Yin, Shan Li, Zhengxia Wang, Lianzheng Zhou, Xueqin Chen, Yuan Ma, Jinhai Tang, Yun Liu, Liang Liu, Mao Huang
Emerging Microbes & Infections.2022; 11(1): 1115. CrossRef - Is imaging-based muscle quantity associated with risk of diabetes? A meta-analysis of cohort studies
Shanhu Qiu, Xue Cai, Yang Yuan, Bo Xie, Zilin Sun, Tongzhi Wu
Diabetes Research and Clinical Practice.2022; 189: 109939. CrossRef - Lipotoxicity in a Vicious Cycle of Pancreatic Beta Cell Exhaustion
Vladimir Grubelnik, Jan Zmazek, Matej Završnik, Marko Marhl
Biomedicines.2022; 10(7): 1627. CrossRef - Association of cluster determinant 36, scavenger receptor class B type 1, and major facilitator superfamily domain containing the 2a genetic polymorphism with serum lipid profile in aging population with type 2 diabetes mellitus
Xixiang Wang, Xiaojun Ma, Jingjing Xu, Yujie Guo, Shaobo Zhou, Huiyan Yu, Linhong Yuan
Frontiers in Nutrition.2022;[Epub] CrossRef - CD36-Fatty Acid-Mediated Metastasis via the Bidirectional Interactions of Cancer Cells and Macrophages
Noorzaileen Eileena Zaidi, Nur Aima Hafiza Shazali, Thean-Chor Leow, Mohd Azuraidi Osman, Kamariah Ibrahim, Wan-Hee Cheng, Kok-Song Lai, Nik Mohd Afizan Nik Abd Rahman
Cells.2022; 11(22): 3556. CrossRef - The Past and Present Lives of the Intraocular Transmembrane Protein CD36
Rucui Yang, Qingping Liu, Mingzhi Zhang
Cells.2022; 12(1): 171. CrossRef - Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus
Sachin Kumar, Tapan Behl, Monika Sachdeva, Aayush Sehgal, Shilpa Kumari, Arun Kumar, Gagandeep Kaur, Harlokesh Narayan Yadav, Simona Bungau
Life Sciences.2021; 264: 118661. CrossRef - Contribution of rs3211938 polymorphism at CD36 to glucose levels, oxidized low-density lipoproteins, insulin resistance, and body mass index in Mexican mestizos with type-2 diabetes from western Mexico
Beatriz Teresita Martín-Márquez, Flavio Sandoval-Garcia, Mónica Vazquez-Del Mercado, Erika-Aurora Martínez-García, Fernanda-Isadora Corona-Meraz, Ana-Lilia Fletes-Rayas, Soraya-Amalí Zavaleta-Muñiz
Nutrición Hospitalaria.2021;[Epub] CrossRef - Investigating the association of CD36 gene polymorphisms (rs1761667 and rs1527483) with T2DM and dyslipidemia: Statistical analysis, machine learning based prediction, and meta-analysis
Ma’mon M. Hatmal, Walhan Alshaer, Ismail S. Mahmoud, Mohammad A. I. Al-Hatamleh, Hamzeh J. Al-Ameer, Omar Abuyaman, Malek Zihlif, Rohimah Mohamud, Mais Darras, Mohammad Al Shhab, Rand Abu-Raideh, Hilweh Ismail, Ali Al-Hamadi, Ali Abdelhay, Kanhaiya Singh
PLOS ONE.2021; 16(10): e0257857. CrossRef - Misregulation of Wnt Signaling Pathways at the Plasma Membrane in Brain and Metabolic Diseases
Mustafa Karabicici, Yagmur Azbazdar, Evin Iscan, Gunes Ozhan
Membranes.2021; 11(11): 844. CrossRef
- Nrf2 inhibition regulates intracellular lipid accumulation in mouse insulinoma cells and improves insulin secretory function
- Basic Research
- Notch1 Has an Important Role in β-Cell Mass Determination and Development of Diabetes
- Young Sil Eom, A-Ryeong Gwon, Kyung Min Kwak, Jin-Young Youn, Heekyoung Park, Kwang-Won Kim, Byung-Joon Kim
- Diabetes Metab J. 2021;45(1):86-96. Published online February 26, 2020
- DOI: https://doi.org/10.4093/dmj.2019.0160
- 6,317 View
- 185 Download
- 7 Web of Science
- 6 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
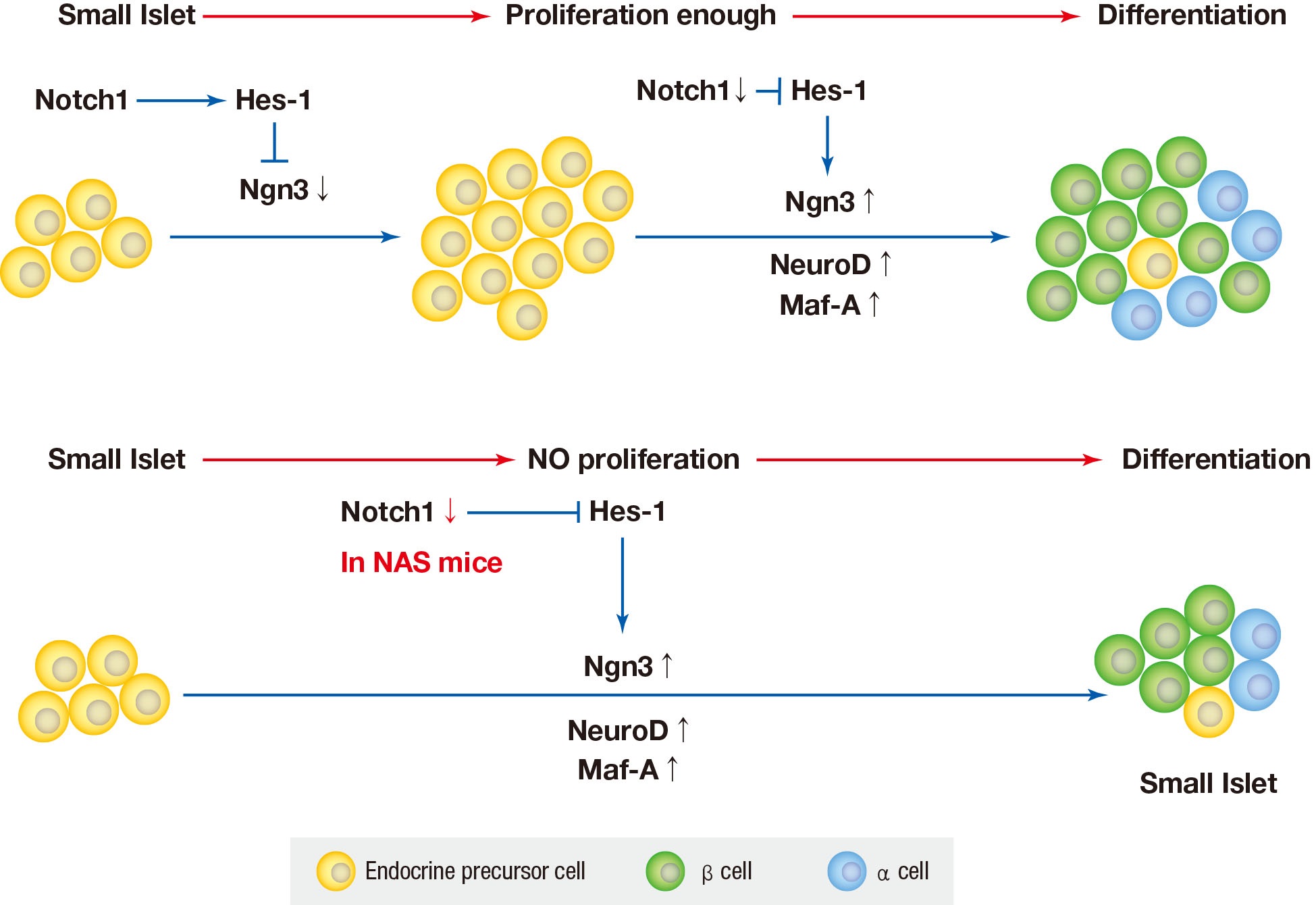
Background Notch signaling pathway plays an important role in regulating pancreatic endocrine and exocrine cell fate during pancreas development. Notch signaling is also expressed in adult pancreas. There are few studies on the effect of Notch on adult pancreas. Here, we investigated the role of Notch in islet mass and glucose homeostasis in adult pancreas using Notch1 antisense transgenic (NAS).
Methods Western blot analysis was performed for the liver of 8-week-old male NAS mice. We also conducted an intraperitoneal glucose tolerance test (IPGTT) and intraperitoneal insulin tolerance test in 8-week-old male NAS mice and male C57BL/6 mice (control). Morphologic observation of pancreatic islet and β-cell was conducted in two groups. Insulin secretion capacity in islets was measured by glucose-stimulated insulin secretion (GSIS) and perifusion.
Results NAS mice showed higher glucose levels and lower insulin secretion in IPGTT than the control mice. There was no significant difference in insulin resistance. Total islet and β-cell masses were decreased in NAS mice. The number of large islets (≥250 µm) decreased while that of small islets (<250 µm) increased. Reduced insulin secretion was observed in GSIS and perifusion. Neurogenin3, neurogenic differentiation, and MAF bZIP transcription factor A levels increased in NAS mice.
Conclusion Our study provides that Notch1 inhibition decreased insulin secretion and decreased islet and β-cell masses. It is thought that Notch1 inhibition suppresses islet proliferation and induces differentiation of small islets. In conclusion, Notch signaling pathway may play an important role in β-cell mass determination and diabetes.
-
Citations
Citations to this article as recorded by- N6-methylation of RNA-bound adenosine regulator HNRNPC promotes vascular endothelial dysfunction in type 2 diabetes mellitus by activating the PSEN1-mediated Notch pathway
Ying Cai, Tao Chen, Mingzhu Wang, Lihua Deng, Cui Li, Siqian Fu, Kangling Xie
Diabetes Research and Clinical Practice.2023; 197: 110261. CrossRef - Single‐cell RNA sequencing: Inhibited Notch2 signalling underlying the increased lens fibre cells differentiation in high myopia
Yunqian Yao, Ling Wei, Zhenhua Chen, Hao Li, Jiao Qi, Qingfeng Wu, Xingtao Zhou, Yi Lu, Xiangjia Zhu
Cell Proliferation.2023;[Epub] CrossRef - Micro ribonucleic acid‐363 regulates the phosphatidylinositol 3‐kinase/threonine protein kinase axis by targeting NOTCH1 and forkhead box C2, leading to hepatic glucose and lipids metabolism disorder in type 2 diabetes mellitus
Yu‐Huan Peng, Ping Wang, Xiao‐Qun He, Ming‐Zhao Hong, Feng Liu
Journal of Diabetes Investigation.2022; 13(2): 236. CrossRef - Soluble T-cadherin promotes pancreatic β-cell proliferation by upregulating Notch signaling
Tomonori Okita, Shunbun Kita, Shiro Fukuda, Keita Fukuoka, Emi Kawada-Horitani, Masahito Iioka, Yuto Nakamura, Yuya Fujishima, Hitoshi Nishizawa, Dan Kawamori, Taka-aki Matsuoka, Maeda Norikazu, Iichiro Shimomura
iScience.2022; 25(11): 105404. CrossRef - Comparison of islet isolation result and clinical applicability according to GMP‐grade collagenase enzyme blend in adult porcine islet isolation and culture
Kyungmin Kwak, Jae‐kyung Park, Joohyun Shim, Nayoung Ko, Hyoung‐Joo Kim, Yongjin Lee, Jun‐Hyeong Kim, Michael Alexander, Jonathan R. T. Lakey, Hyunil Kim, Kimyung Choi
Xenotransplantation.2021;[Epub] CrossRef - Genome-Wide Meta-analysis Identifies Genetic Variants Associated With Glycemic Response to Sulfonylureas
Adem Y. Dawed, Sook Wah Yee, Kaixin Zhou, Nienke van Leeuwen, Yanfei Zhang, Moneeza K. Siddiqui, Amy Etheridge, Federico Innocenti, Fei Xu, Josephine H. Li, Joline W. Beulens, Amber A. van der Heijden, Roderick C. Slieker, Yu-Chuan Chang, Josep M. Mercade
Diabetes Care.2021; 44(12): 2673. CrossRef
- N6-methylation of RNA-bound adenosine regulator HNRNPC promotes vascular endothelial dysfunction in type 2 diabetes mellitus by activating the PSEN1-mediated Notch pathway
- Pathophysiology
- Metformin Ameliorates Lipotoxic β-Cell Dysfunction through a Concentration-Dependent Dual Mechanism of Action
- Hong Il Kim, Ji Seon Lee, Byung Kook Kwak, Won Min Hwang, Min Joo Kim, Young-Bum Kim, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2019;43(6):854-866. Published online June 27, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0179
- 6,651 View
- 115 Download
- 14 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Chronic exposure to elevated levels of free fatty acids contributes to pancreatic β-cell dysfunction. Although it is well known that metformin induces cellular energy depletion and a concomitant activation of AMP-activated protein kinase (AMPK) through inhibition of the respiratory chain, previous studies have shown inconsistent results with regard to the action of metformin on pancreatic β-cells. We therefore examined the effects of metformin on pancreatic β-cells under lipotoxic stress.
Methods NIT-1 cells and mouse islets were exposed to palmitate and treated with 0.05 and 0.5 mM metformin. Cell viability, glucose-stimulated insulin secretion, cellular adenosine triphosphate, reactive oxygen species (ROS) levels and Rho kinase (ROCK) activities were measured. The phosphorylation of AMPK was evaluated by Western blot analysis and mRNA levels of endoplasmic reticulum (ER) stress markers and NADPH oxidase (NOX) were measured by real-time quantitative polymerase chain reaction analysis.
Results We found that metformin has protective effects on palmitate-induced β-cell dysfunction. Metformin at a concentration of 0.05 mM inhibits NOX and suppresses the palmitate-induced elevation of ER stress markers and ROS levels in a AMPK-independent manner, whereas 0.5 mM metformin inhibits ROCK activity and activates AMPK.
Conclusion This study suggests that the action of metformin on β-cell lipotoxicity was implemented by different molecular pathways depending on its concentration. Metformin at a usual therapeutic dose is supposed to alleviate lipotoxic β-cell dysfunction through inhibition of oxidative stress and ER stress.
-
Citations
Citations to this article as recorded by- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
Si-min Zhou, Xin-ming Yao, Yi Cheng, Yu-jie Xing, Yue Sun, Qiang Hua, Shu-jun Wan, Xiang-jian Meng
Heliyon.2024; 10(2): e24432. CrossRef - Reduced Expression Level of Protein PhosphatasePPM1EServes to Maintain Insulin Secretion in Type 2 Diabetes
Sevda Gheibi, Luis Rodrigo Cataldo, Alexander Hamilton, Mi Huang, Sebastian Kalamajski, Malin Fex, Hindrik Mulder
Diabetes.2023; 72(4): 455. CrossRef - Metformin restores prohormone processing enzymes and normalizes aberrations in secretion of proinsulin and insulin in palmitate‐exposed human islets
Quan Wen, Azazul Islam Chowdhury, Banu Aydin, Mudhir Shekha, Rasmus Stenlid, Anders Forslund, Peter Bergsten
Diabetes, Obesity and Metabolism.2023; 25(12): 3757. CrossRef - Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach
Priyamvada Amol Arte, Kanchanlata Tungare, Mustansir Bhori, Renitta Jobby, Jyotirmoi Aich
Human Cell.2023; 37(1): 54. CrossRef - Metformin disrupts insulin secretion, causes proapoptotic and oxidative effects in rat pancreatic beta‐cells in vitro
Maíra M.R. Valle, Eloisa Aparecida Vilas‐Boas, Camila F. Lucena, Simone A. Teixeira, Marcelo N. Muscara, Angelo R. Carpinelli
Journal of Biochemical and Molecular Toxicology.2022;[Epub] CrossRef - Protection by metformin against severe Covid-19: An in-depth mechanistic analysis
Nicolas Wiernsperger, Abdallah Al-Salameh, Bertrand Cariou, Jean-Daniel Lalau
Diabetes & Metabolism.2022; 48(4): 101359. CrossRef - Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis
Lei Wei, Jianjian Shi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Overexpression of miR-297b-5p Promotes Metformin-Mediated Protection Against Stearic Acid-Induced Senescence by Targeting Igf1r
Qingrui Zhao, Shenghan Su, Yuqing Lin, Xuebei Li, Lingfeng Dan, Yunjin Zhang, Chunxiao Yang, Xiaohan Li, Yimeng Dong, Chenchen Geng, Changhao Sun, Xia Chu, Huimin Lu
SSRN Electronic Journal .2022;[Epub] CrossRef - Metformin Dysregulates the Unfolded Protein Response and the WNT/β-Catenin Pathway in Endometrial Cancer Cells through an AMPK-Independent Mechanism
Domenico Conza, Paola Mirra, Gaetano Calì, Luigi Insabato, Francesca Fiory, Francesco Beguinot, Luca Ulianich
Cells.2021; 10(5): 1067. CrossRef - NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction
Suma Elumalai, Udayakumar Karunakaran, Jun-Sung Moon, Kyu-Chang Won
Cells.2021; 10(7): 1573. CrossRef - Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: a nationwide cohort study
Min Ho Kim, Hyung Jung Oh, Soon Hyo Kwon, Jin Seok Jeon, Hyunjin Noh, Dong Cheol Han, Hyoungnae Kim, Dong-Ryeol Ryu
Kidney Research and Clinical Practice.2021; 40(4): 660. CrossRef - Different Effects of Metformin and A769662 on Sodium Iodate-Induced Cytotoxicity in Retinal Pigment Epithelial Cells: Distinct Actions on Mitochondrial Fission and Respiration
Chi-Ming Chan, Ponarulselvam Sekar, Duen-Yi Huang, Shu-Hao Hsu, Wan-Wan Lin
Antioxidants.2020; 9(11): 1057. CrossRef - Metformin Reduces Lipotoxicity-Induced Meta-Inflammation in β-Cells through the Activation of GPR40-PLC-IP3 Pathway
Ximei Shen, Beibei Fan, Xin Hu, Liufen Luo, Yuanli Yan, Liyong Yang
Journal of Diabetes Research.2019; 2019: 1. CrossRef
- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
- Islet Studies and Transplantation
-
- Myricetin Protects Against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5
- Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Jae-Han Jeon, Nam Doo Kim, Keun-Gyu Park, Kyu Chang Won, Jaechan Leem, In-Kyu Lee
- Diabetes Metab J. 2019;43(2):192-205. Published online January 16, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0052
- 4,904 View
- 106 Download
- 33 Web of Science
- 32 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Chronic hyperglycemia has deleterious effects on pancreatic β-cell function and turnover. Recent studies support the view that cyclin-dependent kinase 5 (CDK5) plays a role in β-cell failure under hyperglycemic conditions. However, little is known about how CDK5 impair β-cell function. Myricetin, a natural flavonoid, has therapeutic potential for the treatment of type 2 diabetes mellitus. In this study, we examined the effect of myricetin on high glucose (HG)-induced β-cell apoptosis and explored the relationship between myricetin and CDK5.
Methods To address this question, we subjected INS-1 cells and isolated rat islets to HG conditions (30 mM) in the presence or absence of myricetin. Docking studies were conducted to validate the interaction between myricetin and CDK5. Gene expression and protein levels of endoplasmic reticulum (ER) stress markers were measured by real-time reverse transcription polymerase chain reaction and Western blot analysis.
Results Activation of CDK5 in response to HG coupled with the induction of ER stress via the down regulation of sarcoendoplasmic reticulum calcium ATPase 2b (
SERCA2b ) gene expression and reduced the nuclear accumulation of pancreatic duodenal homeobox 1 (PDX1) leads to β-cell apoptosis. Docking study predicts that myricetin inhibit CDK5 activation by direct binding in the ATP-binding pocket. Myricetin counteracted the decrease in the levels of PDX1 and SERCA2b by HG. Moreover, myricetin attenuated HG-induced apoptosis in INS-1 cells and rat islets and reduce the mitochondrial dysfunction by decreasing reactive oxygen species production and mitochondrial membrane potential (Δψm) loss.Conclusion Myricetin protects the β-cells against HG-induced apoptosis by inhibiting ER stress, possibly through inactivation of CDK5 and consequent upregulation of PDX1 and SERCA2b.
-
Citations
Citations to this article as recorded by- Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches
Nazmun Nahar, Md. Nazmul Hasan Zilani, Partha Biswas, Md. Morsaline Billah, Shabana Bibi, Norah A. Albekairi, Abdulrahman Alshammari, Md. Nazmul Hasan
Saudi Pharmaceutical Journal.2024; 32(1): 101887. CrossRef - Mitochondrial aldehyde dehydrogenase-2 coordinates the hydrogen sulfide - AMPK axis to attenuate high glucose-induced pancreatic β-cell dysfunction by glutathione antioxidant system
Udayakumar Karunakaran, Suma Elumalai, Seung Min Chung, Kathrin Maedler, Kyu Chang Won, Jun Sung Moon
Redox Biology.2024; 69: 102994. CrossRef - Network-based identification and mechanism exploration of active ingredients against Alzheimer’s disease via targeting endoplasmic reticulum stress from traditional chinese medicine
Zhao Dai, Tian Hu, Junwen Wei, Xue Wang, Chuipu Cai, Yong Gu, Yunhui Hu, Wenjia Wang, Qihui Wu, Jiansong Fang
Computational and Structural Biotechnology Journal.2024; 23: 506. CrossRef - Myricetin as a Promising Flavonoid with Multitargeted Biological Activity
A.S. Chiriapkin
Juvenis Scientia.2024; 10(1): 5. CrossRef - Naturally occurring small molecules with dual effect upon inflammatory signaling pathways and endoplasmic reticulum stress response
Daniela Correia da Silva, Patrícia Valentão, David M. Pereira
Journal of Physiology and Biochemistry.2024;[Epub] CrossRef - Omnifarious fruit polyphenols: an omnipotent strategy to prevent and intervene diabetes and related complication?
Yao Chen, Xuejiao Qie, Wei Quan, Maomao Zeng, Fang Qin, Jie Chen, Benu Adhikari, Zhiyong He
Critical Reviews in Food Science and Nutrition.2023; 63(20): 4288. CrossRef - Regulation of reactive oxygen species by phytochemicals for the management of cancer and diabetes
Heui Min Lim, See-Hyoung Park
Critical Reviews in Food Science and Nutrition.2023; 63(22): 5911. CrossRef - Bioactive compounds from Polygonatum genus as anti-diabetic agents with future perspectives
Yan Shi, Dun Si, Donghong Chen, Xinfeng Zhang, Zhigang Han, Qiang Yu, Jingjing Liu, Jinping Si
Food Chemistry.2023; 408: 135183. CrossRef - Venom Peptides, Polyphenols and Alkaloids: Are They the Next Antidiabetics That Will Preserve β-Cell Mass and Function in Type 2 Diabetes?
Michele Lodato, Valérie Plaisance, Valérie Pawlowski, Maxime Kwapich, Alexandre Barras, Emeline Buissart, Stéphane Dalle, Sabine Szunerits, Jérôme Vicogne, Rabah Boukherroub, Amar Abderrahmani
Cells.2023; 12(6): 940. CrossRef - TFP5 attenuates cyclin‐dependent kinase 5‐mediated islet β‐cell damage in diabetes
Shunyao Liu, Bo Li, Danna Ma, Yuejia Tao, Jiang Song, Li Bao, Guoqing Zhang, Hongyan Luo, Shilu Cao, Jing E, Yali Zheng
Chemical Biology & Drug Design.2023; 102(1): 76. CrossRef - Antiviral and Possible Prophylactic Significance of Myricetin for COVID-19
Pawan K. Agrawal, Chandan Agrawal, Gerald Blunden
Natural Product Communications.2023; 18(4): 1934578X2311662. CrossRef - In Vitro and In Silico Protocols for the Assessment of Anti-Tick Compounds from Pinus roxburghii against Rhipicephalus (Boophilus) microplus Ticks
Sana Ayub, Nosheen Malak, Raquel Cossío-Bayúgar, Nasreen Nasreen, Afshan Khan, Sadaf Niaz, Adil Khan, Abdallah D. Alanazi, Mourad Ben Said
Animals.2023; 13(8): 1388. CrossRef - Protective Effect of Myricetin Against Experimentally Induced Torsion in Rats
M. Tatar, Z. Polat, J. Öner, H. Öner
Biology Bulletin.2023; 50(6): 1338. CrossRef - The pharmacological mechanism of Abelmoschus manihot in the treatment of chronic kidney disease
Cuiting Wei, Chao Wang, Run Li, Yunfeng Bai, Xue Wang, Qingyun Fang, Xiangmei Chen, Ping Li
Heliyon.2023; 9(11): e22017. CrossRef - Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases
Jana Viskupicova, Petronela Rezbarikova
Molecules.2022; 27(16): 5095. CrossRef - Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus
SuFang You, JingYi Zheng, YuPing Chen, HuiBin Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Myricetin inhibits pseudorabies virus infection through direct inactivation and activating host antiviral defense
Huaiyue Hu, Zhiqiang Hu, Yingying Zhang, Hongping Wan, Zhongqiong Yin, Lixia Li, Xiaoxia Liang, Xinghong Zhao, Lizi Yin, Gang Ye, Yuan-Feng Zou, Huaqiao Tang, Renyong Jia, Yaqin Chen, Hao Zhou, Xu Song
Frontiers in Microbiology.2022;[Epub] CrossRef - Effects of myricetin against cadmium-induced neurotoxicity in PC12 cells
Azadeh Aminzadeh, Ayda Salarinejad
Toxicology Research.2021; 10(1): 84. CrossRef - Pioglitazone-induced AMPK-Glutaminase-1 prevents high glucose-induced pancreatic β-cell dysfunction by glutathione antioxidant system
Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Redox Biology.2021; 45: 102029. CrossRef - Chlorogenic acid and β-glucan from highland barley grain ameliorate β-cell dysfunction via inhibiting apoptosis and improving cell proliferation
Ze-Hua Liu, Bo Li
Food & Function.2021; 12(20): 10040. CrossRef - The cyclin dependent kinase inhibitor Roscovitine prevents diet-induced metabolic disruption in obese mice
Nabil Rabhi, Kathleen Desevin, Briana Noel Cortez, Ryan Hekman, Jean Z. Lin, Andrew Emili, Stephen R. Farmer
Scientific Reports.2021;[Epub] CrossRef - AdipoRon promotes diabetic fracture repair through endochondral ossification-based bone repair by enhancing survival and differentiation of chondrocytes
Zhongyi Wang, Jinxin Tang, Ying Li, Yu Wang, Yanyang Guo, Qisheng Tu, Jake Chen, Chen Wang
Experimental Cell Research.2020; 387(2): 111757. CrossRef - A kinase of many talents: non-neuronal functions of CDK5 in development and disease
Samanta Sharma, Piotr Sicinski
Open Biology.2020; 10(1): 190287. CrossRef - Mitochondrial dysfunction in the fetoplacental unit in gestational diabetes mellitus
Luis Sobrevia, Paola Valero, Adriana Grismaldo, Roberto Villalobos-Labra, Fabián Pardo, Mario Subiabre, Gael Armstrong, Fernando Toledo, Sofía Vega, Marcelo Cornejo, Gonzalo Fuentes, Reinaldo Marín
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2020; 1866(12): 165948. CrossRef - Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications
Yasaman Taheri, Hafiz Ansar Rasul Suleria, Natália Martins, Oksana Sytar, Ahmet Beyatli, Balakyz Yeskaliyeva, Gulnaz Seitimova, Bahare Salehi, Prabhakar Semwal, Sakshi Painuli, Anuj Kumar, Elena Azzini, Miquel Martorell, William N. Setzer, Alfred Maroyi,
BMC Complementary Medicine and Therapies.2020;[Epub] CrossRef - Current Pharmacological Trends on Myricetin
Gudiya Gupta, Mohd Aftab Siddiqui, Mohd Muazzam Khan, Mohd Ajmal, Rabiya Ahsan, Md Azizur Rahaman, Md Afroz Ahmad, Md Arshad, Mohammad Khushtar
Drug Research.2020;[Epub] CrossRef - Silencing cyclophilin A improves insulin secretion, reduces cell apoptosis, and alleviates inflammation as well as oxidant stress in high glucose-induced pancreatic β-cells via MAPK/NF-kb signaling pathway
Tangying Li, Huibiao Quan, Huachuan Zhang, Leweihua Lin, Qianying Ou, Kaining Chen
Bioengineered.2020; 11(1): 1047. CrossRef - Endoplasmic reticulum stress contributes to NMDA-induced pancreatic β-cell dysfunction in a CHOP-dependent manner
Xiao-Ting Huang, Wei Liu, Yong Zhou, Mei Sun, Chen-Chen Sun, Chen-Yu Zhang, Si-Yuan Tang
Life Sciences.2019; 232: 116612. CrossRef - Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death
Ryo Shibusawa, Eijiro Yamada, Shuichi Okada, Yasuyo Nakajima, Claire C. Bastie, Akito Maeshima, Kyoichi Kaira, Masanobu Yamada
Scientific Reports.2019;[Epub] CrossRef - Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic β-cell dysfunction
Udayakumar Karunakaran, Ji Eun Lee, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Free Radical Biology and Medicine.2019; 141: 59. CrossRef - CDK5: Key Regulator of Apoptosis and Cell Survival
Rabih Roufayel, Nimer Murshid
Biomedicines.2019; 7(4): 88. CrossRef - Oral DhHP-6 for the Treatment of Type 2 Diabetes Mellitus
Kai Wang, Yu Su, Yuting Liang, Yanhui Song, Liping Wang
International Journal of Molecular Sciences.2019; 20(6): 1517. CrossRef
- Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches
- Clinical Diabetes & Therapeutics
- Latent Autoimmune Diabetes in Adults: A Review on Clinical Implications and Management
- Silvia Pieralice, Paolo Pozzilli
- Diabetes Metab J. 2018;42(6):451-464. Published online December 17, 2018
- DOI: https://doi.org/10.4093/dmj.2018.0190
- 12,888 View
- 499 Download
- 51 Web of Science
- 60 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Latent autoimmune diabetes in adults (LADA) is a heterogeneous disease characterized by a less intensive autoimmune process and a broad clinical phenotype compared to classical type 1 diabetes mellitus (T1DM), sharing features with both type 2 diabetes mellitus (T2DM) and T1DM. Since patients affected by LADA are initially insulin independent and recognizable only by testing for islet-cell autoantibodies, it could be difficult to identify LADA in clinical setting and a high misdiagnosis rate still remains among patients with T2DM. Ideally, islet-cell autoantibodies screening should be performed in subjects with newly diagnosed T2DM, ensuring a closer monitoring of those resulted positive and avoiding treatment of hyperglycaemia which might increase the rate of β-cells loss. Thus, since the autoimmune process in LADA seems to be slower than in classical T1DM, there is a wider window for new therapeutic interventions that may slow down β-cell failure. This review summarizes the current understanding of LADA, by evaluating data from most recent studies, the actual gaps in diagnosis and management. Finally, we critically highlight and discuss novel findings and future perspectives on the therapeutic approach in LADA.
-
Citations
Citations to this article as recorded by- A Comprehensive Survey on Diabetes Type-2 (T2D) Forecast Using Machine Learning
Satyanarayana Murthy nimmagadda, Gunnam Suryanarayana, Gangu Bharath Kumar, Ganta Anudeep, Gedela Vinay Sai
Archives of Computational Methods in Engineering.2024;[Epub] CrossRef - Ensemble machine learning reveals key features for diabetes duration from electronic health records
Gabriel Cerono, Davide Chicco
PeerJ Computer Science.2024; 10: e1896. CrossRef - LADA 30th anniversary: A growing form of diabetes with persistent unresolved questions
Ivy Lee Jia Jia, Raffaella Buzzetti, Richard David Leslie, Paolo Pozzilli
Diabetes/Metabolism Research and Reviews.2024;[Epub] CrossRef - A Curious Case of New-Onset Diabetes
Kristina Hernandez, Charity L. Tan
The Journal for Nurse Practitioners.2023; 19(3): 104411. CrossRef - Comprehensive review: Frailty in pancreas transplant candidates and recipients
Ronald F. Parsons, Ekamol Tantisattamo, Wisit Cheungpasitporn, Arpita Basu, Yee Lu, Krista L. Lentine, Kenneth J. Woodside, Neeraj Singh, Joseph Scalea, Tarek Alhamad, Ty B. Dunn, Franco H. Cabeza Rivera, Sandesh Parajuli, Martha Pavlakis, Matthew Cooper
Clinical Transplantation.2023;[Epub] CrossRef - Managing latent autoimmune disease in adults
David Morris
Independent Nurse.2023; 2023(1): 18. CrossRef - Development and validation of a clinical score for identifying patients with high risk of latent autoimmune adult diabetes (LADA): The LADA primary care-protocol study
Pilar Vich-Pérez, Juan Carlos Abánades-Herranz, Gustavo Mora-Navarro, Ángela María Carrasco-Sayalero, Miguel Ángel Salinero-Fort, Ignacio Sevilla-Machuca, Mar Sanz-Pascual, Cristina Álvarez Hernández-Cañizares, Carmen de Burgos-Lunar, Vijayaprakash Suppia
PLOS ONE.2023; 18(2): e0281657. CrossRef - Diagnostic Dilemmas and Current Treatment Approaches in Latent Onset
Autoimmune Diabetes in Adults: A Concise Review
Lakshmi Chandran, Ankul Singh S., Chitra Vellapandian
Current Diabetes Reviews.2023;[Epub] CrossRef - Anti-glutamic Acid Decarboxylase Antibody-Positive Gestational Diabetes Mellitus with Autoimmune Type 1 Diabetes Mellitus in the Early Postpartum Period: A Case Report and Literature Review
Akiko Fujishima, Yohei Onodera, Hiroshi Miura, Yukihiro Terada
The Tohoku Journal of Experimental Medicine.2023; 259(4): 327. CrossRef - Een 54-jarige man met onverklaard gewichtsverlies
N. Mutebi, A. Kharagjitsing
Tijdschrift voor Geneeskunde.2023;[Epub] CrossRef - Effects of Bacillus Calmette-Guérin on immunometabolism, microbiome and liver diseases
Muhammad Umair Ijaz, Farzam Vaziri, Yu-Jui Yvonne Wan
Liver Research.2023; 7(2): 116. CrossRef - The Etiological Diagnosis of Diabetes: Still a Challenge for the Clinician
Danièle Dubois-Laforgue, José Timsit
Endocrines.2023; 4(2): 437. CrossRef - Type 1 diabetes mellitus: features of differential diagnosis
EV Gantsgorn, OV Denisenko, YaO Osipenko, DA Kalmykova, AV Ivanov, SS Gerasyuta, GA Bulguryan, MH Ivanova, DA Saakyan
Bulletin of Russian State Medical University.2023;[Epub] CrossRef - A 1‐year pilot study of intralymphatic injections of GAD‐alum in individuals with latent autoimmune diabetes in adults (LADA) with signs of high immunity: No safety concerns and resemblance to juvenile type 1 diabetes
Ingrid K. Hals, Chandima Balasuriya, Rosaura Casas, Johnny Ludvigsson, Anneli Björklund, Valdemar Grill
Diabetes, Obesity and Metabolism.2023; 25(11): 3400. CrossRef - The relationship between red blood cell distribution width and islet β-cell function indexes in patients with latent autoimmune diabetes in adults
Xiuli Fu, Qin Tan, Wei Wei, Sheng Ding, Zhongjing Wang
BMC Endocrine Disorders.2023;[Epub] CrossRef - A Review on Latent Autoimmune Diabetes in Adults
Vijay Ravikumar, Ariba Ahmed, Ashish Anjankar
Cureus.2023;[Epub] CrossRef - Neutrophils and their role in the aetiopathogenesis of type 1 and type 2 diabetes
Anna Giovenzana, Debora Carnovale, Brett Phillips, Alessandra Petrelli, Nick Giannoukakis
Diabetes/Metabolism Research and Reviews.2022;[Epub] CrossRef - Prevalence of Latent Autoimmune Diabetes in Adult Based on the Presence of GAD 65 Antibodies in North-Eastern Uttar Pradesh, India
Himalina Sangma, Anshul Singh, Anubha Srivastava, Vatsala Misra
Annals of the National Academy of Medical Sciences (India).2022; 58(01): 017. CrossRef - Serum Bile Acid Profiles in Latent Autoimmune Diabetes in Adults and Type 2 Diabetes Patients
Yu Zhou, Deli Ye, Xiaofen Yuan, Yonglie Zhou, Jun Xia, Giuseppe Pugliese
Journal of Diabetes Research.2022; 2022: 1. CrossRef - Early life Bacillus Calmette-Guerin vaccination and incidence of type 1, type 2, and latent autoimmune diabetes in adulthood
Philippe Corsenac, Marie-Élise Parent, Hélène Mansaray, Andrea Benedetti, Hugues Richard, Simona Stäger, Marie-Claude Rousseau
Diabetes & Metabolism.2022; 48(3): 101337. CrossRef - Atypical Diabetes and Management Considerations
Shivajirao Prakash Patil
Primary Care: Clinics in Office Practice.2022; 49(2): 225. CrossRef - Efficacy and safety of sitagliptin and insulin for latent autoimmune diabetes in adults: A systematic review and meta‐analysis
Tong Lin, Yinhe Cai, Liting Tang, Youwei Lian, Min Liu, Chaonan Liu
Journal of Diabetes Investigation.2022; 13(9): 1506. CrossRef - Latent autoimmune diabetes in youth shows greater autoimmunity than latent autoimmune diabetes in adults: Evidence from a nationwide, multicenter, cross‐sectional study
Jin Cheng, Xiaohan Tang, Xiang Yan, Gan Huang, Shuoming Luo, Zhiguang Zhou, Xia Li
Pediatric Diabetes.2022; 23(5): 578. CrossRef - Latent Autoimmune Diabetes in Adults and Metabolic Syndrome—A Mini Review
Niansi Pan, Shimei Yang, Xiaohong Niu
Frontiers in Endocrinology.2022;[Epub] CrossRef - Lifestyle or Environmental Influences and Their Interaction With Genetic Susceptibility on the Risk of LADA
Sofia Carlsson
Frontiers in Endocrinology.2022;[Epub] CrossRef - Antigen-specific immunotherapies in type 1 diabetes
Xuejiao Zhang, Ying Dong, Dianyuan Liu, Liu Yang, Jiayi Xu, Qing Wang
Journal of Trace Elements in Medicine and Biology.2022; 73: 127040. CrossRef - Latent autoimmune diabetes in adults: a focus on β-cell protection and therapy
Wenfeng Yin, Shuoming Luo, Zilin Xiao, Ziwei Zhang, Bingwen Liu, Zhiguang Zhou
Frontiers in Endocrinology.2022;[Epub] CrossRef - Diagnostic camouflage: A case report on Latent autoimmune diabetics of adulthood
Sandhya kiran Neupane, Prakash Paudel Jaishi, Divyaa Koirala, Arjun Kandel, Prabhat Kiran Neupane
Annals of Medicine & Surgery.2022;[Epub] CrossRef - Clinical characteristics and cardiovascular risk profile in children and adolescents with latent autoimmune diabetes: Results from the German/Austrian prospective diabetes follow‐up registry
Alena Welters, Sascha R. Tittel, Thomas Reinehr, Daniel Weghuber, Susanna Wiegand, Wolfram Karges, Clemens Freiberg, Thomas Meissner, Nanette C. Schloot, Reinhard W. Holl
Pediatric Diabetes.2022; 23(8): 1602. CrossRef - The utility of assessing C-peptide in patients with insulin-treated type 2 diabetes: a cross-sectional study
Tuccinardi Dario, Giorgino Riccardo, Pieralice Silvia, Watanabe Mikiko, Maggi Daria, Palermo Andrea, Defeudis Giuseppe, Fioriti Elvira, Pozzilli Paolo, Manfrini Silvia
Acta Diabetologica.2021; 58(4): 411. CrossRef - Cross-reactive peptide epitopes of Enterovirus Coxsackie B4 and human glutamic acid decarboxylase detecting antibodies in latent autoimmune diabetes in adults versus type 1 diabetes
Feliciana Real-Fernández, Alessandra Gallo, Francesca Nuti, Lorenzo Altamore, Gloria Giovanna Del Vescovo, Pietro Traldi, Eugenio Ragazzi, Paolo Rovero, Annunziata Lapolla, Anna Maria Papini
Clinica Chimica Acta.2021; 515: 73. CrossRef - Treating latent autoimmune diabetes in adults in the era of cardiovascular outcomes trials: Old dog should learn new tricks
Theocharis Koufakis, Prashanth Vas, Kalliopi Kotsa
Diabetic Medicine.2021;[Epub] CrossRef - Defining and Classifying New Subgroups of Diabetes
Ashok Balasubramanyam
Annual Review of Medicine.2021; 72(1): 63. CrossRef - Slowly evolving, immune-mediated diabetes in 14-year-old patient: a case report
M. R. Ragimov, D. D. Omelchuk, L. I. Ibragimova, O. S. Derevyanko, T. V. Nikonova
Diabetes mellitus.2021; 24(1): 70. CrossRef - The role of autoimmunity in the pathophysiology of type 2 diabetes: Looking at the other side of the moon
Theocharis Koufakis, George Dimitriadis, Symeon Metallidis, Pantelis Zebekakis, Kalliopi Kotsa
Obesity Reviews.2021;[Epub] CrossRef - Dapagliflozin as an Adjunct Therapy to Insulin in Patients with Type 1 Diabetes Mellitus: Efficacy and Safety of this Combination
Johan H Jendle, Francisco J Ampudia-Blasco, Martin Füchtenbusch, Paolo Pozzilli
European Endocrinology.2021; 1(1): 12. CrossRef - Dapagliflozin as an Adjunct Therapy to Insulin in Patients with Type 1 Diabetes Mellitus: Efficacy and Safety of this Combination
Johan H Jendle, Francisco J Ampudia-Blasco, Martin Füchtenbusch, Paolo Pozzilli
touchREVIEWS in Endocrinology.2021; 17(1): 12. CrossRef - Toward an Improved Classification of Type 2 Diabetes: Lessons From Research into the Heterogeneity of a Complex Disease
Maria J Redondo, Ashok Balasubramanyam
The Journal of Clinical Endocrinology & Metabolism.2021; 106(12): e4822. CrossRef - Bacillus Calmette-Guerin 's beneficial impact on glucose metabolism: Evidence for broad based applications
Gabriella F. Shpilsky, Hiroyuki Takahashi, Anna Aristarkhova, Michele Weil, Nathan Ng, Kacie J. Nelson, Amanda Lee, Hui Zheng, Willem M. Kühtreiber, Denise L. Faustman
iScience.2021; 24(10): 103150. CrossRef - Masqueraders: how to identify atypical diabetes in primary care
Sumera Ahmed, Sana Saeed, Jay H. Shubrook
Journal of Osteopathic Medicine.2021; 121(12): 899. CrossRef - Lada or Type 2 Diabetes Mellitus - A Challenging Diagnosis in Clinical Approach
Lucia Mihaela Custură, Oana Deteşan, Raluca Maria Tilinca, Reka Annamaria Schmiedt, Brigitta Irén Bacso, Mariana Cornelia Tilinca
Acta Medica Transilvanica.2021; 26(3): 55. CrossRef - Antioxidant, Anti-Inflammatory, and Immunomodulatory Properties of Tea—The Positive Impact of Tea Consumption on Patients with Autoimmune Diabetes
Anna Winiarska-Mieczan, Ewa Tomaszewska, Karolina Jachimowicz
Nutrients.2021; 13(11): 3972. CrossRef - A Review of Statistical and Machine Learning Techniques for Microvascular Complications in Type 2 Diabetes
Nitigya Sambyal, Poonam Saini, Rupali Syal
Current Diabetes Reviews.2021; 17(2): 143. CrossRef - Bariatric Surgery Outcomes in Patients with Latent Autoimmune Diabetes of the Adult
Marta Guimarães, Sofia S. Pereira, Mário Nora, Mariana P. Monteiro
Obesity Facts.2021; 14(4): 425. CrossRef - Особливості перебігу діабетичної хвороби нирок у хворих на латентний автоімунний діабет дорослих
I.O. Tsaryk, N.V. Pashkovska
INTERNATIONAL JOURNAL OF ENDOCRINOLOGY (Ukraine).2021; 17(2): 116. CrossRef - Latenter Autoimmundiabetes im Erwachsenen- und Kindesalter
Alena Welters, Nanette C. Schloot
Der Diabetologe.2020; 16(1): 27. CrossRef - A treatment-based algorithm for identification of diabetes type in the National Health and Nutrition Examination Survey
Mitra Mosslemi, Hannah L. Park, Christine E. McLaren, Nathan D. Wong
Cardiovascular Endocrinology & Metabolism.2020; 9(1): 9. CrossRef - Case 6-2020: A 34-Year-Old Woman with Hyperglycemia
Richard C. Cabot, Eric S. Rosenberg, Virginia M. Pierce, David M. Dudzinski, Meridale V. Baggett, Dennis C. Sgroi, Jo-Anne O. Shepard, Kathy M. Tran, Emily K. McDonald, Tara Corpuz, Miriam S. Udler, Camille E. Powe, Christina A. Austin-Tse
New England Journal of Medicine.2020; 382(8): 745. CrossRef - Therapeutic approaches for latent autoimmune diabetes in adults: One size does not fit all
Theocharis Koufakis, Niki Katsiki, Pantelis Zebekakis, George Dimitriadis, Kalliopi Kotsa
Journal of Diabetes.2020; 12(2): 110. CrossRef - Grenzformen zwischen Typ-1- und Typ-2-Diabetes
Michael Hummel
MMW - Fortschritte der Medizin.2020; 162(11): 60. CrossRef - The Differential Expression of Long Noncoding RNAs in Type 2 Diabetes Mellitus and Latent Autoimmune Diabetes in Adults
Zhang Pengyu, Yan Yan, Fu Xiying, Yang Maoguang, Li Mo, Cheng Yan, Shen Hong, Wang Lijuan, Zhang Xiujuan, Cai Hanqing
International Journal of Endocrinology.2020; 2020: 1. CrossRef - A convenient diagnostic tool for discriminating adult-onset glutamic acid decarboxylase antibody-positive autoimmune diabetes from type 2 diabetes: a retrospective study
Hon-Ke Sia, Shih-Te Tu, Pei-Yung Liao, Kuan-Han Lin, Chew-Teng Kor, Ling-Ling Yeh
PeerJ.2020; 8: e8610. CrossRef - Correct Diabetes Diagnosis and Treatment Allows Sailor to Remain on Active Duty
Andrea R Frazier
Military Medicine.2020; 185(9-10): e1843. CrossRef - Hombre de 28 años con presentación de diabetes
M. Ricci, J. Sanz Cánovas, V. Buonaluto, R. Gómez Huelgas
Medicine - Programa de Formación Médica Continuada Acreditado.2020; 13(16): 942.e1. CrossRef - Uncommon Presentations of Diabetes: Zebras in the Herd
Karen L. Shidler, Lisa R. Letourneau, Lucia M. Novak
Clinical Diabetes.2020; 38(1): 78. CrossRef - Ketogenic Diet as a Trigger for Diabetic Ketoacidosis in a Misdiagnosis of Diabetes: A Case Report
Alexander J. White-Cotsmire, Amber M. Healy
Clinical Diabetes.2020; 38(3): 318. CrossRef - Subphänotypen des Diabetes
Oana Patricia Zaharia, Julia Szendrödi
Der Diabetologe.2019; 15(4): 288. CrossRef - Autoimmunity in latent autoimmune diabetes in adults
Alessandro P. Delitala
AIMS Medical Science.2019; 6(2): 132. CrossRef - Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study
Oana P Zaharia, Klaus Strassburger, Alexander Strom, Gidon J Bönhof, Yanislava Karusheva, Sofia Antoniou, Kálmán Bódis, Daniel F Markgraf, Volker Burkart, Karsten Müssig, Jong-Hee Hwang, Olof Asplund, Leif Groop, Emma Ahlqvist, Jochen Seissler, Peter Nawr
The Lancet Diabetes & Endocrinology.2019; 7(9): 684. CrossRef - The Influence of Type 2 Diabetes–Associated Factors on Type 1 Diabetes
Maria J. Redondo, Carmella Evans-Molina, Andrea K. Steck, Mark A. Atkinson, Jay Sosenko
Diabetes Care.2019; 42(8): 1357. CrossRef
- A Comprehensive Survey on Diabetes Type-2 (T2D) Forecast Using Machine Learning
- Obesity and Metabolic Syndrome
In Vitro Effect of Fatty Acids Identified in the Plasma of Obese Adolescents on the Function of Pancreatic β-Cells- Claudia Velasquez, Juan Sebastian Vasquez, Norman Balcazar
- Diabetes Metab J. 2017;41(4):303-315. Published online May 24, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.4.303
- 3,768 View
- 38 Download
- 8 Web of Science
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The increase in circulating free fatty acid (FFA) levels is a major factor that induces malfunction in pancreatic β-cells. We evaluated the effect of FFAs reconstituted according to the profile of circulating fatty acids found in obese adolescents on the viability and function of the murine insulinoma cell line (mouse insulinoma [MIN6]).
Methods From fatty acids obtained commercially, plasma-FFA profiles of three different youth populations were reconstituted: obese with metabolic syndrome; obese without metabolic syndrome; and normal weight without metabolic syndrome. MIN6 cells were treated for 24 or 48 hours with the three FFA profiles, and glucose-stimulated insulin secretion, cell viability, mitochondrial function and antioxidant activity were evaluated.
Results The high FFA content and high polyunsaturated ω6/ω3 ratio, present in plasma of obese adolescents with metabolic syndrome had a toxic effect on MIN6 cell viability and function, increasing oxidative stress and decreasing glucose-dependent insulin secretion.
Conclusion These results could help to guide nutritional management of obese young individuals, encouraging the increase of ω-3-rich food consumption in order to reduce the likelihood of deterioration of β-cells and the possible development of type 2 diabetes mellitus.
-
Citations
Citations to this article as recorded by- The reversible effects of free fatty acids on sulfonylurea-stimulated insulin secretion are related to the expression and dynamin-mediated endocytosis of KATP channels in pancreatic β cells
Chenmin Wei, Zichen Zhang, Qi Fu, Yunqiang He, Tao Yang, Min Sun
Endocrine Connections.2023;[Epub] CrossRef - Preparation of fatty acid solutions exerts significant impact on experimental outcomes in cell culture models of lipotoxicity
Axel Römer, Divya Rawat, Thomas Linn, Sebastian F Petry
Biology Methods and Protocols.2022;[Epub] CrossRef - Lipotoxic Impairment of Mitochondrial Function in β-Cells: A Review
Axel Römer, Thomas Linn, Sebastian F. Petry
Antioxidants.2021; 10(2): 293. CrossRef - Contribution of Oxidative Stress and Impaired Biogenesis of Pancreatic β-Cells to Type 2 Diabetes
Petr Ježek, Martin Jabůrek, Lydie Plecitá-Hlavatá
Antioxidants & Redox Signaling.2019; 31(10): 722. CrossRef - The role of polyunsaturated fatty acids (n-3 PUFAs) on the pancreatic β-cells and insulin action
Habtamu Wondifraw Baynes, Seifu Mideksa, Sintayehu Ambachew
Adipocyte.2018; : 1. CrossRef - Fatty Acid-Stimulated Insulin Secretion vs. Lipotoxicity
Petr Ježek, Martin Jabůrek, Blanka Holendová, Lydie Plecitá-Hlavatá
Molecules.2018; 23(6): 1483. CrossRef
- The reversible effects of free fatty acids on sulfonylurea-stimulated insulin secretion are related to the expression and dynamin-mediated endocytosis of KATP channels in pancreatic β cells
- Pancreatic α-Cell Dysfunction in Type 2 Diabetes: Old Kids on the Block
- Jun Sung Moon, Kyu Chang Won
- Diabetes Metab J. 2015;39(1):1-9. Published online February 16, 2015
- DOI: https://doi.org/10.4093/dmj.2015.39.1.1
- 4,933 View
- 82 Download
- 38 Web of Science
- 37 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Type 2 diabetes (T2D) has been known as 'bi-hormonal disorder' since decades ago, the role of glucagon from α-cell has languished whereas β-cell taking center stage. Recently, numerous findings indicate that the defects of glucagon secretion get involve with development and exacerbation of hyperglycemia in T2D. Aberrant α-cell responses exhibit both fasting and postprandial states: hyperglucagonemia contributes to fasting hyperglycemia caused by inappropriate hepatic glucose production, and to postprandial hyperglycemia owing to blunted α-cell suppression. During hypoglycemia, insufficient counter-regulation response is also observed in advanced T2D. Though many debates still remained for exact mechanisms behind the dysregulation of α-cell in T2D, it is clear that the blockade of glucagon receptor or suppression of glucagon secretion from α-cell would be novel therapeutic targets for control of hyperglycemia. Whereas there have not been remarkable advances in developing new class of drugs, currently available glucagon-like peptide-1 and dipeptidyl peptidase-IV inhibitors could be options for treatment of hyperglucagonemia. In this review, we focus on α-cell dysfunction and therapeutic potentials of targeting α-cell in T2D.
-
Citations
Citations to this article as recorded by- An extended minimal model of OGTT: estimation of α- and β-cell dysfunction, insulin resistance, and the incretin effect
Vijaya Subramanian, Jonatan I. Bagger, Vinayak Harihar, Jens J. Holst, Filip K. Knop, Tina Villsbøll
American Journal of Physiology-Endocrinology and Metabolism.2024; 326(2): E182. CrossRef - Inhibition of Sodium-Glucose Cotransporter-2 during Serum Deprivation Increases Hepatic Gluconeogenesis via the AMPK/AKT/FOXO Signaling Pathway
Jinmi Lee, Seok-Woo Hong, Min-Jeong Kim, Yu-Mi Lim, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2024; 39(1): 98. CrossRef - Childhood Obesity, Diabetes, and Cardiovascular Disease Risk
Mostafa Salama, Babu Balagopal, Ilene Fennoy, Seema Kumar
The Journal of Clinical Endocrinology & Metabolism.2023; 108(12): 3051. CrossRef - Heterogeneity of Diabetes: β-Cells, Phenotypes, and Precision Medicine: Proceedings of an International Symposium of the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes and the U.S. National Institutes of Health’s
William T. Cefalu, Dana K. Andersen, Guillermo Arreaza-Rubín, Christopher L. Pin, Sheryl Sato, C. Bruce Verchere, Minna Woo, Norman D. Rosenblum
Diabetes.2022; 71(1): 1. CrossRef - Heterogeneity of Diabetes: β-Cells, Phenotypes, and Precision Medicine: Proceedings of an International Symposium of the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes and the U.S. National Institutes of Health’s
William T. Cefalu, Dana K. Andersen, Guillermo Arreaza-Rubín, Christopher L. Pin, Sheryl Sato, C. Bruce Verchere, Minna Woo, Norman D. Rosenblum
Diabetes Care.2022; 45(1): 3. CrossRef - Daily Treatment of Mice with Type 2 Diabetes with Adropin for Four Weeks Improves Glucolipid Profile, Reduces Hepatic Lipid Content and Restores Elevated Hepatic Enzymes in Serum
Marek Skrzypski, Paweł A. Kołodziejski, Ewa Pruszyńska-Oszmałek, Tatiana Wojciechowicz, Paulina Janicka, Małgorzata Krążek, Emilian Małek, Mathias Z. Strowski, Krzysztof W. Nowak
International Journal of Molecular Sciences.2022; 23(17): 9807. CrossRef - A glucose-insulin-glucagon coupled model of the isoglycemic intravenous glucose infusion experiment
Vijaya Subramanian, Jonatan I. Bagger, Jens J. Holst, Filip K. Knop, Tina Vilsbøll
Frontiers in Physiology.2022;[Epub] CrossRef - Increased serum cystatin C levels and responses of pancreatic α- and β-cells in type 2 diabetes
Hui-qing Yuan, Jia-xi Miao, Jia-ping Xu, Su-xiang Zhu, Feng Xu, Xiao-hua Wang, Chun-hua Wang, Chao Yu, Xue-qin Wang, Jian-bin Su, Dong-mei Zhang
Endocrine Connections.2022;[Epub] CrossRef - Effect of dipeptidyl peptidase-4 inhibitors on postprandial glucagon level in patients with type 2 diabetes mellitus: A systemic review and meta-analysis
Shangyu Chai, Ruya Zhang, Ye Zhang, Richard David Carr, Yiman Zheng, Swapnil Rajpathak, Linong Ji
Frontiers in Endocrinology.2022;[Epub] CrossRef - The Change in Glucagon Following Meal Ingestion Is Associated with Glycemic Control, but Not with Incretin, in People with Diabetes
Soyeon Yoo, Dongkyu Kim, Gwanpyo Koh
Journal of Clinical Medicine.2021; 10(11): 2487. CrossRef - Serum fatty acid-binding protein 4 levels and responses of pancreatic islet β-cells and α-cells in patients with type 2 diabetes
Hong Wang, Jie Cao, Jian-bin Su, Xue-qin Wang, Xing Wang, Dong-mei Zhang, Xiao-hua Wang
Diabetology & Metabolic Syndrome.2021;[Epub] CrossRef - Angiopoietins stimulate pancreatic islet development from stem cells
Soujanya S. Karanth, Shuofei Sun, Huanjing Bi, Kaiming Ye, Sha Jin
Scientific Reports.2021;[Epub] CrossRef - A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives
Josh Reed, Stephen Bain, Venkateswarlu Kanamarlapudi
Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy.2021; Volume 14: 3567. CrossRef - The Role of the α Cell in the Pathogenesis of Diabetes: A World beyond the Mirror
María Sofía Martínez, Alexander Manzano, Luis Carlos Olivar, Manuel Nava, Juan Salazar, Luis D’Marco, Rina Ortiz, Maricarmen Chacín, Marion Guerrero-Wyss, Mayela Cabrera de Bravo, Clímaco Cano, Valmore Bermúdez, Lisse Angarita
International Journal of Molecular Sciences.2021; 22(17): 9504. CrossRef - Heterogeneity of Diabetes: β-Cells, Phenotypes, and Precision Medicine: Proceedings of an International Symposium of the Canadian Institutes of Health Research’s Institute of Nutrition, Metabolism and Diabetes and the U.S. National Institutes of Health’s
William T. Cefalu, Dana K. Andersen, Guillermo Arreaza-Rubín, Christopher L. Pin, Sheryl Sato, C. Bruce Verchere, Minna Woo, Norman D. Rosenblum, Norman Rosenblum, William Cefalu, Dana K. Andersen, Guillermo Arreaza-Rubín, Christine Dhara, Stephen P. Jame
Canadian Journal of Diabetes.2021; 45(8): 697. CrossRef - The role of GIP in α-cells and glucagon secretion
Kimberley El, Jonathan E. Campbell
Peptides.2020; 125: 170213. CrossRef - Recommendations for improving clinical trial design to facilitate the study of youth-onset type 2 diabetes
Muhammad Yazid Jalaludin, Margarita Barrientos-Pérez, Mona Hafez, Jane Lynch, Naim Shehadeh, Serap Turan, Daniel Weghuber
Clinical Trials.2020; 17(1): 87. CrossRef - Recent advances in understanding the role of glucagon-like peptide 1
Josh Reed, Stephen Bain, Venkateswarlu Kanamarlapudi
F1000Research.2020; 9: 239. CrossRef - Prediabetes diagnosis criteria, type 2 diabetes risk and dietary modulation: The CORDIOPREV study
Irene Roncero-Ramos, Juan F. Alcala-Diaz, Oriol A. Rangel-Zuñiga, Francisco Gomez-Delgado, Rosa Jimenez-Lucena, Antonio García-Rios, Cristina Vals-Delgado, Cristina Romero-Baldonado, Raul M. Luque, Jose M. Ordovas, Pablo Perez-Martinez, Antonio Camargo, J
Clinical Nutrition.2020; 39(2): 492. CrossRef - Comparison of the effects of gemigliptin and dapagliflozin on glycaemic variability in type 2 diabetes: A randomized, open‐label, active‐controlled, 12‐week study (STABLE II study)
Soo Heon Kwak, You‐Cheol Hwang, Jong Chul Won, Ji Cheol Bae, Hyun Jin Kim, Sunghwan Suh, Eun Young Lee, Subin Lee, Sang‐Yong Kim, Jae Hyeon Kim
Diabetes, Obesity and Metabolism.2020; 22(2): 173. CrossRef - Pathophysiology of Type 2 Diabetes in Children and Adolescents
Badhma Valaiyapathi, Barbara Gower , Ambika P. Ashraf
Current Diabetes Reviews.2020; 16(3): 220. CrossRef - Decellularized Tissue Matrix Enhances Self-Assembly of Islet Organoids from Pluripotent Stem Cell Differentiation
Huanjing Bi, Soujanya S. Karanth, Kaiming Ye, Roland Stein, Sha Jin
ACS Biomaterials Science & Engineering.2020; 6(7): 4155. CrossRef - Short Chain Fatty Acids, pancreatic dysfunction and type 2 diabetes
Dipeeka K. Mandaliya, Sriram Seshadri
Pancreatology.2019; 19(4): 617. CrossRef - A contemporary biological pathway of islet amyloid polypeptide for the management of diabetic dementia
Sushil Kumar Sah, Vijaya Paul Samuel, Sunita Dahiya, Yogendar Singh, Ritu M. Gilhotra, Gaurav Gupta, Anurag Mishra, Rakesh Kumar Sharma, Gubbiyappa Shiva Kumar, Nagaraja SreeHarsha, Dinesh Kumar Chellappan, Kamal Dua
Chemico-Biological Interactions.2019; 306: 117. CrossRef - Short Chain Fatty Acids, pancreatic dysfunction and type 2 diabetes
Dipeeka K. Mandaliya, Sriram Seshadri
Pancreatology.2019; 19(2): 280. CrossRef - Higher glucagon-to-insulin ratio is associated with elevated glycated hemoglobin levels in type 2 diabetes patients
Minyoung Lee, Minkyung Kim, Jong Suk Park, Sangbae Lee, Jihong You, Chul Woo Ahn, Kyung Rae Kim, Shinae Kang
The Korean Journal of Internal Medicine.2019; 34(5): 1068. CrossRef - Insulin regulates glucagon-like peptide-1 secretion by pancreatic alpha cells
Pan Liu, Jia Song, He Liu, Fei Yan, Tianyi He, Lingshu Wang, Huying Shen, Xinguo Hou, Li Chen
Endocrine.2018; 62(2): 394. CrossRef - Bridging Type 2 Diabetes and Alzheimer's Disease: Assembling the Puzzle Pieces in the Quest for the Molecules With Therapeutic and Preventive Potential
Ana Marta de Matos, Maria Paula de Macedo, Amélia Pilar Rauter
Medicinal Research Reviews.2018; 38(1): 261. CrossRef - Alpha cell function interacts with diet to modulate prediabetes and Type 2 diabetes
Irene Roncero-Ramos, Rosa Jimenez-Lucena, Juan F. Alcala-Diaz, Cristina Vals-Delgado, Antonio P. Arenas-Larriva, Oriol A. Rangel-Zuñiga, Ana Leon-Acuña, María M. Malagon, Javier Delgado-Lista, Pablo Perez-Martinez, Jose M. Ordovas, Antonio Camargo, Jose L
The Journal of Nutritional Biochemistry.2018; 62: 247. CrossRef - Targetting PED/PEA-15 for diabetes treatment
Francesca Fiory, Rosa Spinelli, Gregory Alexander Raciti, Luca Parrillo, Vittoria D’esposito, Pietro Formisano, Claudia Miele, Francesco Beguinot
Expert Opinion on Therapeutic Targets.2017; 21(6): 571. CrossRef - PANCREATIC ALPHA CELL DYSFUNCTION IN DIABETES MELLITUS AND ITS MANAGEMENT STRATEGIES
Butungeshwar Pradhan, Chakradhar Majhi
Journal of Evidence Based Medicine and Healthcare.2017; 4(45): 2775. CrossRef - Glucagon and heart in type 2 diabetes: new perspectives
Antonio Ceriello, Stefano Genovese, Edoardo Mannucci, Edoardo Gronda
Cardiovascular Diabetology.2016;[Epub] CrossRef - Potential Drug Combinations to Reduce Cardiovascular Disease Burden in Diabetes
Sivaram Pillarisetti
Trends in Pharmacological Sciences.2016; 37(3): 207. CrossRef - The biology of glucagon and the consequences of hyperglucagonemia
Nicolai J Wewer Albrechtsen, Rune E Kuhre, Jens Pedersen, Filip K Knop, Jens J Holst
Biomarkers in Medicine.2016; 10(11): 1141. CrossRef - Increasing GLP-1 Circulating Levels by Bariatric Surgery or by GLP-1 Receptor Agonists Therapy: Why Are the Clinical Consequences so Different?
Chloé Amouyal, Fabrizio Andreelli
Journal of Diabetes Research.2016; 2016: 1. CrossRef - Prediabetes linked to excess glucagon in transgenic mice with pancreatic active AKT1
Toya M Albury-Warren, Veethika Pandey, Lina P Spinel, Michal M Masternak, Deborah A Altomare
Journal of Endocrinology.2016; 228(1): 49. CrossRef - Blood Glucagon Levels Predict the Hemoglobin A1c Response to Saxagliptin in Patients with Type 2 Diabetes Inadequately Controlled with Metformin
Hao Liu, Yun Hu, Feng-fei Li, Bing-li Liu, Xiao-fei Su, Jian-hua Ma
Diabetes Therapy.2016; 7(4): 743. CrossRef
- An extended minimal model of OGTT: estimation of α- and β-cell dysfunction, insulin resistance, and the incretin effect
- The Effects of Glyburide on Apoptosis and Endoplasmic Reticulum Stress in INS-1 Cells in a Glucolipotoxic Condition
- Min Jeong Kwon, Hye Suk Chung, Chang Shin Yoon, Jung Hae Ko, Hae Jung Jun, Tae Kyun Kim, Soon Hee Lee, Kyung Soo Ko, Byoung Doo Rhee, Mi Kyung Kim, Jeong Hyun Park
- Diabetes Metab J. 2011;35(5):480-488. Published online October 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.5.480
- 3,912 View
- 43 Download
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background β-cell death due to endoplasmic reticulum (ER) stress has been regarded as an important pathogenic component of type 2 diabetes. The possibility has been suggested that sulfonylurea, currently being used as one of the main oral hypoglycemic agents of type 2 diabetes, increases ER stress, which could lead to sulfonylurea failure. The authors of the present study examined ER stress of β-cells in a glucolipotoxic condition using glyburide (GB) in an environment mimicking type 2 diabetes.
Methods Apoptosis was induced by adding various concentrations of GB (0.001 to 200 µM) to a glucolipotoxic condition using 33 mM glucose, and the effects of varied concentrations of palmitate were evaluated via annexin V staining. The markers of ER stress and pro-apoptotic markers were assessed by Western blotting and semi-quantitative reverse transcription-polymerase chain reaction. Additionally, the anti-apoptotic markers were evaluated.
Results Addition of any concentration of GB in 150 µM palmitate and 33 mM glucose did not increase apoptosis. The expression of phosphorylated eukaryotic initiation factor (eIF-2α) was increased and cleaved caspase 3 was decreased by adding GB to a glucolipotoxic condition. However, other ER stress-associated markers such as Bip-1, X-box binding protein-1, ATF-4 and C/EBP-homologous protein transcription factor and anti-apoptotic markers phosphor-p85 phosphatidylinositol 3-kinase and phosphorylation of Akt did not change significantly.
Conclusion GB did not show further deleterious effects on the degree of apoptosis or ER stress of INS-1 cells in a glucolipotoxic condition. Increased phosphorylation of eIF-2α may attenuate ER stress for adaptation to increased ER protein load.
-
Citations
Citations to this article as recorded by- The antagonistic atorvastatin-glibenclamide interactions suppressed the atorvastatin-induced Bax/cytochrome c/p53 mRNA expressions and increased Rho A mRNA expression in B16f10 melanoma cell culture
Maryam Malek, Nasim Dana, Ahmad Ghasemi, Maedeh Ghasemi
Gene Reports.2021; 23: 101156. CrossRef - Expression profiles of stress-related genes in islets from donors with progressively impaired glucose metabolism
Marcus Lundberg, Anton Stenwall, Angie Tegehall, Olle Korsgren, Oskar Skog
Islets.2018; 10(2): 69. CrossRef - Pharmacological Modulators of Endoplasmic Reticulum Stress in Metabolic Diseases
Tae Jung, Kyung Choi
International Journal of Molecular Sciences.2016; 17(2): 192. CrossRef - The TRPA1 channel and oral hypoglycemic agents
Carlos Manlio Diaz-Garcia
Channels.2013; 7(6): 420. CrossRef - Dimethyl sulfoxide reduces hepatocellular lipid accumulation through autophagy induction
Young Mi Song, Sun-Ok Song, Yong-Keun Jung, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2012; 8(7): 1085. CrossRef - The Duration of Sulfonylurea Treatment Is Associated withβ-Cell Dysfunction in Patients with Type 2 Diabetes Mellitus
Mi-Seon Shin, Jee Hee Yu, Chang Hee Jung, Jenie Yoonoo Hwang, Woo Je Lee, Min-Seon Kim, Joong-Yeol Park
Diabetes Technology & Therapeutics.2012; 14(11): 1033. CrossRef
- The antagonistic atorvastatin-glibenclamide interactions suppressed the atorvastatin-induced Bax/cytochrome c/p53 mRNA expressions and increased Rho A mRNA expression in B16f10 melanoma cell culture
- Autophagy in Diabetes.
- Hye Seung Jung, Myung Shik Lee
- Korean Diabetes J. 2009;33(6):453-457. Published online December 1, 2009
- DOI: https://doi.org/10.4093/kdj.2009.33.6.453
- 1,843 View
- 25 Download
-
 Abstract
Abstract
 PDF
PDF - Diabetes mellitus is characterized by decreased insulin secretion and action. Decreased insulin secretion results from a reduction in mass and/or function of pancreatic beta-cells. Apoptosis, oxidative stress, mitochondrial dysfunction, and endoplasmic reticulum (ER) stress responses have been suggested as mechanisms for the changes in beta-cells in type 2 diabetes; however, the underlying causes have not been clearly elucidated. Autophagy is an intracellular process that maintains cellular homeostasis through degradation and recycling of organelles. Recently, we reported reduction of beta-cell mass in autophagy-deficient mice. Pancreatic insulin content was also decreased due to the decreased beta-cell mass and the reduced number of insulin granules. Morphological analysis of these beta-cells revealed an accumulation of ubiquitinated proteins, swollen mitochondria, and distended ER. Insulin secretory function ex vivo was also impaired. As a result, autophagy-deficient mice showed hypoinsulinemia and hyperglycemia. These results suggested that autophagy is necessary to maintain the structure, mass and function of beta-cells. In addition, as autophagy may play a protective role against ER stress and rejuvenate organelle function, impaired autophagy may lead to mitochondrial dysfunction and ER stress, which have been implicated as causes of insulin resistance. Therefore, in addition to beta-cell homeostasis, dysregulated autophagy may possibly be involved in insulin resistance.

 KDA
KDA
 First
First Prev
Prev





