
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Type 1 Diabetes
- Real-World Analysis of Therapeutic Outcome in Type 1 Diabetes Mellitus at a Tertiary Care Center
- Antonia Kietaibl, Michaela Riedl, Latife Bozkurt
- Diabetes Metab J. 2022;46(1):149-153. Published online July 6, 2021
- DOI: https://doi.org/10.4093/dmj.2020.0267
- 4,361 View
- 144 Download
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
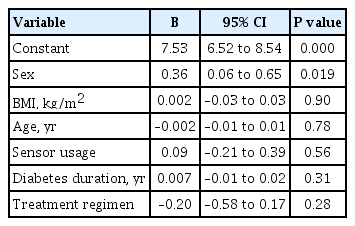
ePub - Insulin replacement in type 1 diabetes mellitus (T1DM) needs intensified treatment, which can either be performed by multiple daily injections (MDI) or continuous subcutaneous insulin infusion (CSII). This retrospective analysis of a real-world scenario aimed to evaluate whether glycaemic and cardiovascular risk factors could be controlled with CSII outclass MDI as suggested by recent evidence. Data from patients with either insulin pump (n=68) or injection (n=224) therapy at an Austrian tertiary care centre were analysed between January 2016 and December 2017. There were no significant differences with regard to the latest glycosylated hemoglobin, cardiovascular risk factor control or diabetes-associated late complications. Hypoglycaemia was less frequent (P<0.001), sensor-augmented therapy was more common (P=0.003) and mean body mass index (BMI) was higher (P=0.002) with CSII treatment. This retrospective analysis of real-world data in T1DM did not demonstrate the superiority of insulin pump treatment with regard to glycaemic control or cardiovascular risk factor control.
- Drug/Regimen
- Efficacy and Safety of Treatment with Quadruple Oral Hypoglycemic Agents in Uncontrolled Type 2 Diabetes Mellitus: A Multi-Center, Retrospective, Observational Study
- Jun Sung Moon, Sunghwan Suh, Sang Soo Kim, Heung Yong Jin, Jeong Mi Kim, Min Hee Jang, Kyung Ae Lee, Ju Hyung Lee, Seung Min Chung, Young Sang Lyu, Jin Hwa Kim, Sang Yong Kim, Jung Eun Jang, Tae Nyun Kim, Sung Woo Kim, Eonju Jeon, Nan Hee Cho, Mi-Kyung Kim, Hye Soon Kim, Il Seong Nam-Goong, Eun Sook Kim, Jin Ook Chung, Dong-Hyeok Cho, Chang Won Lee, Young Il Kim, Dong Jin Chung, Kyu Chang Won, In Joo Kim, Tae Sun Park, Duk Kyu Kim, Hosang Shon
- Diabetes Metab J. 2021;45(5):675-683. Published online August 12, 2020
- DOI: https://doi.org/10.4093/dmj.2020.0107
- 35,361 View
- 367 Download
- 9 Web of Science
- 5 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader  ePub
ePub 
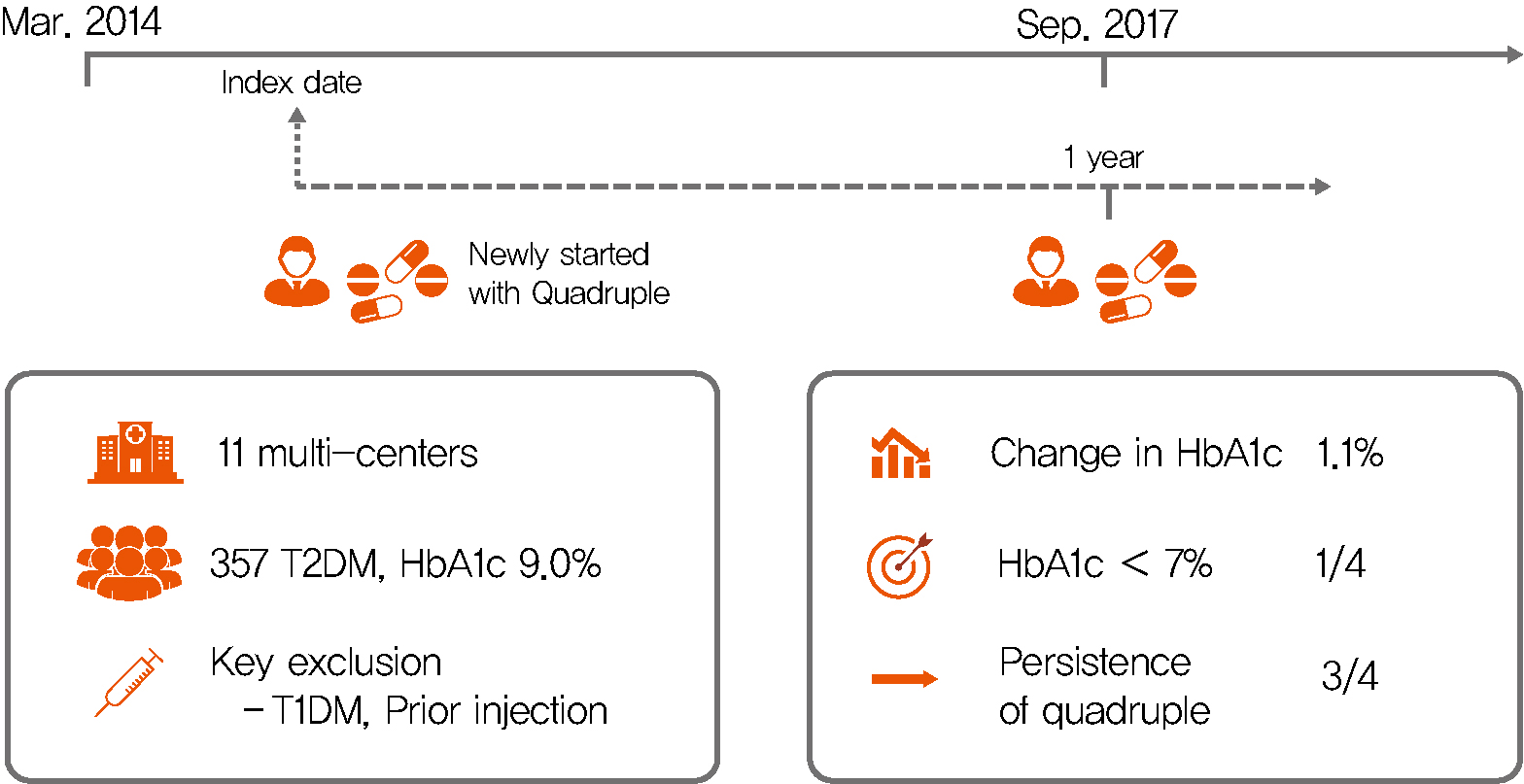
Background Only few studies have shown the efficacy and safety of glucose-control strategies using the quadruple drug combination. Therefore, the aim of the present study was to investigate the usefulness of the quadruple combination therapy with oral hypoglycemic agents (OHAs) in patients with uncontrolled type 2 diabetes mellitus (T2DM).
Methods From March 2014 to December 2018, data of patients with T2DM, who were treated with quadruple hypoglycemic medications for over 12 months in 11 hospitals in South Korea, were reviewed retrospectively. We compared glycosylated hemoglobin (HbA1c) levels before and 12 months after quadruple treatment with OHAs. The safety, maintenance rate, and therapeutic patterns after failure of the quadruple therapy were also evaluated.
Results In total, 357 patients were enrolled for quadruple OHA therapy, and the baseline HbA1c level was 9.0%±1.3% (74.9±14.1 mmol/mol). After 12 months, 270 patients (75.6%) adhered to the quadruple therapy and HbA1c was significantly reduced from 8.9%±1.2% to 7.8%±1.3% (mean change, −1.1%±1.2%;
P <0.001). The number of patients with HbA1c <7% increased significantly from 5 to 68 (P <0.005). In addition, lipid profiles and liver enzyme levels were also improved whereas no changes in body weight. There was no significant safety issue in patients treated with quadruple OHA therapy.Conclusion This study shows the therapeutic efficacy of the quadruple OHA regimen T2DM and demonstrates that it can be an option for the management of T2DM patients who cannot use insulin or reject injectable therapy.
-
Citations
Citations to this article as recorded by- Estimating Type 2 Diabetes Prevalence: A Model of Drug Consumption Data
Rita Oliveira, Matilde Monteiro-Soares, José Pedro Guerreiro, Rúben Pereira, António Teixeira-Rodrigues
Pharmacy.2024; 12(1): 18. CrossRef - Efficacy and safety of enavogliflozin versus dapagliflozin added to metformin plus gemigliptin treatment in patients with type 2 diabetes: A double-blind, randomized, comparator-active study: ENHANCE-D study
Kyung-Soo Kim, Kyung Ah Han, Tae Nyun Kim, Cheol-Young Park, Jung Hwan Park, Sang Yong Kim, Yong Hyun Kim, Kee Ho Song, Eun Seok Kang, Chul Sik Kim, Gwanpyo Koh, Jun Goo Kang, Mi Kyung Kim, Ji Min Han, Nan Hee Kim, Ji Oh Mok, Jae Hyuk Lee, Soo Lim, Sang S
Diabetes & Metabolism.2023; 49(4): 101440. CrossRef - Effectiveness and safety of teneligliptin added to patients with type 2 diabetes inadequately controlled by oral triple combination therapy: A multicentre, randomized, double‐blind, and placebo‐controlled study
Minyoung Lee, Woo‐je Lee, Jae Hyeon Kim, Byung‐Wan Lee
Diabetes, Obesity and Metabolism.2022; 24(6): 1105. CrossRef - A double‐blind, Randomized controlled trial on glucose‐lowering EFfects and safety of adding 0.25 or 0.5 mg lobeglitazone in type 2 diabetes patients with INadequate control on metformin and dipeptidyl peptidase‐4 inhibitor therapy: REFIND study
Soree Ryang, Sang Soo Kim, Ji Cheol Bae, Ji Min Han, Su Kyoung Kwon, Young Il Kim, Il Seong Nam‐Goong, Eun Sook Kim, Mi‐kyung Kim, Chang Won Lee, Soyeon Yoo, Gwanpyo Koh, Min Jeong Kwon, Jeong Hyun Park, In Joo Kim
Diabetes, Obesity and Metabolism.2022; 24(9): 1800. CrossRef - Glycaemic control with add‐on thiazolidinedione or a sodium‐glucose co‐transporter‐2 inhibitor in patients with type 2 diabetes after the failure of an oral triple antidiabetic regimen: A 24‐week, randomized controlled trial
Jaehyun Bae, Ji Hye Huh, Minyoung Lee, Yong‐Ho Lee, Byung‐Wan Lee
Diabetes, Obesity and Metabolism.2021; 23(2): 609. CrossRef
- Estimating Type 2 Diabetes Prevalence: A Model of Drug Consumption Data
- Others
- Generation of Insulin-Expressing Cells in Mouse Small Intestine by Pdx1, MafA, and BETA2/NeuroD
- So-Hyun Lee, Marie Rhee, Ji-Won Kim, Kun-Ho Yoon
- Diabetes Metab J. 2017;41(5):405-416. Published online September 5, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.5.405
- 5,106 View
- 64 Download
- 5 Web of Science
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background To develop surrogate insulin-producing cells for diabetes therapy, adult stem cells have been identified in various tissues and studied for their conversion into β-cells. Pancreatic progenitor cells are derived from the endodermal epithelium and formed in a manner similar to gut progenitor cells. Here, we generated insulin-producing cells from the intestinal epithelial cells that induced many of the specific pancreatic transcription factors using adenoviral vectors carrying three genes: PMB (pancreatic and duodenal homeobox 1 [Pdx1], V-maf musculoaponeurotic fibrosarcoma oncogene homolog A [MafA], and BETA2/NeuroD).
Methods By direct injection into the intestine through the cranial mesenteric artery, adenoviruses (Ad) were successfully delivered to the entire intestine. After virus injection, we could confirm that the small intestine of the mouse was appropriately infected with the Ad-Pdx1 and triple Ad-PMB.
Results Four weeks after the injection, insulin mRNA was expressed in the small intestine, and the insulin gene expression was induced in Ad-Pdx1 and Ad-PMB compared to control Ad-green fluorescent protein. In addition, the conversion of intestinal cells into insulin-expressing cells was detected in parts of the crypts and villi located in the small intestine.
Conclusion These data indicated that PMB facilitate the differentiation of mouse intestinal cells into insulin-expressing cells. In conclusion, the small intestine is an accessible and abundant source of surrogate insulin-producing cells.
-
Citations
Citations to this article as recorded by- Harnessing gut cells for functional insulin production: Strategies and challenges
Kelvin Baafi, John C. March
Biotechnology Notes.2023; 4: 7. CrossRef - Differential Morphological Diagnosis of Various Forms of Congenital Hyperinsulinism in Children
Lubov Borisovna Mitrofanova, Anastasia Arkadyevna Perminova, Daria Viktorovna Ryzhkova, Anna Andreyevna Sukhotskaya, Vladimir Gireyevich Bairov, Irina Leorovna Nikitina
Frontiers in Endocrinology.2021;[Epub] CrossRef - Generation of iPSC-derived insulin-producing cells from patients with type 1 and type 2 diabetes compared with healthy control
Min Jung Kim, Eun Young Lee, Young-Hye You, Hae Kyung Yang, Kun-Ho Yoon, Ji-Won Kim
Stem Cell Research.2020; 48: 101958. CrossRef - ERK Regulates NeuroD1-mediated Neurite Outgrowth via Proteasomal Degradation
Tae-young Lee, In-Su Cho, Narayan Bashyal, Francisco J Naya, Ming-Jer Tsai, Jeong Seon Yoon, Jung-Mi Choi, Chang-Hwan Park, Sung-Soo Kim, Haeyoung Suh-Kim
Experimental Neurobiology.2020; 29(3): 189. CrossRef - Generation of a PDX1–EGFP reporter human induced pluripotent stem cell line, KSCBi005-A-3, using the CRISPR/Cas9 system
Youngsun Lee, Hye Young Choi, Ara Kwon, Hyeyeon Park, Mi-Hyun Park, Ji-Won Kim, Min Jung Kim, Yong-Ou Kim, Sungwook Kwak, Soo Kyung Koo
Stem Cell Research.2019; 41: 101632. CrossRef
- Harnessing gut cells for functional insulin production: Strategies and challenges

 KDA
KDA
 First
First Prev
Prev





