
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
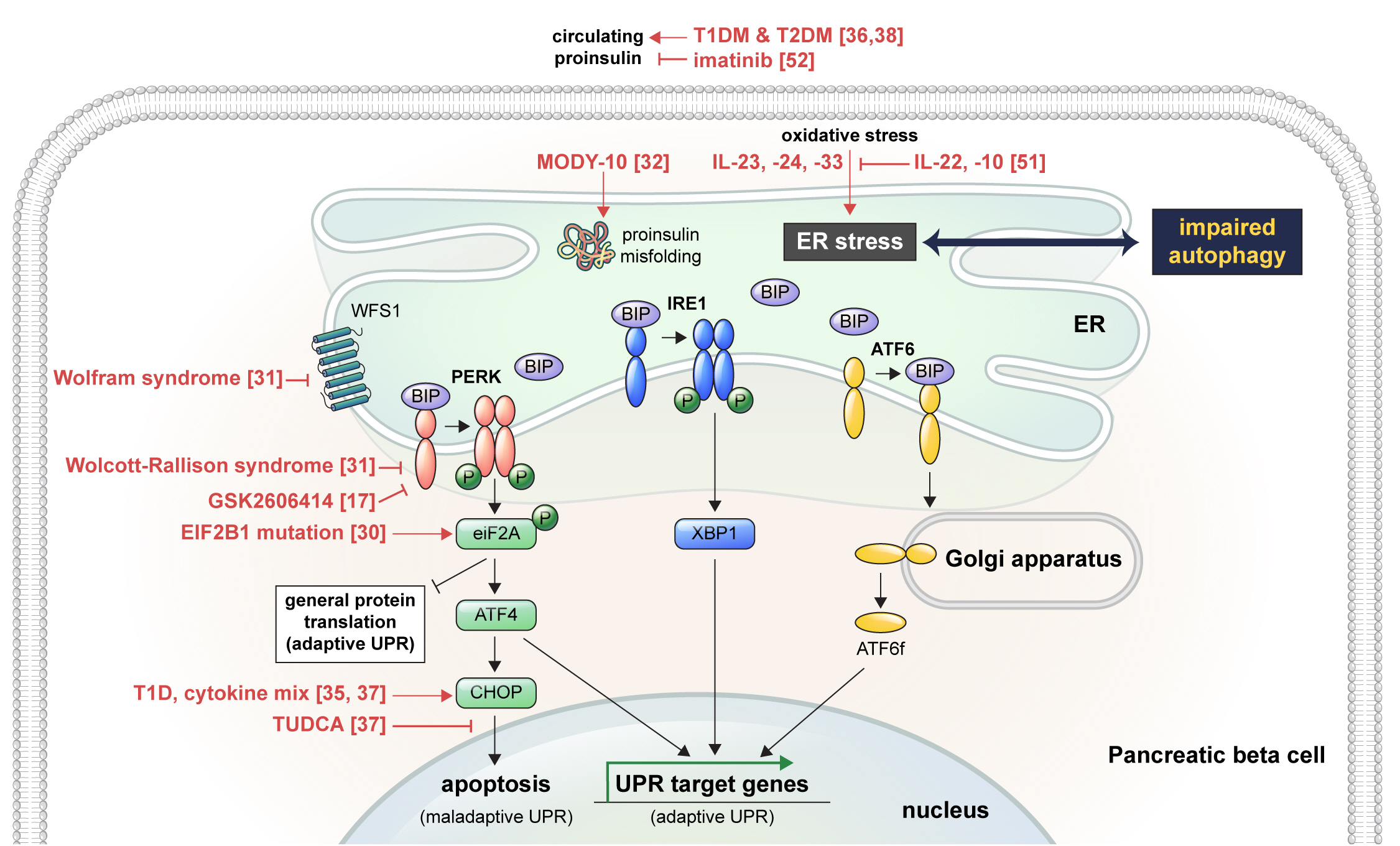
- Pathophysiology
- Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells
- Seoil Moon, Hye Seung Jung
- Diabetes Metab J. 2022;46(4):533-542. Published online July 27, 2022
- DOI: https://doi.org/10.4093/dmj.2022.0070
- 4,537 View
- 250 Download
- 8 Web of Science
- 10 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub - Pancreatic beta cell homeostasis is crucial for the synthesis and secretion of insulin; disruption of homeostasis causes diabetes, and is a treatment target. Adaptation to endoplasmic reticulum (ER) stress through the unfolded protein response (UPR) and adequate regulation of autophagy, which are closely linked, play essential roles in this homeostasis. In diabetes, the UPR and autophagy are dysregulated, which leads to beta cell failure and death. Various studies have explored methods to preserve pancreatic beta cell function and mass by relieving ER stress and regulating autophagic activity. To promote clinical translation of these research results to potential therapeutics for diabetes, we summarize the current knowledge on ER stress and autophagy in human insulin-secreting cells.
-
Citations
Citations to this article as recorded by- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
Seoil Moon, Ji Yoon Lim, Mirang Lee, Youngmin Han, Hongbeom Kim, Wooil Kwon, Jin-Young Jang, Mi Na Kim, Kyong Soo Park, Hye Seung Jung
Diabetes & Metabolism Journal.2024; 48(2): 231. CrossRef - Endoplasmic reticulum stress: A possible connection between intestinal inflammation and neurodegenerative disorders
Giorgio Vivacqua, Romina Mancinelli, Stefano Leone, Rosa Vaccaro, Ludovica Garro, Simone Carotti, Ludovica Ceci, Paolo Onori, Luigi Pannarale, Antonio Franchitto, Eugenio Gaudio, Arianna Casini
Neurogastroenterology & Motility.2024;[Epub] CrossRef - Docosahexanoic Acid Attenuates Palmitate-Induced Apoptosis by Autophagy Upregulation via GPR120/mTOR Axis in Insulin-Secreting Cells
Seok-Woo Hong, Jinmi Lee, Sun Joon Moon, Hyemi Kwon, Se Eun Park, Eun-Jung Rhee, Won-Young Lee
Endocrinology and Metabolism.2024; 39(2): 353. CrossRef - Pancreatic islet remodeling in cotadutide-treated obese mice
Renata Spezani, Thatiany Souza Marinho, Luiz E. Macedo Cardoso, Marcia Barbosa Aguila, Carlos Alberto Mandarim-de-Lacerda
Life Sciences.2023; 327: 121858. CrossRef - Modulation of Unfolded Protein Response Restores Survival and Function of β-Cells Exposed to the Endocrine Disruptor Bisphenol A
Laura Maria Daian, Gabriela Tanko, Andrei Mircea Vacaru, Luiza Ghila, Simona Chera, Ana-Maria Vacaru
International Journal of Molecular Sciences.2023; 24(3): 2023. CrossRef - Interplay of skeletal muscle and adipose tissue: sarcopenic obesity
Min Jeong Park, Kyung Mook Choi
Metabolism.2023; 144: 155577. CrossRef - Identification and analysis of type 2 diabetes-mellitus-associated autophagy-related genes
Kun Cui, Zhizheng Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Sestrin2 in diabetes and diabetic complications
Xiaodan Zhang, Zirui Luo, Jiahong Li, Yaxuan Lin, Yu Li, Wangen Li
Frontiers in Endocrinology.2023;[Epub] CrossRef - Crosstalk between autophagy and insulin resistance: evidence from different tissues
Asie Sadeghi, Maryam Niknam, Mohammad Amin Momeni-Moghaddam, Maryam Shabani, Hamid Aria, Alireza Bastin, Maryam Teimouri, Reza Meshkani, Hamed Akbari
European Journal of Medical Research.2023;[Epub] CrossRef - Beta cell lipotoxicity in the development of type 2 diabetes: the need for species-specific understanding
Patricia Thomas, Meurig T. Gallagher, Gabriela Da Silva Xavier
Frontiers in Endocrinology.2023;[Epub] CrossRef
- Glucolipotoxicity Suppressed Autophagy and Insulin Contents in Human Islets, and Attenuation of PERK Activity Enhanced Them in an ATG7-Dependent Manner
- Pathophysiology
- Metformin Ameliorates Lipotoxic β-Cell Dysfunction through a Concentration-Dependent Dual Mechanism of Action
- Hong Il Kim, Ji Seon Lee, Byung Kook Kwak, Won Min Hwang, Min Joo Kim, Young-Bum Kim, Sung Soo Chung, Kyong Soo Park
- Diabetes Metab J. 2019;43(6):854-866. Published online June 27, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0179
- 6,655 View
- 115 Download
- 14 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Chronic exposure to elevated levels of free fatty acids contributes to pancreatic β-cell dysfunction. Although it is well known that metformin induces cellular energy depletion and a concomitant activation of AMP-activated protein kinase (AMPK) through inhibition of the respiratory chain, previous studies have shown inconsistent results with regard to the action of metformin on pancreatic β-cells. We therefore examined the effects of metformin on pancreatic β-cells under lipotoxic stress.
Methods NIT-1 cells and mouse islets were exposed to palmitate and treated with 0.05 and 0.5 mM metformin. Cell viability, glucose-stimulated insulin secretion, cellular adenosine triphosphate, reactive oxygen species (ROS) levels and Rho kinase (ROCK) activities were measured. The phosphorylation of AMPK was evaluated by Western blot analysis and mRNA levels of endoplasmic reticulum (ER) stress markers and NADPH oxidase (NOX) were measured by real-time quantitative polymerase chain reaction analysis.
Results We found that metformin has protective effects on palmitate-induced β-cell dysfunction. Metformin at a concentration of 0.05 mM inhibits NOX and suppresses the palmitate-induced elevation of ER stress markers and ROS levels in a AMPK-independent manner, whereas 0.5 mM metformin inhibits ROCK activity and activates AMPK.
Conclusion This study suggests that the action of metformin on β-cell lipotoxicity was implemented by different molecular pathways depending on its concentration. Metformin at a usual therapeutic dose is supposed to alleviate lipotoxic β-cell dysfunction through inhibition of oxidative stress and ER stress.
-
Citations
Citations to this article as recorded by- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
Si-min Zhou, Xin-ming Yao, Yi Cheng, Yu-jie Xing, Yue Sun, Qiang Hua, Shu-jun Wan, Xiang-jian Meng
Heliyon.2024; 10(2): e24432. CrossRef - Reduced Expression Level of Protein PhosphatasePPM1EServes to Maintain Insulin Secretion in Type 2 Diabetes
Sevda Gheibi, Luis Rodrigo Cataldo, Alexander Hamilton, Mi Huang, Sebastian Kalamajski, Malin Fex, Hindrik Mulder
Diabetes.2023; 72(4): 455. CrossRef - Metformin restores prohormone processing enzymes and normalizes aberrations in secretion of proinsulin and insulin in palmitate‐exposed human islets
Quan Wen, Azazul Islam Chowdhury, Banu Aydin, Mudhir Shekha, Rasmus Stenlid, Anders Forslund, Peter Bergsten
Diabetes, Obesity and Metabolism.2023; 25(12): 3757. CrossRef - Treatment of type 2 diabetes mellitus with stem cells and antidiabetic drugs: a dualistic and future-focused approach
Priyamvada Amol Arte, Kanchanlata Tungare, Mustansir Bhori, Renitta Jobby, Jyotirmoi Aich
Human Cell.2023; 37(1): 54. CrossRef - Metformin disrupts insulin secretion, causes proapoptotic and oxidative effects in rat pancreatic beta‐cells in vitro
Maíra M.R. Valle, Eloisa Aparecida Vilas‐Boas, Camila F. Lucena, Simone A. Teixeira, Marcelo N. Muscara, Angelo R. Carpinelli
Journal of Biochemical and Molecular Toxicology.2022;[Epub] CrossRef - Protection by metformin against severe Covid-19: An in-depth mechanistic analysis
Nicolas Wiernsperger, Abdallah Al-Salameh, Bertrand Cariou, Jean-Daniel Lalau
Diabetes & Metabolism.2022; 48(4): 101359. CrossRef - Insight Into Rho Kinase Isoforms in Obesity and Energy Homeostasis
Lei Wei, Jianjian Shi
Frontiers in Endocrinology.2022;[Epub] CrossRef - Overexpression of miR-297b-5p Promotes Metformin-Mediated Protection Against Stearic Acid-Induced Senescence by Targeting Igf1r
Qingrui Zhao, Shenghan Su, Yuqing Lin, Xuebei Li, Lingfeng Dan, Yunjin Zhang, Chunxiao Yang, Xiaohan Li, Yimeng Dong, Chenchen Geng, Changhao Sun, Xia Chu, Huimin Lu
SSRN Electronic Journal .2022;[Epub] CrossRef - Metformin Dysregulates the Unfolded Protein Response and the WNT/β-Catenin Pathway in Endometrial Cancer Cells through an AMPK-Independent Mechanism
Domenico Conza, Paola Mirra, Gaetano Calì, Luigi Insabato, Francesca Fiory, Francesco Beguinot, Luca Ulianich
Cells.2021; 10(5): 1067. CrossRef - NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction
Suma Elumalai, Udayakumar Karunakaran, Jun-Sung Moon, Kyu-Chang Won
Cells.2021; 10(7): 1573. CrossRef - Metformin use and cardiovascular outcomes in patients with diabetes and chronic kidney disease: a nationwide cohort study
Min Ho Kim, Hyung Jung Oh, Soon Hyo Kwon, Jin Seok Jeon, Hyunjin Noh, Dong Cheol Han, Hyoungnae Kim, Dong-Ryeol Ryu
Kidney Research and Clinical Practice.2021; 40(4): 660. CrossRef - Different Effects of Metformin and A769662 on Sodium Iodate-Induced Cytotoxicity in Retinal Pigment Epithelial Cells: Distinct Actions on Mitochondrial Fission and Respiration
Chi-Ming Chan, Ponarulselvam Sekar, Duen-Yi Huang, Shu-Hao Hsu, Wan-Wan Lin
Antioxidants.2020; 9(11): 1057. CrossRef - Metformin Reduces Lipotoxicity-Induced Meta-Inflammation in β-Cells through the Activation of GPR40-PLC-IP3 Pathway
Ximei Shen, Beibei Fan, Xin Hu, Liufen Luo, Yuanli Yan, Liyong Yang
Journal of Diabetes Research.2019; 2019: 1. CrossRef
- Metformin enhances METTL14-Mediated m6A methylation to alleviate NIT-1 cells apoptosis induced by hydrogen peroxide
- Islet Studies and Transplantation
-
- Myricetin Protects Against High Glucose-Induced β-Cell Apoptosis by Attenuating Endoplasmic Reticulum Stress via Inactivation of Cyclin-Dependent Kinase 5
- Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Jae-Han Jeon, Nam Doo Kim, Keun-Gyu Park, Kyu Chang Won, Jaechan Leem, In-Kyu Lee
- Diabetes Metab J. 2019;43(2):192-205. Published online January 16, 2019
- DOI: https://doi.org/10.4093/dmj.2018.0052
- 4,913 View
- 106 Download
- 33 Web of Science
- 32 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material PubReader
PubReader Background Chronic hyperglycemia has deleterious effects on pancreatic β-cell function and turnover. Recent studies support the view that cyclin-dependent kinase 5 (CDK5) plays a role in β-cell failure under hyperglycemic conditions. However, little is known about how CDK5 impair β-cell function. Myricetin, a natural flavonoid, has therapeutic potential for the treatment of type 2 diabetes mellitus. In this study, we examined the effect of myricetin on high glucose (HG)-induced β-cell apoptosis and explored the relationship between myricetin and CDK5.
Methods To address this question, we subjected INS-1 cells and isolated rat islets to HG conditions (30 mM) in the presence or absence of myricetin. Docking studies were conducted to validate the interaction between myricetin and CDK5. Gene expression and protein levels of endoplasmic reticulum (ER) stress markers were measured by real-time reverse transcription polymerase chain reaction and Western blot analysis.
Results Activation of CDK5 in response to HG coupled with the induction of ER stress via the down regulation of sarcoendoplasmic reticulum calcium ATPase 2b (
SERCA2b ) gene expression and reduced the nuclear accumulation of pancreatic duodenal homeobox 1 (PDX1) leads to β-cell apoptosis. Docking study predicts that myricetin inhibit CDK5 activation by direct binding in the ATP-binding pocket. Myricetin counteracted the decrease in the levels of PDX1 and SERCA2b by HG. Moreover, myricetin attenuated HG-induced apoptosis in INS-1 cells and rat islets and reduce the mitochondrial dysfunction by decreasing reactive oxygen species production and mitochondrial membrane potential (Δψm) loss.Conclusion Myricetin protects the β-cells against HG-induced apoptosis by inhibiting ER stress, possibly through inactivation of CDK5 and consequent upregulation of PDX1 and SERCA2b.
-
Citations
Citations to this article as recorded by- Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches
Nazmun Nahar, Md. Nazmul Hasan Zilani, Partha Biswas, Md. Morsaline Billah, Shabana Bibi, Norah A. Albekairi, Abdulrahman Alshammari, Md. Nazmul Hasan
Saudi Pharmaceutical Journal.2024; 32(1): 101887. CrossRef - Mitochondrial aldehyde dehydrogenase-2 coordinates the hydrogen sulfide - AMPK axis to attenuate high glucose-induced pancreatic β-cell dysfunction by glutathione antioxidant system
Udayakumar Karunakaran, Suma Elumalai, Seung Min Chung, Kathrin Maedler, Kyu Chang Won, Jun Sung Moon
Redox Biology.2024; 69: 102994. CrossRef - Network-based identification and mechanism exploration of active ingredients against Alzheimer’s disease via targeting endoplasmic reticulum stress from traditional chinese medicine
Zhao Dai, Tian Hu, Junwen Wei, Xue Wang, Chuipu Cai, Yong Gu, Yunhui Hu, Wenjia Wang, Qihui Wu, Jiansong Fang
Computational and Structural Biotechnology Journal.2024; 23: 506. CrossRef - Myricetin as a Promising Flavonoid with Multitargeted Biological Activity
A.S. Chiriapkin
Juvenis Scientia.2024; 10(1): 5. CrossRef - Naturally occurring small molecules with dual effect upon inflammatory signaling pathways and endoplasmic reticulum stress response
Daniela Correia da Silva, Patrícia Valentão, David M. Pereira
Journal of Physiology and Biochemistry.2024;[Epub] CrossRef - Omnifarious fruit polyphenols: an omnipotent strategy to prevent and intervene diabetes and related complication?
Yao Chen, Xuejiao Qie, Wei Quan, Maomao Zeng, Fang Qin, Jie Chen, Benu Adhikari, Zhiyong He
Critical Reviews in Food Science and Nutrition.2023; 63(20): 4288. CrossRef - Regulation of reactive oxygen species by phytochemicals for the management of cancer and diabetes
Heui Min Lim, See-Hyoung Park
Critical Reviews in Food Science and Nutrition.2023; 63(22): 5911. CrossRef - Bioactive compounds from Polygonatum genus as anti-diabetic agents with future perspectives
Yan Shi, Dun Si, Donghong Chen, Xinfeng Zhang, Zhigang Han, Qiang Yu, Jingjing Liu, Jinping Si
Food Chemistry.2023; 408: 135183. CrossRef - Venom Peptides, Polyphenols and Alkaloids: Are They the Next Antidiabetics That Will Preserve β-Cell Mass and Function in Type 2 Diabetes?
Michele Lodato, Valérie Plaisance, Valérie Pawlowski, Maxime Kwapich, Alexandre Barras, Emeline Buissart, Stéphane Dalle, Sabine Szunerits, Jérôme Vicogne, Rabah Boukherroub, Amar Abderrahmani
Cells.2023; 12(6): 940. CrossRef - TFP5 attenuates cyclin‐dependent kinase 5‐mediated islet β‐cell damage in diabetes
Shunyao Liu, Bo Li, Danna Ma, Yuejia Tao, Jiang Song, Li Bao, Guoqing Zhang, Hongyan Luo, Shilu Cao, Jing E, Yali Zheng
Chemical Biology & Drug Design.2023; 102(1): 76. CrossRef - Antiviral and Possible Prophylactic Significance of Myricetin for COVID-19
Pawan K. Agrawal, Chandan Agrawal, Gerald Blunden
Natural Product Communications.2023; 18(4): 1934578X2311662. CrossRef - In Vitro and In Silico Protocols for the Assessment of Anti-Tick Compounds from Pinus roxburghii against Rhipicephalus (Boophilus) microplus Ticks
Sana Ayub, Nosheen Malak, Raquel Cossío-Bayúgar, Nasreen Nasreen, Afshan Khan, Sadaf Niaz, Adil Khan, Abdallah D. Alanazi, Mourad Ben Said
Animals.2023; 13(8): 1388. CrossRef - Protective Effect of Myricetin Against Experimentally Induced Torsion in Rats
M. Tatar, Z. Polat, J. Öner, H. Öner
Biology Bulletin.2023; 50(6): 1338. CrossRef - The pharmacological mechanism of Abelmoschus manihot in the treatment of chronic kidney disease
Cuiting Wei, Chao Wang, Run Li, Yunfeng Bai, Xue Wang, Qingyun Fang, Xiangmei Chen, Ping Li
Heliyon.2023; 9(11): e22017. CrossRef - Natural Polyphenols as SERCA Activators: Role in the Endoplasmic Reticulum Stress-Related Diseases
Jana Viskupicova, Petronela Rezbarikova
Molecules.2022; 27(16): 5095. CrossRef - Research progress on the mechanism of beta-cell apoptosis in type 2 diabetes mellitus
SuFang You, JingYi Zheng, YuPing Chen, HuiBin Huang
Frontiers in Endocrinology.2022;[Epub] CrossRef - Myricetin inhibits pseudorabies virus infection through direct inactivation and activating host antiviral defense
Huaiyue Hu, Zhiqiang Hu, Yingying Zhang, Hongping Wan, Zhongqiong Yin, Lixia Li, Xiaoxia Liang, Xinghong Zhao, Lizi Yin, Gang Ye, Yuan-Feng Zou, Huaqiao Tang, Renyong Jia, Yaqin Chen, Hao Zhou, Xu Song
Frontiers in Microbiology.2022;[Epub] CrossRef - Effects of myricetin against cadmium-induced neurotoxicity in PC12 cells
Azadeh Aminzadeh, Ayda Salarinejad
Toxicology Research.2021; 10(1): 84. CrossRef - Pioglitazone-induced AMPK-Glutaminase-1 prevents high glucose-induced pancreatic β-cell dysfunction by glutathione antioxidant system
Udayakumar Karunakaran, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Redox Biology.2021; 45: 102029. CrossRef - Chlorogenic acid and β-glucan from highland barley grain ameliorate β-cell dysfunction via inhibiting apoptosis and improving cell proliferation
Ze-Hua Liu, Bo Li
Food & Function.2021; 12(20): 10040. CrossRef - The cyclin dependent kinase inhibitor Roscovitine prevents diet-induced metabolic disruption in obese mice
Nabil Rabhi, Kathleen Desevin, Briana Noel Cortez, Ryan Hekman, Jean Z. Lin, Andrew Emili, Stephen R. Farmer
Scientific Reports.2021;[Epub] CrossRef - AdipoRon promotes diabetic fracture repair through endochondral ossification-based bone repair by enhancing survival and differentiation of chondrocytes
Zhongyi Wang, Jinxin Tang, Ying Li, Yu Wang, Yanyang Guo, Qisheng Tu, Jake Chen, Chen Wang
Experimental Cell Research.2020; 387(2): 111757. CrossRef - A kinase of many talents: non-neuronal functions of CDK5 in development and disease
Samanta Sharma, Piotr Sicinski
Open Biology.2020; 10(1): 190287. CrossRef - Mitochondrial dysfunction in the fetoplacental unit in gestational diabetes mellitus
Luis Sobrevia, Paola Valero, Adriana Grismaldo, Roberto Villalobos-Labra, Fabián Pardo, Mario Subiabre, Gael Armstrong, Fernando Toledo, Sofía Vega, Marcelo Cornejo, Gonzalo Fuentes, Reinaldo Marín
Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease.2020; 1866(12): 165948. CrossRef - Myricetin bioactive effects: moving from preclinical evidence to potential clinical applications
Yasaman Taheri, Hafiz Ansar Rasul Suleria, Natália Martins, Oksana Sytar, Ahmet Beyatli, Balakyz Yeskaliyeva, Gulnaz Seitimova, Bahare Salehi, Prabhakar Semwal, Sakshi Painuli, Anuj Kumar, Elena Azzini, Miquel Martorell, William N. Setzer, Alfred Maroyi,
BMC Complementary Medicine and Therapies.2020;[Epub] CrossRef - Current Pharmacological Trends on Myricetin
Gudiya Gupta, Mohd Aftab Siddiqui, Mohd Muazzam Khan, Mohd Ajmal, Rabiya Ahsan, Md Azizur Rahaman, Md Afroz Ahmad, Md Arshad, Mohammad Khushtar
Drug Research.2020;[Epub] CrossRef - Silencing cyclophilin A improves insulin secretion, reduces cell apoptosis, and alleviates inflammation as well as oxidant stress in high glucose-induced pancreatic β-cells via MAPK/NF-kb signaling pathway
Tangying Li, Huibiao Quan, Huachuan Zhang, Leweihua Lin, Qianying Ou, Kaining Chen
Bioengineered.2020; 11(1): 1047. CrossRef - Endoplasmic reticulum stress contributes to NMDA-induced pancreatic β-cell dysfunction in a CHOP-dependent manner
Xiao-Ting Huang, Wei Liu, Yong Zhou, Mei Sun, Chen-Chen Sun, Chen-Yu Zhang, Si-Yuan Tang
Life Sciences.2019; 232: 116612. CrossRef - Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death
Ryo Shibusawa, Eijiro Yamada, Shuichi Okada, Yasuyo Nakajima, Claire C. Bastie, Akito Maeshima, Kyoichi Kaira, Masanobu Yamada
Scientific Reports.2019;[Epub] CrossRef - Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic β-cell dysfunction
Udayakumar Karunakaran, Ji Eun Lee, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Free Radical Biology and Medicine.2019; 141: 59. CrossRef - CDK5: Key Regulator of Apoptosis and Cell Survival
Rabih Roufayel, Nimer Murshid
Biomedicines.2019; 7(4): 88. CrossRef - Oral DhHP-6 for the Treatment of Type 2 Diabetes Mellitus
Kai Wang, Yu Su, Yuting Liang, Yanhui Song, Liping Wang
International Journal of Molecular Sciences.2019; 20(6): 1517. CrossRef
- Profiling of secondary metabolite and evaluation of anti-diabetic potency of Crotalaria quinquefolia (L): In-vitro, in-vivo, and in-silico approaches
- Pathophysiology
- Nuclear Receptors Resolve Endoplasmic Reticulum Stress to Improve Hepatic Insulin Resistance
- Jae Man Lee
- Diabetes Metab J. 2017;41(1):10-19. Published online February 16, 2017
- DOI: https://doi.org/10.4093/dmj.2017.41.1.10
- 4,592 View
- 94 Download
- 13 Web of Science
- 13 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Chronic endoplasmic reticulum (ER) stress culminating in proteotoxicity contributes to the development of insulin resistance and progression to type 2 diabetes mellitus. Pharmacologic interventions targeting several different nuclear receptors have emerged as potential treatments for insulin resistance. The mechanistic basis for these antidiabetic effects has primarily been attributed to multiple metabolic and inflammatory functions. Here we review recent advances in our understanding of the association of ER stress with insulin resistance and the role of nuclear receptors in promoting ER stress resolution and improving insulin resistance in the liver.
-
Citations
Citations to this article as recorded by- Duality of Nrf2 in iron-overload cardiomyopathy
Enrica Federti, Francesca Vinchi, Iana Iatcenko, Alessandra Ghigo, Alessandro Matte, Serge Cedrick Mbiandjeu Toya, Angela Siciliano, Deborah Chiabrando, Emanuela Tolosano, Steven Zebulon Vance, Veronica Riccardi, Immacolata Andolfo, Manuela Iezzi, Alessia
Haematologica.2023; 108(5): 1335. CrossRef - Endoplasmic Reticulum Stress and Its Impact on Adipogenesis: Molecular Mechanisms Implicated
Gyuhui Kim, Jiyoon Lee, Joohun Ha, Insug Kang, Wonchae Choe
Nutrients.2023; 15(24): 5082. CrossRef - Qingluotongbi formula regulates the LXRα-ERS-SREBP-1c pathway in hepatocytes to alleviate the liver injury caused by Tripterygium wilfordii Hook. f.
Zhichao Yu, Zhe Feng, Ling Fu, Jing Wang, Changqing Li, Huaxu Zhu, Tong Xie, Jie Zhou, Lingling Zhou, Xueping Zhou
Journal of Ethnopharmacology.2022; 287: 114952. CrossRef - Nuclear‐mitochondrial crosstalk: On the role of the nuclear receptor liver receptor homolog‐1 (NR5A2) in the regulation of mitochondrial metabolism, cell survival, and cancer
Svenja Michalek, Thomas Brunner
IUBMB Life.2021; 73(3): 592. CrossRef - NGBR is required to ameliorate type 2 diabetes in mice by enhancing insulin sensitivity
Yi Chen, Wenquan Hu, Qi Li, Shiwei Zhao, Dan Zhao, Shuang Zhang, Zhuo Wei, Xiaoxiao Yang, Yuanli Chen, Xiaoju Li, Chenzhong Liao, Jihong Han, Qing Robert Miao, Yajun Duan
Journal of Biological Chemistry.2021; 296: 100624. CrossRef - Impaired ferritinophagy flux induced by high fat diet mediates hepatic insulin resistance via endoplasmic reticulum stress
Chunjie Jiang, Shanshan Zhang, Dan Li, Li Chen, Ying Zhao, Guibin Mei, Jingjing Liu, Yuhan Tang, Chao Gao, Ping Yao
Food and Chemical Toxicology.2020; 140: 111329. CrossRef - Dipeptidyl peptidase-4 inhibitor protects against non-alcoholic steatohepatitis in mice by targeting TRAIL receptor-mediated lipoapoptosis via modulating hepatic dipeptidyl peptidase-4 expression
Minyoung Lee, Eugene Shin, Jaehyun Bae, Yongin Cho, Ji-Yeon Lee, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
Scientific Reports.2020;[Epub] CrossRef - Use of fenofibrate on cardiovascular outcomes in statin users with metabolic syndrome: propensity matched cohort study
Nam Hoon Kim, Ki Hoon Han, Jimi Choi, Juneyoung Lee, Sin Gon Kim
BMJ.2019; : l5125. CrossRef - Inhibition of the Low Molecular Weight Protein Tyrosine Phosphatase (LMPTP) as a Potential Therapeutic Strategy for Hepatic Progenitor Cells Lipotoxicity—Short Communication
Michalina Alicka, Katarzyna Kornicka-Garbowska, Michael Roecken, Krzysztof Marycz
International Journal of Molecular Sciences.2019; 20(23): 5873. CrossRef - Myricetin prevents thapsigargin-induced CDK5-P66Shc signalosome mediated pancreatic β-cell dysfunction
Udayakumar Karunakaran, Ji Eun Lee, Suma Elumalai, Jun Sung Moon, Kyu Chang Won
Free Radical Biology and Medicine.2019; 141: 59. CrossRef - Spontaneous ketonuria and risk of incident diabetes: a 12 year prospective study
Gyuri Kim, Sang-Guk Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha, Ele Ferrannini, Yong-ho Lee, Nam H. Cho
Diabetologia.2019; 62(5): 779. CrossRef - CCAAT/enhancer binding protein homologous protein knockdown alleviates hypoxia-induced myocardial injury in rat cardiomyocytes exposed to high glucose
Wenqi Yang, Fang Wu, Ting Luo, Yuelan Zhang
Experimental and Therapeutic Medicine.2018;[Epub] CrossRef - Association of changes in ER stress-mediated signaling pathway with lead-induced insulin resistance and apoptosis in rats and their prevention by A-type dimeric epigallocatechin-3-gallate
Chan-Min Liu, Jie-Qiong Ma, Jian-Mei Sun, Zhao-Jun Feng, Chao Cheng, Wei Yang, Hong Jiang
Food and Chemical Toxicology.2017; 110: 325. CrossRef
- Duality of Nrf2 in iron-overload cardiomyopathy
- The Effects of Glyburide on Apoptosis and Endoplasmic Reticulum Stress in INS-1 Cells in a Glucolipotoxic Condition
- Min Jeong Kwon, Hye Suk Chung, Chang Shin Yoon, Jung Hae Ko, Hae Jung Jun, Tae Kyun Kim, Soon Hee Lee, Kyung Soo Ko, Byoung Doo Rhee, Mi Kyung Kim, Jeong Hyun Park
- Diabetes Metab J. 2011;35(5):480-488. Published online October 31, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.5.480
- 3,915 View
- 43 Download
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background β-cell death due to endoplasmic reticulum (ER) stress has been regarded as an important pathogenic component of type 2 diabetes. The possibility has been suggested that sulfonylurea, currently being used as one of the main oral hypoglycemic agents of type 2 diabetes, increases ER stress, which could lead to sulfonylurea failure. The authors of the present study examined ER stress of β-cells in a glucolipotoxic condition using glyburide (GB) in an environment mimicking type 2 diabetes.
Methods Apoptosis was induced by adding various concentrations of GB (0.001 to 200 µM) to a glucolipotoxic condition using 33 mM glucose, and the effects of varied concentrations of palmitate were evaluated via annexin V staining. The markers of ER stress and pro-apoptotic markers were assessed by Western blotting and semi-quantitative reverse transcription-polymerase chain reaction. Additionally, the anti-apoptotic markers were evaluated.
Results Addition of any concentration of GB in 150 µM palmitate and 33 mM glucose did not increase apoptosis. The expression of phosphorylated eukaryotic initiation factor (eIF-2α) was increased and cleaved caspase 3 was decreased by adding GB to a glucolipotoxic condition. However, other ER stress-associated markers such as Bip-1, X-box binding protein-1, ATF-4 and C/EBP-homologous protein transcription factor and anti-apoptotic markers phosphor-p85 phosphatidylinositol 3-kinase and phosphorylation of Akt did not change significantly.
Conclusion GB did not show further deleterious effects on the degree of apoptosis or ER stress of INS-1 cells in a glucolipotoxic condition. Increased phosphorylation of eIF-2α may attenuate ER stress for adaptation to increased ER protein load.
-
Citations
Citations to this article as recorded by- The antagonistic atorvastatin-glibenclamide interactions suppressed the atorvastatin-induced Bax/cytochrome c/p53 mRNA expressions and increased Rho A mRNA expression in B16f10 melanoma cell culture
Maryam Malek, Nasim Dana, Ahmad Ghasemi, Maedeh Ghasemi
Gene Reports.2021; 23: 101156. CrossRef - Expression profiles of stress-related genes in islets from donors with progressively impaired glucose metabolism
Marcus Lundberg, Anton Stenwall, Angie Tegehall, Olle Korsgren, Oskar Skog
Islets.2018; 10(2): 69. CrossRef - Pharmacological Modulators of Endoplasmic Reticulum Stress in Metabolic Diseases
Tae Jung, Kyung Choi
International Journal of Molecular Sciences.2016; 17(2): 192. CrossRef - The TRPA1 channel and oral hypoglycemic agents
Carlos Manlio Diaz-Garcia
Channels.2013; 7(6): 420. CrossRef - Dimethyl sulfoxide reduces hepatocellular lipid accumulation through autophagy induction
Young Mi Song, Sun-Ok Song, Yong-Keun Jung, Eun-Seok Kang, Bong Soo Cha, Hyun Chul Lee, Byung-Wan Lee
Autophagy.2012; 8(7): 1085. CrossRef - The Duration of Sulfonylurea Treatment Is Associated withβ-Cell Dysfunction in Patients with Type 2 Diabetes Mellitus
Mi-Seon Shin, Jee Hee Yu, Chang Hee Jung, Jenie Yoonoo Hwang, Woo Je Lee, Min-Seon Kim, Joong-Yeol Park
Diabetes Technology & Therapeutics.2012; 14(11): 1033. CrossRef
- The antagonistic atorvastatin-glibenclamide interactions suppressed the atorvastatin-induced Bax/cytochrome c/p53 mRNA expressions and increased Rho A mRNA expression in B16f10 melanoma cell culture
- The Effect of Tribbles-Related Protein 3 on ER Stress-Suppressed Insulin Gene Expression in INS-1 Cells
- Young Yun Jang, Nam Keong Kim, Mi Kyung Kim, Ho Young Lee, Sang Jin Kim, Hye Soon Kim, Hye-Young Seo, In Kyu Lee, Keun Gyu Park
- Korean Diabetes J. 2010;34(5):312-319. Published online October 31, 2010
- DOI: https://doi.org/10.4093/kdj.2010.34.5.312
- 3,541 View
- 32 Download
- 5 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background The highly developed endoplasmic reticulum (ER) structure in pancreatic beta cells is heavily involved in insulin biosynthesis. Thus, any perturbation in ER function inevitably impacts insulin biosynthesis. Recent studies showed that the expression of tribbles-related protein 3 (TRB3), a mammalian homolog of Drosophilia tribbles, in various cell types is induced by ER stress. Here, we examined whether ER stress induces TRB3 expression in INS-1 cells and found that TRB3 mediates ER stress-induced suppression of insulin gene expression.
Methods The effects of tunicamycin and thapsigargin on insulin and TRB3 expression in INS-1 cells were measured by Northern and Western blot analysis, respectively. The effects of adenovirus-mediated overexpression of TRB3 on insulin, PDX-1 and MafA gene expression in INS-1 cells were measured by Northern blot analysis. The effect of TRB3 on insulin promoter was measured by transient transfection study with constructs of human insulin promoter.
Results The treatment of INS-1 cells with tunicamycin and thapsigargin decreased insulin mRNA expression, but increased TRB3 protein expression. Adenovirus-mediated overexpression of TRB3 decreased insulin gene expression in a dose-dependent manner. A transient transfection study showed that TRB3 inhibited insulin promoter activity, suggesting that TRB3 inhibited insulin gene expression at transcriptional level. Adenovirus-mediated overexpression of TRB3 also decreased PDX-1 mRNA expression, but did not influence MafA mRNA expression.
Conclusions This study showed that ER stress induced TRB3 expression, but decreased both insulin and PDX-1 gene expression in INS-1 cells. Our data suggest that TRB3 plays an important role in ER stress-induced beta cell dysfunction.
-
Citations
Citations to this article as recorded by- Endoplasmic reticulum stress causes insulin resistance by inhibiting delivery of newly synthesized insulin receptors to the cell surface
Max Brown, Samantha Dainty, Natalie Strudwick, Adina D. Mihai, Jamie N. Watson, Robina Dendooven, Adrienne W. Paton, James C. Paton, Martin Schröder, James Arthur Olzmann
Molecular Biology of the Cell.2020; 31(23): 2597. CrossRef - PTB and TIAR binding to insulin mRNA 3′- and 5′UTRs; implications for insulin biosynthesis and messenger stability
Rikard G. Fred, Syrina Mehrabi, Christopher M. Adams, Nils Welsh
Heliyon.2016; 2(9): e00159. CrossRef - Asna1/TRC40 Controls β-Cell Function and Endoplasmic Reticulum Homeostasis by Ensuring Retrograde Transport
Stefan Norlin, Vishal S. Parekh, Peter Naredi, Helena Edlund
Diabetes.2016; 65(1): 110. CrossRef - Role of the Unfolded Protein Response inβCell Compensation and Failure during Diabetes
Nabil Rabhi, Elisabet Salas, Philippe Froguel, Jean-Sébastien Annicotte
Journal of Diabetes Research.2014; 2014: 1. CrossRef - Endoplasmic Reticulum Stress and Insulin Biosynthesis: A Review
Mi-Kyung Kim, Hye-Soon Kim, In-Kyu Lee, Keun-Gyu Park
Experimental Diabetes Research.2012; 2012: 1. CrossRef
- Endoplasmic reticulum stress causes insulin resistance by inhibiting delivery of newly synthesized insulin receptors to the cell surface
- The Effect of Chronic High Glucose Concentration on Endoplasmic Reticulum Stress in INS-1 Cells.
- Mi Kyung Kim, Hye Young Seo, Tae Sung Yun, Nam Kyung Kim, Yu Jin Hah, Yun Jung Kim, Ho Chan Cho, Young Yun Jang, Hye Soon Kim, Seong Yeol Ryu, In Kyu Lee, Keun Gyu Park
- Korean Diabetes J. 2008;32(2):112-120. Published online April 1, 2008
- DOI: https://doi.org/10.4093/kdj.2008.32.2.112
- 2,755 View
- 30 Download
-
 Abstract
Abstract
 PDF
PDF - BACKGROUND
The highly developed endoplasmic reticulum (ER) structure is one of the characteristic features of pancreatic beta-cells. Recent study showed that ER stress causes beta-cell dysfunction. However, little is known about the effects of high glucose concentration on induction of ER stress in pancreatic beta-cells. Therefore, this study was designed to evaluate whether exposure of high glucose concentration in rat insulinoma cell line, INS-1 cell induces ER stress and whether ER stress decreases insulin gene expression. METHODS: The effect of 30 mM glucose on insulin expression and secretion in INS-1 cells was evaluated by Northern blot analysis and glucose-stimulated insulin secretion (GSIS). Cell viability was evaluated by XTT assay. The effect of 30 mM glucose on phosphorylation of eIF2alpha and CHOP expression, which are markers of ER stress were evaluated by Western blot analysis. RT-PCR analysis was performed to determine whether high glucose concentration induces XBP-1 splicing. To investigate whether ER stress decreases insulin gene expression, the effect of tunicamycin on insulin mRNA expression was evaluated by Northern blot analysis. RESULTS: The prolonged exposure of INS-1 cells with the 30 mM glucose concentration decreased insulin mRNA expression in a time dependent manner and impaired GSIS while did not influence on cell viability. 30 mM glucose increased phosphorylation of eIF2alpha, XBP-1 splicing and CHOP expression in INS-1 cells. Tunicamycin-treated INS-1 increased XBP-1 splicing and decreased insulin mRNA expression in a dose dependent manner. CONCLUSION: This study showed that prolonged exposure of INS-1 with high glucose concentration induces ER stress and ER stress decreases insulin gene expression. Further studies about underlying molecular mechanism by which ER stress induces beta-cell dysfunction are needed.

 KDA
KDA
 First
First Prev
Prev





